Simultaneous Determination of Levamisole, Mebendazole, and the Two Metabolite Residues of Mebendazole in Poultry Eggs by High-Performance Liquid Chromatography–Tandem Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chemicals and Reagents
2.3. Solution Preparation
2.4. HPLC–MS/MS Conditions
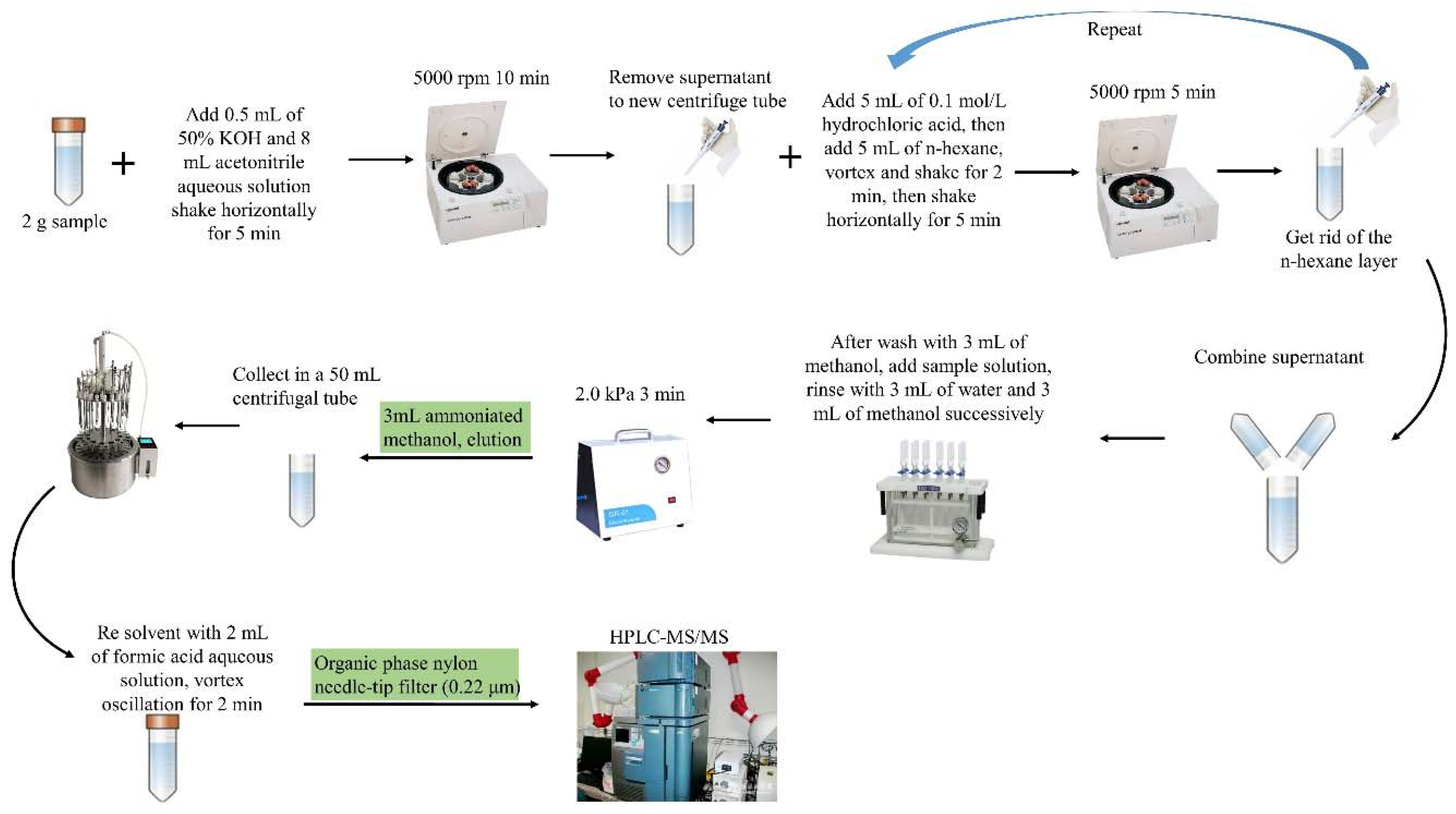
2.5. Sample Preparation
2.6. Method Validation
2.6.1. Specificity
2.6.2. Linearity
2.6.3. Accuracy and Precision
2.6.4. LOD and LOQ
3. Results and Discussion
3.1. Determination of Precursor Ions and Product Ions
3.2. Selection of Solvents
3.3. Optimization of the Sample Pretreatment Method
3.4. Optimization of Chromatographic Conditions
3.5. Methodology Validation
3.5.1. Specificity
3.5.2. Linearity
3.5.3. Accuracy and Precision
3.5.4. LOD and LOQ
3.6. Comparison of Different Detection Methods
3.7. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotterill, O.J.; Glauert, J.; Froning, G.W. Nutrient Composition of Commercially Spray-Dried Egg Products. Poult. Sci. 1978, 57, 439–442. [Google Scholar] [CrossRef]
- Swendseid, M.E.; Fe Eley, R.J.; Harris, C.L.; Tuttle, S.G. Egg protein as a source of the essential amino acids: Requirement for nitrogen balance in young adults studied at two levels of nitrogen intake. J. Nutr. 1959, 68, 203. [Google Scholar] [CrossRef]
- Feyera, T.; Ruhnke, I.; Sharpe, B.; Elliott, T.; Walkden-Brown, S.W. Comparative therapeutic efficacies of oral and in-water administered levamisole, piperazine and fenbendazole against experimental Ascaridia galli infection in chickens. Vet. Parasitol. 2021, 298, 109514. [Google Scholar] [CrossRef]
- Chai, J.-Y.; Jung, B.-K.; Hong, S.-J. Albendazole and Mebendazole as Anti-Parasitic and Anti-Cancer Agents: An Update. Korean J. Parasitol. 2021, 59, 189–225. [Google Scholar] [CrossRef]
- Friedman, P.A.; Platzer, E.G. Interaction of anthelmintic benzimidazoles with Ascaris suum embryonic tubulin. BBA Gen. Subj. 1980, 630, 271–278. [Google Scholar] [CrossRef]
- Albonico, M.; Bickle, Q.; Ramsan, M.; Montresor, A.; Savioli, L.; Taylor, M. Efficacy of Mebendazole and Levamisole Alone or in Combination against Intestinal Nematode Infections after Repeated Targeted Mebendazole Treatment in Zanzibar. (Research). Bull. World Health Organ. 2003, 81, 343–352. [Google Scholar] [CrossRef]
