Abstract
In an effort to combat the risks associated with traditional tobacco products, tobacco product innovation has been redirected towards reducing the consumer’s potential exposure to harmful or potentially harmful constituents (HPHCs). Among these innovations are modern oral nicotine products (MONPs). This product class aims to deliver nicotine while limiting the consumer’s potential toxicant exposure. This body of work sought to investigate the potential for select HPHC exposure (tobacco-specific nitrosamines, carbonyls, benzo[a]pyrene, nitrite, and metals) from MONPs and to compare it to that from traditional tobacco products. This work expands on previously published studies both in terms of diversity of products assessed and analytes tested. In total, twenty-one unique MONPs were assessed and compared to four traditional tobacco products. We found that there was a difference in the potential exposure based on the MONP filler—plant material vs. granulate/powder. Typically, the HPHC levels observed in plant-based MONPs were higher than those observed for granulate/powder products, most notably within the metals analysis, for which the levels were occasionally greater than those seen in traditional smokeless tobacco products. Generally, the overall HPHC levels observed in MONP were at or below those levels observed in traditional tobacco products.
1. Introduction
It is generally acknowledged that use of tobacco products is associated with risks. In an effort to combat these risks, tobacco science and production have refocused their efforts to provide consumers with products that may limit their potential exposure to harmful or potentially harmful constituents (HPHCs). In 2009, the Family Smoking Prevention and Tobacco Control Act (‘Tobacco Control Act’) was passed in which control over regulatory oversight was given to the US Food and Drug Administration (FDA) [1,2]. Included in this act were specific requirements for the language to be included on warning labels for various tobacco products and the need for scientific rigor when making claims for any modified risk profile a product may offer [3]. To be able to claim a modified risk profile, manufacturers must submit scientific evidence to support the claim as part of a Modified-Risk Tobacco Product (MRTP) application, and FDA permission must be received. The Tobacco Control Act further required the FDA to establish a list of harmful or potentially harmful constituents to human health found in mainstream smoke and tobacco products (referred to as the ‘HPHC list’) [4].
Reducing the consumer’s exposure to compounds on the HPHC list is one way that risk can conceivably be lowered. Given that combustion is the main source for many of the HPHC compounds, alternative means of delivering nicotine are being promoted and developed. Examples of such products are electronic nicotine delivery systems (ENDS) and heated tobacco products (HTP), which both produce aerosolized nicotine for inhalation. An alternative to smoking altogether is the use of traditional smokeless tobacco products (STPs), such as chewing tobacco and snuff. STPs have seen a 23.1% increase in total usage over the same period that cigarette consumption has declined by 38.7%, though whether the two are linked is unclear given that there is a perception among some US smokers that STPs do not provide any reduction in toxicant exposure [5,6,7]. Indeed, STPs are known to contain a number of HPHCs categorized by the IARC (International Agency for Research on Cancer) as being Group 1 carcinogens (e.g., formaldehyde, N-Nitrosonornicotine (NNN), 4-(N-Methylnitroamino)-1-(3-pyridyl)-1-butanone (NNK), and cadmium) [8,9,10,11]. The production techniques for STPs vary considerably and can include curing, fermentation, and pasteurization processes that will influence the HPHC profile of the resulting product, creating a broad spectrum of potential risk among this product class. For instance, typical US moist snuff (including snus) uses a fermentation process that leads to high levels of tobacco-specific nitrosamines (TSNAs) whereas Swedish-style moist snuff is heat-treated (pasteurized) and contains lower TSNA levels [12]. Of the few tobacco products that have been granted MRTP designation by the FDA, eight are Swedish-style snus products manufactured by Swedish Match USA, Inc., which can state they present a reduced risk for certain cancers and diseases when compared with cigarettes [13,14]. Similar to cigarettes, there has been an emergence of alternative products that replicate the STP usage experience with a lowered HPHC exposure risk, namely modern oral nicotine products.
Modern oral nicotine products (MONPs), also known as tobacco-free nicotine products (TFNPs), are a novel class of nicotine-containing products aimed at further reducing toxicant exposure while still delivering the desired nicotine dosage. These products are intended to be consumed in a similar way as STPs, with placement between the gum and the lip/cheek. MONPs are produced in two main formulations—white granular powders (WGP) and plant-based versions. The white granular powder MONPs are pre-portioned and composed of a number of ingredients that include a stabilized form of nicotine (e.g., nicotine salt, nicotine-polacrilex), pH-adjusting agents (e.g., sodium carbonates), filler materials (e.g., modified cellulose, microcrystalline cellulose), sweeteners, and flavorings. Plant-based MONPs more closely mimic traditional STPs in that they are moist products produced using many of the same techniques and are packaged as either long-cut (loose) or pre-portioned pouches. Plant-based MONPs differ from STPs in that they are made from non-tobacco plant-based materials with pharmaceutical-grade nicotine added during the production process. Both forms of MONPs are typically sold in a variety of flavors with some also having the option of multiple nicotine strengths, granting the consumer a variety of options to choose from. At the time of publication, no MRTP applications for any MONPs have been made public on the FDA website (as is required by the Tobacco Control Act).
With the requirements set forth by the FDA pertaining to the classification of modified-risk products, the challenges of accurately assessing potential HPHC exposure comes to the forefront of any analytical testing laboratory. Currently, there are a number of published, standardized methods for the analysis of HPHCs found in smokeless tobacco products. However, as potential HPHC levels are expected to be low, the question of whether these standardized methods are “fit for purpose” must be addressed. A 2021 collaborative study undertaken by the Tobacco and Tobacco Products Analytes (TTPA) sub-group of CORESTA (Cooperation Centre for Scientific Research Relative to Tobacco) examined the suitability of existing CORESTA recommended methods (CRMs) for the analysis of select HPHCs (nicotine, TSNAs, carbonyls, benzo[a]pyrene (B[a]P), and metals (arsenic and cadmium)) in nicotine pouches [15]. This study found that the methods were suitable and the nicotine pouch matrix was subsequently added to the scope of the respective CRMs in December 2021.
A survey of the current literature turns up few studies that focus on the assessment of MONPs and their potential HPHC exposure risk [16,17,18,19,20]. Further, these studies tend to have a narrow focus on a single product brand, limiting their overall scope. It is the intention of this publication to address this deficiency. Herein we describe the screening of seven brands of modern oral nicotine products. Within each brand, where possible, multiple flavors were analyzed. In addition to these products, two CORESTA smokeless tobacco reference products were screened, along with two smokeless tobacco products that are currently on the US market. In all, 25 unique products were assessed for a select list of HPHCs including TSNAs, carbonyls, nitrite, benzo[a]pyrene, and metals.
2. Materials and Methods
2.1. Materials
Standards were prepared either from neat materials (Sigma-Aldrich (St. Louis, MO, USA), Alfa Aesar (Tewksbury, MA, USA)) or an ISO 17034-certified reference standard solution containing the analytes of interest (Cerilliant (Round Rock, TX, USA), Toronto Research Chemicals (North York, ON, Canada), Inorganic Ventures (Christiansburg, VA, USA), Restek (Bellefonte, PA, USA), Accustandard (New Haven, CT, USA)). Where required, labelled internal standards were obtained from CDN Isotopes (Pointe-Clare, QC, Canada). For sample preparation, all reagents were sourced through Thomas Scientific (Swedesboro, NJ, USA) and were of American Chemical Society (ACS) grade or better where available, except for Type 1 water (18.2 MΩ⋅cm), which was generated in-house [21].
2.2. Test Products
All modern oral nicotine products were purchased by the authors through the online retailers, Northerner and Nicokick, with the exception of Black Buffalo products, which were purchased directly from the manufacturer. All test products were stored refrigerated and brought to room temperature prior to extraction.
2.3. Method Summaries
All product analysis was conducted using Enthalpy’s in-house methods, which are fully validated for the analysis of smokeless tobacco products and have been shown to be suitable for the analysis of modern oral nicotine products. Where the pouch weight exceeded the stated sample size, a single pouch was extracted and analyzed. All pouched products were cut in half prior to extraction, with both the filler and pouch material analyzed.
2.3.1. Nicotine Analysis
Nicotine was assessed for products where levels were not reported on the packaging from the manufacturer. The method used for analysis of nicotine was based upon the Centers for Disease Control and Prevention (CDC) method [22] and the CORESTA Recommended Method No. 62 (CRM 62) [23]. In brief, an aliquot of 2N sodium hydroxide was added to a pre-weighed sample (approximately 1.0 g). Methyl-t-butyl ether containing quinoline was added and nicotine was extracted into the organic layer via solvent–solvent extraction with mechanical shaking. An aliquot of the organic layer was transferred to a sample vial for analysis via GC-FID.
The analysis of nicotine for select products in this study was carried out using an Agilent (Santa Clara, CA, USA) 6890 gas chromatograph equipped with a flame ionization detector (FID). The analytical column used for analysis was a HP-5, 30 m × 0.32 mm ID × 0.25 µm, with a carrier gas (helium) flow rate of 1.7 mL/min. The injection volume was 2 µL, split 40:1 with an injection port temperature of 250 °C. The following GC oven temperature program was used: initial oven temperature of 110 °C, no hold; 10 °C/min to 185 °C, no hold; ramp 6 °C/min to 240 °C, hold for 10 min. The detector temperature was set to 250 °C.
The calibration curve was constructed with seven points using a linear calibration model with 1/x weighting. The calibration range was 0.0356 to 1.19 mg/mL.
2.3.2. Benzo[a]pyrene Analysis
Benzo[a]pyrene (B[a]P) was extracted from approximately 1.0 g of tobacco or MONP with methanol and mechanical shaking. After centrifuging the sample, the supernatant was collected and evaporated to approximately 1 mL. The sample was then filtered prior to analysis via UPLC-FLR (Ultra Performance Liquid Chromatography with Fluorescence detection). B[a]P was quantitated using B[a]P-d12 as the internal standard.
UPLC was carried out using a Waters (Milford, MA, USA) H-class quaternary pump with a flowthrough needle sample manager. The analytical column used for analysis was an Agilent Zorbax RRHD Eclipse PAH column (150 mm × 2.1 mm, 1.8 µm) with an Agilent Zorbax Eclipse PAH guard column (2.1 mm × 5 mm, 1.8 µm). A 10 µL injection volume was used with a flow rate of 0.5 mL/min and a column temperature of 45 °C. The injection run time was 10 min with an isocratic gradient profile consisting of 20% water and 80% acetonitrile. Samples were detected using a Waters ACQUITY fluorescence detector with an excitation wavelength of 364 nm and an emission wavelength of 405 nm.
The calibration curve was constructed with ten points using a linear calibration model with 1/x weighting. The calibration range used was 0.10 to 100.4 ng/mL.
2.3.3. Nitrite Analysis
Nitrite was extracted from approximately 2 g of tobacco or MONP using water and mechanical shaking. The extracts were centrifuged and the supernatant was filtered prior to analysis via a continuous flow analyzer (CFA). During analysis, nitrite permeated through a dialysis membrane and reacted with sulfanilamide to form a diazonium ion, which was further coupled with N-1-naphthylethyldiamine dihydrogen chloride (NED) to form a purple azo dye. Absorbance was measured at 540 nm and sample extracts were quantitated using external calibration.
The analysis of tobacco extracts was performed using an Astoria 2 continuous flow analyzer (Astoria-Pacific, Clackamas, OR, USA) with a nitrite manifold and 540 nm filter.
The calibration curve was constructed with six points using a first-order polynomial calibration model with no weighting. The calibration range used was 0.10 to 3.0 µg/mL.
2.3.4. Tobacco-Specific Nitrosamine (TSNA) Analysis
The method of extraction was based upon the CORESTA Recommended Method No. 72 (CRM 72) [24]. TSNAs were extracted from approximately 1 g of tobacco or MONP using an aqueous solution of ammonium acetate and shaken mechanically. Extracts were subsequently filtered prior to analysis by UPLC-MSMS. The level of TSNAs present in each brand/sample was quantified using deuterated analogs of each analyte as internal standards (NAB-d4, NAT-d4, NNK-d4, and NNN-d4).
UPLC analysis was carried out using a Waters ACQUITY binary pump. The analytical column used was a Waters ACQUITY BEH C18 (2.2 mm × 100 mm, 1.7 µm) column with matching pre-column. The mobile phases used were A: 0.01% acetic acid in water and B: 0.1% acetic acid in methanol. An injection volume of 10 µL was used with a flow rate of 0.2 mL/min. The gradient profile was: 0–1 min 99% solvent A; 1–4 min 99–10% solvent A; 4–4.01 min 10–1% solvent A; 4.01–5.75 min 1% solvent A; 5.75–5.9 min 1–99% solvent A; 5.9–8 min 99% solvent A. A constant column temperature of 55 °C was maintained throughout the injection.
Detection of the TSNAs was carried out using a Waters (Milford, MA, USA) Xevo-TQ detector. Samples were analyzed using electrospray ionization operating in positive ion mode (ESI+). The source temperature was 150 °C and the desolvation temperature was 500 °C. Nitrogen was used as the desolvation gas (1000 L/h) and argon was used as the collision gas. Analytes were detected in multiple reaction monitoring mode with specific analysis parameters described in Table 1.

Table 1.
MS/MS instrument parameters for the detection of TSNAs.
The calibration curve was constructed with eight points using a quadratic calibration model with 1/x weighting. The calibration range for each of the TSNAs was as follows: NAT, NNK, NNN: 0.48 to 300 ng/mL; NAB: 0.12 to 75 ng/mL.
2.3.5. Carbonyls Analysis
The method of extraction was based upon the CORESTA Recommended Method No. 86 (CRM 86) [25]. Approximately 1 g of tobacco or MONP was suspended in acidic aqueous ammonium formate buffer solution. Any carbonyls that were present were derivatized using 2,4-dinitrophenylhydrazine (DNPH) and then extracted into hexanes in situ via solvent–solvent extraction. The hexane layer was transferred to an autosampler vial for analysis via UPLC-MS/MS. Quantitation was performed using the deuterated internal standards formaldehyde-d2, acetaldehyde-d4, and crotonaldehyde-DNPH-d3.
UPLC was carried out using a Waters ACQUITY binary pump. The analytical column used for analysis was a Waters ACQUITY BEH Shield RP18 column (2.1 mm × 100 mm, 1.7 µm) with a Waters BEH C18 guard column (2.1 mm × 5 mm, 1.7 µm). The mobile phases used were A: 1 mM acetic acid in water and B: 1 mM acetic acid in 93:7 (v/v) methanol:acetonitrile. A 2 µL injection volume was used with a constant flow rate of 0.35 mL/min and a column temperature of 50 °C. The gradient profile used was: 0.0–4.0 min 55–40% solvent A; 4.0–5.5 min 40–37% solvent A; 5.5–7.25 min 37–25% solvent A; 7.25–7.27 min 25–0% solvent A; 7.27–8.25 min hold at 0% solvent A; 8.25–8.27 min 0–55% solvent A; 8.27–10.25 min 55% solvent A.
Detection of the carbonyls was carried out using a Waters Xevo-TQ mass spectrometer. Samples were analyzed using electrospray ionization operating in negative ion mode (ESI-). Source parameters included a 2.00 kV capillary voltage, 150 °C source temperature, and a desolvation temperature of 500 °C. Nitrogen was used as the desolvation gas (1000 L/h) and argon was used as the collision gas. Analytes were detected in multiple reaction monitoring mode and compound specific parameters can be found in Table 2.

Table 2.
MS/MS instrument parameters for the detection of cabonyls.
The calibration curve was constructed with eight points using a linear calibration model with 1/x weighting. The calibration range for each analyte was as follows: acetaldehyde: 0.0101 to 2.01 µg/mL; crotonaldehyde: 0.00548 to 0.199 µg/mL; formaldehyde: 0.0101 to 2.01 µg/mL.
2.3.6. Metals Analysis
The method of extraction was a modified version of the CORESTA Recommended Method No. 93 (CRM 93) [26]. Metals were quantitated from an aqueous digestion of approximately 0.5 g of tobacco or MONP. The digestion was performed using concentrated trace-metal-grade nitric acid utilizing a microwave followed by centrifugation. Samples were quantified by ICP-MS equipped with a dynamic reaction cell. Internal standards were used to correct for instrumental drift and were tailored to the metal being quantitated.
Metals analysis was performed on an Agilent (Santa Clara, CA, USA) 7700 ICP-MS. Self-tuning was performed each day of analysis and 72-Ge, 103-Rh, and 209-Bi were used as internal standards. A complete list of instrument parameters can be found in Table 3 and the masses of measure can be found in Table 4.

Table 3.
ICP-MS operating parameters for the detection of metals.

Table 4.
ICP-MS mass/elements of analytes measured in each operating mode.
The calibration range for the metal analytes was as follows: As, Ni: 0.10 to 50 ng/mL; Be, Cd, Cr, Co: 0.05 to 50 ng/mL; Pb: 0.05 to 30 ng/mL; Se: 0.20 to 50 ng/mL.
3. Results and Discussion
3.1. Study Design
Current product innovation is focused on reducing consumer exposure to HPHCs through the development of modified-risk products. As these products aim to reduce the consumer’s exposure to harmful or potentially harmful constituents, the expected levels of each analyte examined is expected to be lower than those observed in traditional smokeless tobacco products. As MONPs are a relatively new product class, most of the literature published are by the product manufacturers and only focus on their own products, which can make cross product comparisons difficult. This study sought to address this by providing a direct comparison within a single study.
For this work, twenty-five (25) different products from nine individual manufacturers were selected for evaluation (Table 5). Of these twenty-five, four were traditional smokeless tobacco products chosen for comparative purposes. The goal was to obtain products from a range of manufacturers that also varied in flavor and product type (pouch vs. long cut). An effort was made to diversify the flavor profiles, but also to ensure flavor overlap between manufacturers when possible. It must be noted that given the variety of nicotine strengths, not only from brand to brand, but even within a manufacturer’s own flavor, the products selected for analysis were at the maximum nicotine strength available. As this is a relatively new product classification, consideration of overall market share was not a top priority in the selection of brands. As mentioned previously, MONP are produced in two main forms: white granular powders (pouches) and plant-based (pouched or long cut). Based on these forms of consumption (pouch/long cut), four traditional smokeless tobacco products, two commercial brands and two CORESTA reference products (CRP1.1 and CRP2.1), were selected as the comparators. The commercial pouch product, General Wintergreen White Portion, was chosen as this product has received permission from the FDA to be marketed as a modified-risk tobacco product [13]. Each of these products was screened for several HPHCs that are shown in Table 6, along with their IARC designations.

Table 5.
Modern oral nicotine products and smokeless tobacco comparators tested in this study.

Table 6.
IARC designations for the HPHCs of interest.
Our own in-house methods (see Section 2.3) for the analysis of the selected HPHCs are fully validated and based upon the corresponding CORESTA recommended methods, with the exception of B[a]P, where a UHPLC method with fluorescence detection is used, and nitrite, which is based on Astoria-Pacific Method A181 (itself based on EPA method 353.2) [40]. The challenge presented by MONPs is that most HPHCs should be found in levels lower than what are considered typical in tobacco products. As a result, the limits of detection (LOD) and quantitation (LOQ) become more critical for each analytical method and may not be appropriate for the analysis of MONPs should the analytes be present. The 2021 CORESTA collaborative study [15] did not assess the suitability of the respective CRM LOQs, which were set with regard to the typical native levels found in tobacco products, and so this was an additional consideration in our study. The LOQs for our in-house methods are listed in Table 7, along with the LOQ values from the analogous CRM. Except for the metals and nitrite analysis methods, all of the LOQs for the validated methods are comparable to those listed in the analogous CRM. It is worth noting that CRMs are consensus methods that are evaluated by multiple laboratories before approval and publishing. As such, the method LOQs can be much higher than a particular laboratory may be capable of achieving in order to allow for differences in instrumentation and expertise across laboratories.

Table 7.
Limits of detection and quantitation for Enthalpy’s in-house analytical methods (see Section 2.3) and the analogous CRM quantitation limits (note: CRMs do not provide LODs).
Using our in-house methods (see Section 2.3), we analyzed the selected products for the 17 HPHCs listed in Table 7 and, where necessary, nicotine. For ease of comparison, the results were initially calculated on a per-gram basis without correction for water content, a full summary of which can be found in Table S1 of the Supplementary Materials. The results were not corrected for any moisture content since our aim was to examine the consumer’s potential exposure during use. Overall, the general trend observed followed what was expected, with the average levels measured in MONPs being lower than those measured in selected smokeless tobacco products. The trends for each analyte class are discussed in the following sections.
3.2. Nicotine
Since MONPs do not contain tobacco, nicotine is added during their manufacture. For the vast majority of the products, including most of those used in this study, the nicotine levels are reported on the packaging and range from 4 mg to 12 mg per portion (Table 5). This is comparable to the levels observed in the traditional smokeless products used as comparators (7 or 8 mg per portion for the snus products and 10 or 12 mg per portion for the long-cut products). Since some of the products selected for this study did not list their nicotine content on their packaging, they were assessed experimentally, and were found to exhibit comparable nicotine content to the other products being tested, ranging from 3.1 to 8.0 mg/portion.
3.3. Benzo[a]pyrene
Benzo[a]pyrene (B[a]P) is typically produced during combustion or any manufacturing process that may require heat (curing) [44]. Even if some MONPs are exposed to these types of processes, levels are still expected to be low. Of all the MONPs examined, only Fully Loaded, Berry Long Cut (F4) had detectable levels of B[a]P (1.27 ± 0.04 ng/g). This was far below those levels seen in the two long-cut STP comparators, which were 77.2 and 151 ng/g.
3.4. Nitrite and TSNAs
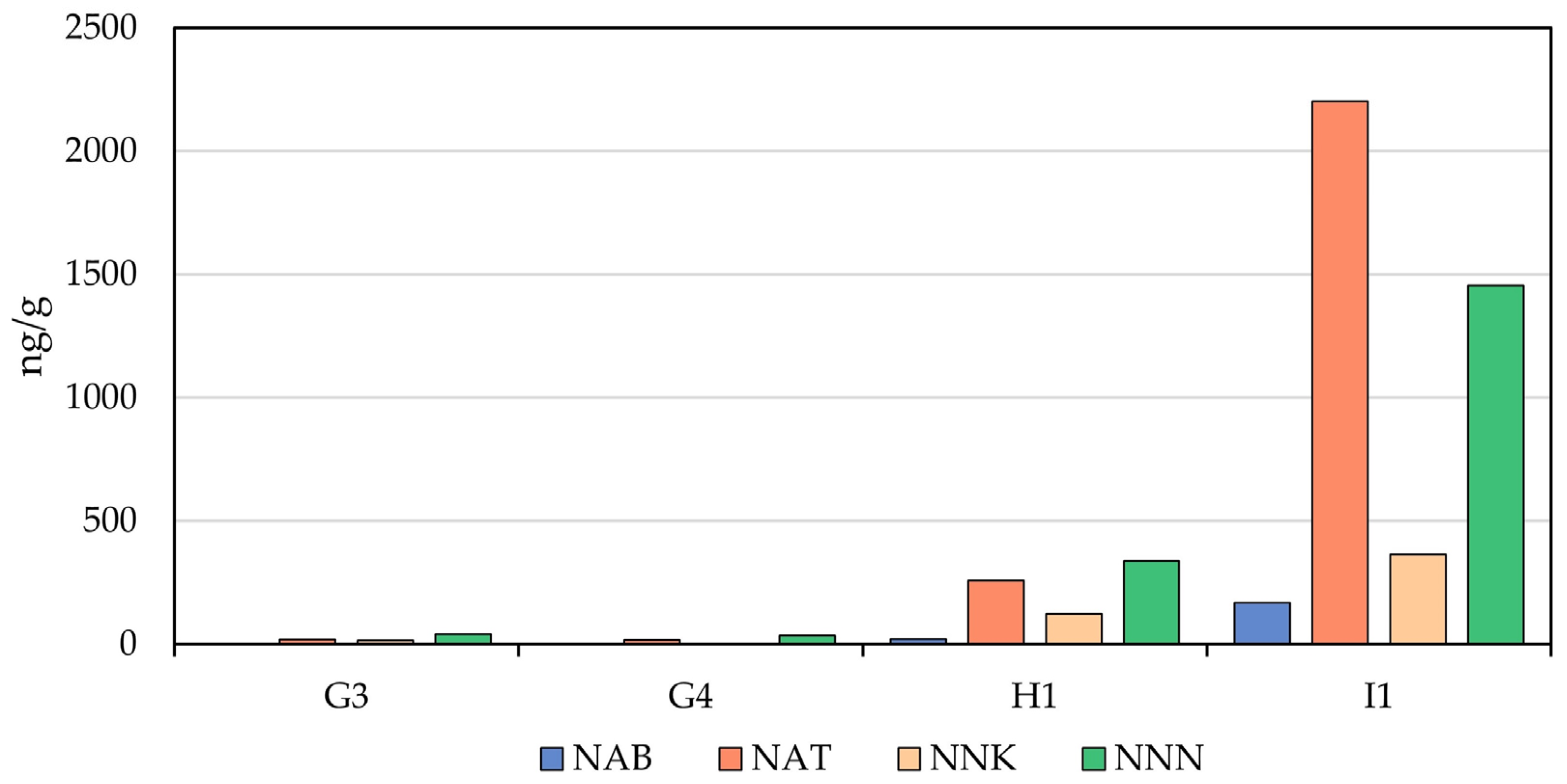
Nitrite is a precursor to TSNA formation [45,46] and is readily found in smokeless tobacco products [47]. Generally, the nitrite observed in MONPs was lower than those levels seen in STPs, except for one product (G2) that contained three-to-four times more nitrite than observed in the STPs. Given the low nitrite observed, it is not surprising that the levels of TSNAs observed for almost all of the MONPs in this study were below the LOQ or non-detectability threshold. The exceptions to this were the two Black Buffalo long-cut products (G3 and G4) that were found to contain NAT (18.5 ± 0.3 and 14.8 ± 1.3 ng/g, respectively), NNK (13.7 ± 0.4 ng/g in G3 only), and NNN (39.4 ± 1.3 and 32.4 ± 0.8 ng/g, respectively), shown in Figure 1. Both the NAT and NNK amounts were close to the respective LOQ values, while the NNN was close to three times the LOQ. In contrast to the MONPs, all four STPs were found to contain the four TSNAs at higher levels, particularly the CRP2.1 reference product (NAB: 9 to 274 ng/g; NAT: 125 to 4168 ng/g; NNK: 40 to 2104 ng/g; NNN: 177 to 3380 ng/g). While our analysis of MONPs shows that the LOQs associated with the method used are suitable for comparison to STPs, lower LOQs may be more beneficial when analyzing for regulatory reporting purposes. Adapting methods for trace TSNA analysis in ENDS e-liquid and aerosol may be a means for achieving this [48].
Figure 1.
Comparison of the observed TSNA levels in the two long-cut Black Buffalo MONPs (G3 and G4) to the two commercial STPs (H1 and I1).
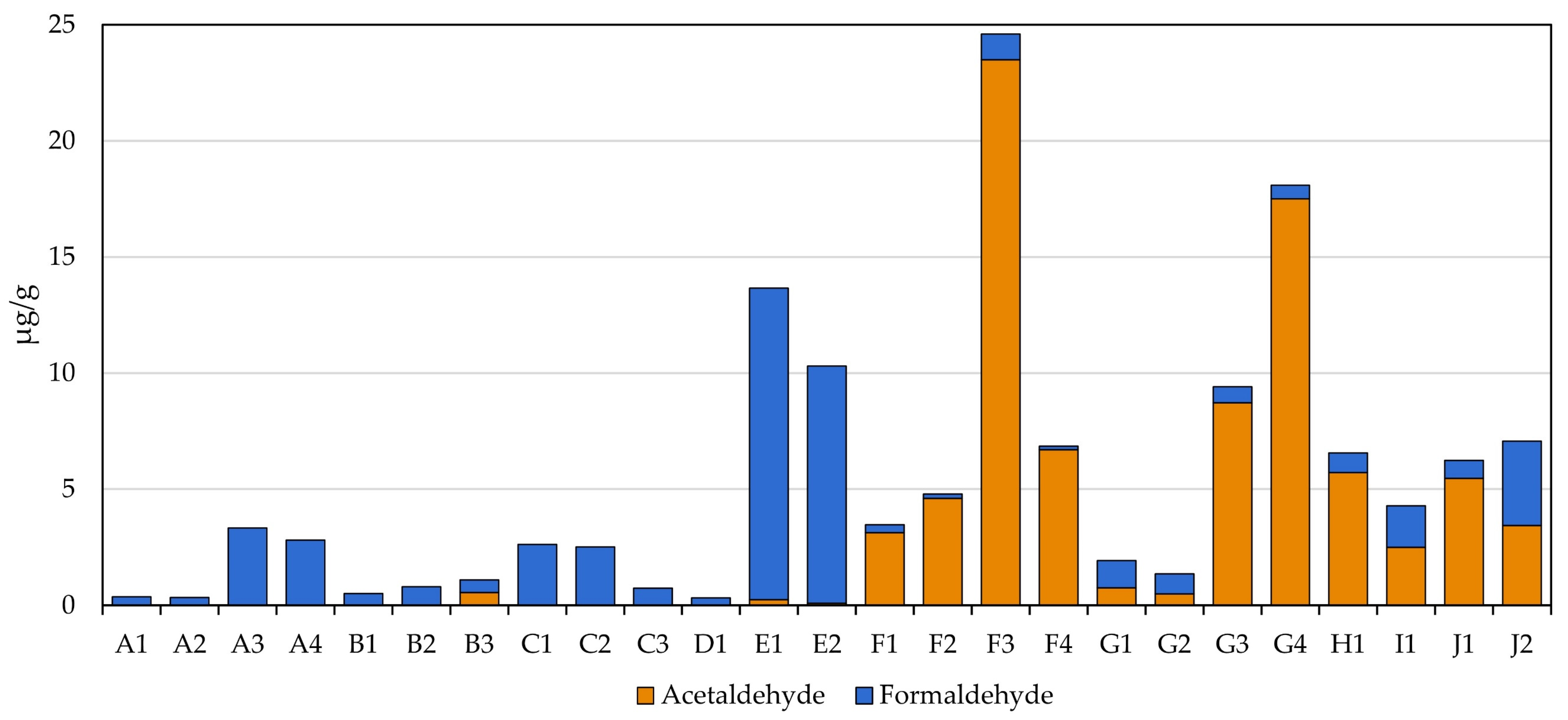
3.5. Carbonyls
One of the more interesting classes of compounds assessed in this study was carbonyls, for which there were distinct trends observed based solely on the filler composition of the MONP (Figure 2). The formaldehyde levels measured in all MONPs were comparable to those measured in the smokeless tobacco products tested (0.33 to 3.33 µg/g for MONPs and 0.78 to 3.64 µg/g for STPs). Two WGP-based MONPs, E1 and E2 (Zyn Nicotine Pouches), exhibited levels that were approximately three-to-four times those seen in STPs, being in the 10 to 14 µg/g range.
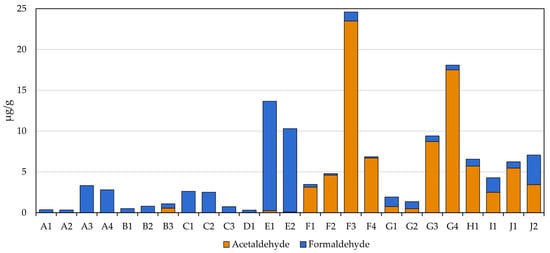
Figure 2.
Observed levels of formaldehyde and acetaldehyde in the MONPs and four STPs.
Although the levels of formaldehyde were fairly consistent among all products screened, acetaldehyde appeared to be composition specific. The acetaldehyde levels measured in WGP-based products were typically below the LOQ or non-detectability except for one product, B3, that contained 0.56 ± 0.06 µg/g. Conversely, the acetaldehyde measured in the plant-based products ranged from lower to substantially higher than the levels observed in STPs, topping out at 23.5 µg/g versus 5.7 µg/g measured in the commercial snus product (H1).
Interestingly, the highest levels of acetaldehyde measured in MONP were observed in products F3 and G4, both of which happen to be peach flavored. It is also noteworthy that one of these products (G4) was the only product tested to have detectable crotonaldehyde, albeit just above the LOQ. Given that manufacturers will likely use the same base composition across their respective products, it is indicative of the elevated acetaldehyde levels observed being due to this particular flavorant.
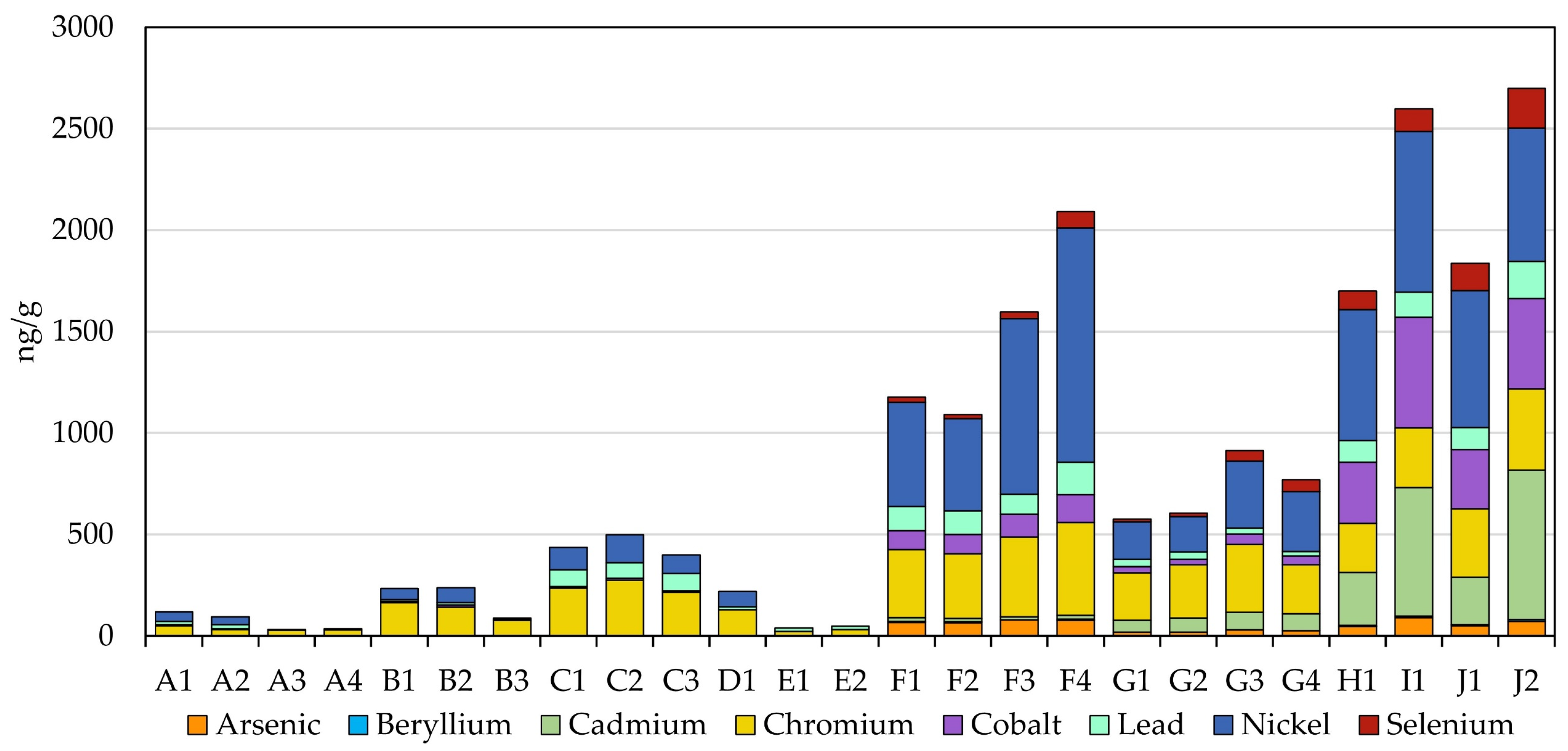
3.6. Metals
Similar to carbonyls, there are clear differences in the metal analyte profiles of the two MONP types (Figure 3). WGP-based MONPs generally show much lower levels of metals, if present at all, than the plant- and tobacco-based products. Arsenic, beryllium, cadmium, and selenium were all either below the LOD or below the LOQ. Cobalt (4.5 to 10.4 ng/g) and lead (3.9 to 19.6) were all within six-times the LOQ (both 3.7 ng/g), except for lead in the Rogue Pouch products, C1 to C3 (77 to 83 ng/g), which were closer to the levels seen in the pouched STPs (approximately 107 ng/g). The more prevalent metals in WGP-MONPs were chromium (22.5 to 274 ng/g) and nickel (39.4 to 138 ng/g), though the levels were, for the most part, much lower than in the pouched STPS (Cr: 242 to 336 ng/g; Ni: 644 to 676 ng/g). The highest levels were seen for the Rogue Pouch products (Cr: 215 to 274 ng/g; Ni: 92.5 to 138 ng/g), with two of the on! Nicotine Pouch products (B1 and B2) and Fré Lush also showing elevated Cr levels relative to the majority of the WGP-MONPs tested.
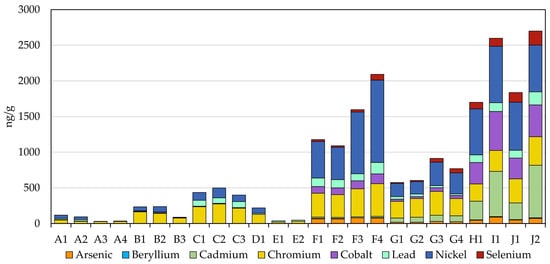
Figure 3.
Observed levels of metals in the MONPs and four STPs.
In contrast to the WGP-based MONPs, the plant-based MONPs showed greater comparability with the tobacco-based products, though overall contained lower metal levels than the STPs with some noted exceptions. Beryllium was at or below the LOQ in all eight plant-based MONPs, and cadmium (16.0 to 86.0 ng/g) and cobalt (29.0 to 139 ng/g) were well below the levels in the STPs (Cd: 233 to 735 ng/g; Co: 293 to 546 ng/g). Arsenic (17.9 to 78.7 ng/g), chromium (233 to 456 ng/g), lead (21.7 to 159 ng/g), and selenium (12.4 to 81.8 ng/g) were observed at levels that were below or comparable to those in the STPs (As: 46.8 to 90.4 ng/g; Cr: 242 to 401 ng/g; Pb: 107 to 182 ng/g; Se: 93.5 to 197 ng/g). Nickel levels ranged from well below (175 ng/g in G2) to well above (1115 ng/g in F4) those in STPs (644 to 792 ng/g). Furthermore, there were clear differences between the two product brands tested, with the Fully Loaded brand generally containing higher metals levels than the Black Buffalo products for all analytes except cadmium and, for select products, selenium. This difference in levels may reflect the plants used to create the products and their respective uptake of metals from the soil and air. The generally higher levels in the Fully Loaded products may have been due to the use of kudzu root [49], compared with edible green leaves for Black Buffalo products [50], since a plant’s root system is responsible for the absorption of nutrients from the surrounding soil. The kudzu plant was even shown to have utility for the lead phytoremediation of soils through rhizofiltration (root absorption) [51]. Finally, the metal content of the long-cut MONP formulations of the products were, with few exceptions, higher than their pouched counterparts. This may have been due to differences in the ingredients—the Fully Loaded long-cut formulation, for instance, contains molasses whereas the pouch formulation does not [49]—or it could simply have been due to batch variations in the base plant matrix used during manufacturing.
3.7. Exposure Assessment
One of the goals of reduced risk products is to be able to provide comparable nicotine delivery with reduced exposure to potential HPHCs. However, assessing potential exposure is not a trivial task. The frequency of use is directly associated with the potential exposure to HPHCs and can vary widely across the globe. For example, a study conducted in the United States examined adult usage of specific brands of snus and found the study subjects consumed an average of 3.3 pouches per day [52]. This is a relatively small amount when compared to the consumption habits found in Sweden where a survey conducted found the average daily consumption to be 11–12 g and 29–32 g for pouched snus and loose snus, respectively [53]. Due to this wide range in consumption habits, where available, the approximate portion size (pouch weight) was obtained and is provided in Table 5, providing context to potential usage. The observed HPHC analyte levels on a per-portion basis can also be found in Table S2 of the Supplementary Materials, and were calculated using the actual number of pouches analyzed per replicate (for long-cut products, a 1 g portion size was used). For the WGP-based MONPs, the HPHC amount per portion will be reduced relative to both the STP amounts and their own per-gram amount since they have portion weights below 0.7 g. The plant-based MONP pouches, on the other hand, are either comparable (Black Buffalo, G1 and G2) or higher (Fully Loaded, F1 and F2) than their per-gram amounts. Relative to the pouched STPs, there are few changes to the trends described above, the exceptions being for the heavier Fully Loaded pouch products in which acetaldehyde becomes comparable, and lead and nickel become higher per portion.
Overall, the MONPs appear to pose a much-reduced exposure risk compared to STPs, though the caveat here is that the sample size for STPs is very limited in this study and does not represent the full range of products available to the consumer. Ultimately, however, it will be the end user’s consumption habits that determine their particular potential HPHC exposure risk.
4. Conclusions
The purpose of this work was to expand upon previous studies examining the potential HPHC content of modern oral nicotine products. With the push towards reducing the consumer’s potential HPHC exposure, interest in the science surrounding modern oral nicotine products has increased. Previous work typically focused on assessing a single product brand, where this study sought to assess 25 unique products, which also includes two CORESTA reference products and two traditional smokeless tobacco products. Generally, products that are composed of powder-based materials displayed much lower levels of the HPHCs being assessed than those observed in the plant-based oral nicotine products. This trend was most evident in the metals and acetaldehyde analysis, and was likely due to a combination of higher native levels in the plants being used and any curing or manufacturing processes employed during production.
Although the levels reported here for MONPs are typically lower than the commercial and reference STPs analyzed, it should be noted that the STPs used in this study do not represent all smokeless tobacco products and these results should be viewed in that context. Further work is suggested to provide a more complete picture of the toxicant exposure risk to consumers with relation to all available marketed products. Potential areas for inclusion would be an examination into how the nicotine level may affect the HPHC amounts and a probe of potential batch-to-batch variability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9030065/s1, Table S1: Tabulated results on a per-gram basis for select HPHC analytes in the chosen MONPs and STP comparators; Table S2: Tabulated results on a per-portion basis for select HPHC analytes in the chosen MONPs and STP comparators; Raw data with calculations.xlsx.
Author Contributions
Conceptualization, J.J.J. and A.G.C.; methodology, J.J.J. and A.G.C.; formal analysis, J.J.J., A.G.C., and A.M.M.; investigation, J.J.J. and A.G.C.; resources, J.J.J., A.G.C., and A.M.M.; data curation, J.J.J. and A.G.C.; writing—original draft preparation, J.J.J. and A.G.C.; writing—review and editing, J.J.J., A.G.C., and A.M.M.; visualization, J.J.J., A.G.C. and A.M.M.; supervision, A.M.M.; project administration, J.J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Enthalpy Analytical, LLC with internal funds. No external funding was received in support of this work. All commercial modern oral nicotine products and smokeless tobacco products were purchased from the retail market by Enthalpy Analytical staff.
Data Availability Statement
All data used in this work are available in the Supplementary Materials.
Conflicts of Interest
The authors declare the following financial/personal relationships that may be considered competing interests: The authors are employed by Enthalpy Analytical, LLC. Enthalpy Analytical, LLC is a commercial testing laboratory with a focus on nicotine-containing products and provides services to a wide range of clients including tobacco manufacturers and regulatory authorities. This study was funded internally by Enthalpy Analytical and no industry funding was accepted for the preparation of this manuscript. All products used in this study were purchased from the retail market by Enthalpy Analytical, LLC.
References
- Family Smoking Prevention and Tobacco Control Act Table of Contents. Available online: https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/family-smoking-prevention-and-tobacco-control-act-table-contents (accessed on 24 January 2022).
- Food and Drug Administration Family Smoking Prevention and Tobacco Control Act—An Overview. Available online: https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/family-smoking-prevention-and-tobacco-control-act-overview#smokeless (accessed on 4 April 2021).
- Food and Drug Administration; US Department of Health and Human Services. Draft Guidance for Industry: Modified Risk Tobacco Product Applications; Availability; Agency Information Collection Activities; Proposed Collection; Comment Request. Fed. Regist. 2012, 77, 20026–20030. [Google Scholar]
- Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke—Established List. Fed. Regist. 2012, 77, 20034–20037. [Google Scholar]
- Wang, T.; Kenemer, B.; Tynan, M.; Singh, T.; King, B. Consumption of Combustible and Smokeless Tobacco—United States, 2000–2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Timberlake, D.S. Are smokers receptive to using smokeless tobacco as a substitute? Prev. Med. 2009, 49, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Sami, M.; Timberlake, D.S.; Nelson, R.; Goettsch, B.; Ataian, N.; Libao, P.; Vassile, E. Smokers’ perceptions of smokeless tobacco and harm reduction. J. Public Health Policy 2012, 33, 188–201. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer List of Classifications—IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 12 April 2021).
- International Agency for Research on Cancer. Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol; International Agency for Research on Cancer: Lyon, France, 2006; ISBN 978-92-832-1288-1. [Google Scholar]
- International Agency for Research on Cancer. Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines; World Health Organization: Geneva, Switzerland, 2006; ISBN 978-92-832-1289-8. [Google Scholar]
- International Agency for Research on Cancer. Arsenic, Metals, Fibres, and Dusts; International Agency for Research on Cancer: Lyon, France, 2012; Volume 1989, ISBN 978-92-832-1320-8. [Google Scholar]
- Borgerding, M.F.; Bodnar, J.A.; Curtin, G.M.; Swauger, J.E. The chemical composition of smokeless tobacco: A survey of products sold in the United States in 2006 and 2007. Regul. Toxicol. Pharmacol. 2012, 64, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration Modified Risk Orders. Available online: https://www.fda.gov/tobacco-products/advertising-and-promotion/modified-risk-orders (accessed on 11 April 2021).
- Food and Drug Administration. FDA Grants First-Ever Modified Risk Orders to Eight Smokeless Tobacco Products. Available online: https://www.fda.gov/news-events/press-announcements/fda-grants-first-ever-modified-risk-orders-eight-smokeless-tobacco-products (accessed on 11 April 2021).
- CORESTA TTPA Sub-Group. Technical Report 2021 Nicotine Pouches Collaborative Study. Available online: https://www.coresta.org/2021-nicotine-pouches-collaborative-study-35369.html (accessed on 24 January 2022).
- Aldeek, F.; McCutcheon, N.; Smith, C.; Miller, J.H.; Danielson, T.L. Dissolution Testing of Nicotine Release from OTDN Pouches: Product Characterization and Product-to-Product Comparison. Separations 2021, 8, 7. [Google Scholar] [CrossRef]
- Wagner, K.A.; Brown, A.P.; Jin, X.C.; Sharifi, M.; Lopez, V.F.; Ballentine, R.M.; Melvin, M.S.; Mcfarlane, C.B.; Morton, M.J.; Danielson, T.L. Characterization of on! ® nicotine pouches—Part 1: HPHCs. In Proceedings of the SRNT 26th Annual Meeting, New Orleans, LA, USA, 11–14 March 2020. [Google Scholar]
- Bishop, E.; East, N.; Bozhilova, S.; Santopietro, S.; Smart, D.; Taylor, M.; Meredith, S.; Baxter, A.; Breheny, D.; Thorne, D.; et al. An approach for the extract generation and toxicological assessment of tobacco-free ‘modern’ oral nicotine pouches. Food Chem. Toxicol. 2020, 145, 111713. [Google Scholar] [CrossRef]
- East, N.; Bishop, E.; Breheny, D.; Gaca, M.; Thorne, D. A screening approach for the evaluation of tobacco-free ‘modern oral’ nicotine products using Real Time Cell Analysis. Toxicol. Rep. 2021, 8, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, D.; Liu, C.; Murphy, J. Chemical characterization of tobacco-free “modern” oral nicotine pouches and their position on the toxicant and risk continuums. Drug Chem. Toxicol. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- ASTM D1193-06(2018); Standard Specification for Reagent Water. ASTM International. Available online: https://www.astm.org/d1193-06r18.html (accessed on 24 January 2022).
- Centers for Disease Control and Prevention (CDC); US Department of Health and Human Services. Notice Regarding Revisions to the Laboratory Protocol To Measure the Quantity of Nicotine Contained in Smokeless Tobacco Products Manufactured, Imported, or Packaged in the United States. Fed. Regist. 2009, 74, 609–768. [Google Scholar]
- Cooperation Centre for Scientific Research Relative to Tobacco CORESTA Recommended Method No. 62 Determination of Nicotine in Tobacco and Tobacco Products by Gas Chromatographic Analysis. Available online: https://www.coresta.org/determination-nicotine-tobacco-and-tobacco-products-gas-chromatographic-analysis-29185.html (accessed on 21 February 2022).
- Cooperation Centre for Scientific Research Relative to Tobacco CORESTA Recommended Method No. 72 Determination of Tobacco Specific Nitrosamines in Tobacco and Tobacco Products by LC-MS/MS. Available online: https://www.coresta.org/determination-tobacco-specific-nitrosamines-tobacco-and-tobacco-products-lc-msms-29195.html (accessed on 24 January 2022).
- Cooperation Centre for Scientific Research Relative to Tobacco CORESTA Recommended Method No. 86 Determination of Select Carbonyls in Tobacco and Tobacco Products by UHPLC-MS/MS. Available online: https://www.coresta.org/determination-select-carbonyls-tobacco-and-tobacco-products-uhplc-msms-30991.html (accessed on 24 January 2022).
- Cooperation Centre for Scientific Research Relative to Tobacco CORESTA Recommended Method No. 93 Determination of Selected Metals in Tobacco Products by ICP-MS. Available online: https://www.coresta.org/determination-selected-metals-tobacco-products-icp-ms-33784.html (accessed on 24 January 2022).
- Brands. Available online: https://www.reynoldsamerican.com/brands (accessed on 25 January 2022).
- Our Companies—Altria. Available online: https://www.altria.com/en/about-altria/our-companies (accessed on 25 January 2022).
- Who is Rogue Holdings, LLC?—Rogue NOD. Available online: https://help.roguenicotine.com/hc/en-us/articles/360054562234-Who-is-Rogue-Holdings-LLC- (accessed on 25 January 2022).
- Turning Point Brands—Our Company—Company Timeline. Available online: https://www.turningpointbrands.com/our-company/company-timeline/default.aspx (accessed on 25 January 2022).
- Smokeless Tobacco Reference Products. Available online: https://strp.wordpress.ncsu.edu/ (accessed on 24 February 2022).
- International Agency for Research on Cancer. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; International Agency for Research on Cancer: Lyon, France, 2005; ISBN 978-92-832-1292-8. [Google Scholar]
- International Agency for Research on Cancer. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins; International Agency for Research on Cancer: Lyon, France, 2010; ISBN 978-92-832-1294-2. [Google Scholar]
- International Agency for Research on Cancer. Re-Evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide (Part 1, Part 2, Part 3); World Health Organization: Geneva, Switzerland; International Agency for Research on Cancer: Lyon, France, 1999; ISBN 978-92-832-1271-3. [Google Scholar]
- International Agency for Research on Cancer. Acrolein, Crotonaldehyde, and Arecoline; International Agency for Research on Cancer: Lyon, France, 2021; ISBN 978-92-832-0195-3. [Google Scholar]
- International Agency for Research on Cancer. Chlorinated Drinking-Water; Chlorination By-Products; Some Other Halogenated Compounds; Cobalt and Cobalt Compounds; IARC: Lyon, France, 1991; ISBN 978-92-832-1252-2. [Google Scholar]
- International Agency for Research on Cancer. Some Metals and Metallic Compounds; International Agency for Research on Cancer: Lyon, France, 1980; Volume 23, ISBN 978-92-832-1223-2. [Google Scholar]
- International Agency for Research on Cancer. Chromium, Nickel and Welding; IARC: Lyon, France, 1990; ISBN 978-92-832-1249-2. [Google Scholar]
- International Agency for Research on Cancer. Some Aziridines, N-, S- and O-Mustards and Selenium; World Health Organization: Geneva, Switzerland, 1975; ISBN 978-92-832-1209-6. [Google Scholar]
- O’Dell, J.W. U.S. Environmental Protection Agency Method 353.2, Revision 2.0: Determination of Nitrate-Nitrite Nitrogen by Automated Colorimetry; US Environmental Protection Agency: Cincinnati, OH, USA, 1993. [Google Scholar]
- Cooperation Centre for Scientific Research Relative to Tobacco CORESTA Recommended Method No. 36 Determination of Nitrate in Tobacco and Smokeless Tobacco Products by Reduction to Nitrite and Continuous Flow Analysis. Available online: https://www.coresta.org/determination-nitrate-tobacco-and-smokeless-tobacco-products-reduction-nitrite-and-continuous-flow (accessed on 24 January 2022).
- Cooperation Centre for Scientific Research Relative to Tobacco CORESTA Recommended Method No. 82 Determination of Benzo[a]pyrene in Tobacco Products by GC-MS. Available online: https://www.coresta.org/determination-benzoapyrene-tobacco-products-gc-ms-29897.html (accessed on 24 January 2022).
- CORESTA TTPA Sub-Group Technical Repor Determination of Nitrite and Nitrate in Smokeless Tobacco Products by Ion Chromatography and Continuous Flow Analysis—2016 Collaborative Study. Available online: https://www.coresta.org/determination-nitrite-and-nitrate-smokeless-tobacco-products-ion-chromatography-and-continuous-flow (accessed on 24 January 2022).
- McAdam, K.G.; Faizi, A.; Kimpton, H.; Porter, A.; Rodu, B. Polycyclic aromatic hydrocarbons in US and Swedish smokeless tobacco products. Chem. Cent. J. 2013, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Nestor, T.B.; Gentry, J.S.; Peele, D.M.; Riddick, M.G.; Conner, B.T.; Edwards, M.E. Role of Oxides of Nitrogen in Tobacco-Specific Nitrosamine Formation in Flue-Cured Tobacco. Beitr. Table Int. 2014, 20, 467–475. [Google Scholar] [CrossRef]
- Burton, H.R.; Dye, N.K.; Bush, L.P. Relationship between Tobacco-Specific Nitrosamines and Nitrite from Different Air-Cured Tobacco Varieties. J. Agric. Food Chem. 1994, 42, 2007–2011. [Google Scholar] [CrossRef]
- Andersen, R.A.; Burton, H.R.; Fleming, P.D.; Hamilton-Kemp, T.R. Effect of Storage Conditions on Nitrosated, Acylated, and Oxidized Pyridine Alkaloid Derivatives in Smokeless Tobacco Products. Cancer Res. 1989, 49, 5895–5900. [Google Scholar]
- Moldoveanu, S.C.; Zhu, J.; Qian, N. Analysis of Traces of Tobacco-Specific Nitrosamines (TSNAs) in USP Grade Nicotine, E-Liquids, and Particulate Phase Generated by the Electronic Smoking Devices. Beiträge zur Table Int. to Tob. Res. 2017, 27, 86–96. [Google Scholar] [CrossRef]
- FAQs—Frequenty Asked Questions—Fully Loaded LLC. Available online: https://fullyloadedchew.com/pages/faq (accessed on 24 January 2022).
- FAQs On Dipping Black Buffalo Long Cut & Pouches. Available online: https://blackbuffalo.com/pages/faq (accessed on 24 January 2022).
- Schwarzauer-Rockett, K.; Al-Hamdani, S.H.; Rayburn, J.R.; Mwebi, N.O. Utilization of kudzu as a lead phytoremediator and the impact of lead on selected physiological responses. Can. J. Plant Sci. 2013, 93, 951–959. [Google Scholar] [CrossRef][Green Version]
- Caraway, J.W.; Chen, P.X. Assessment of Mouth-Level Exposure to Tobacco Constituents in U.S. Snus Consumers. Nicot. Tob. Res. 2013, 15, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Digard, H.; Errington, G.; Richter, A.; McAdam, K. Patterns and behaviors of snus consumption in Sweden. Nicot. Tob. Res. Off. J. Soc. Res. Nicot. Tob. 2009, 11, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).