- Bennet, E.M.; Behm, C.; Bryant, C. Effects of mebendazole and levamisole on tetrathyridia of Mesocestoides corti in the mouse. Int. J. Parasitol. 1978, 8, 463–466. [Google Scholar] [CrossRef]
- Heath, D.D.; Christie, M.J.; Chevis, R. The lethal effect of mebendazole on secondary Echinococcus granulosus, cysticerci of Taenia pisiformis and tetrathyridia of Mesocestoides corti. Parasitology 1975, 70, 273–285. [Google Scholar] [CrossRef]
- Xu, L.; Luan, F.; Wang, L.; Liu, H.; Gao, Y. Development of a Capillary Zone Electrophoresis Method for Determination of Mebendazole and Levamisole Hydrochloride in a Combined Tablet and a Comparison with a LC Method. J. AOAC Int. 2014, 97, 128–132. [Google Scholar] [CrossRef]
- Ejlertsen, B.; Mouridsen, H.T.; Jensen, M.-B.; Andersen, J.; Andersson, M.; Kamby, C.; Knoop, A.S.; Danish Breast Cancer Cooperative Group. Cyclophosphamide, methotrexate, and fluorouracil; oral cyclophosphamide; levamisole; or no adjuvant therapy for patients with high-risk, premenopausal breast cancer. Cancer 2010, 116, 2081–2089. [Google Scholar] [CrossRef]
- Commission, E. Commission Decision (EU) No 37/2010 of December 2009 on Pharmacologically Active Substances and their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. 2010. Available online: https://eur-lex.europa.eu/eli/reg/2010/37(1)/oj (accessed on 15 February 2022).
- FDA. CFR-Code of Federal Regulations Title 21. 2019. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=556&showFR=1 (accessed on 15 June 2020).
- Korea Food & Drug Administration. Notice No. 2012-59 of the Korea Food & Drug Administration. 2012. Available online: https://www.mfds.go.kr/eng/brd/m_60/view.do?seq=67277 (accessed on 15 June 2020).
- Guo, L.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Gold Nanoparticle-Based Paper Sensor for Simultaneous Detection of 11 Benzimidazoles by One Monoclonal Antibody. Small 2017, 14, 1701782. [Google Scholar] [CrossRef]
- Jinxin, H.; Yan, Z.; Xiaorong, C.; Na, L.; Fang, T.; Nairui, H.; Jianzhong, W.; Shaopeng, G. Development of an enzyme-linked immunosorbent assay for the detection of mebendazole in chicken and mutton. Anal. Methods Adv. Methods Appl. 2021, 13, 1740–1746. [Google Scholar] [CrossRef]
- Woestenborghs, R.; Michielsen, L.; Heykants, J. Determination of levamisole in plasma and animal tissues by gas chromatography with thermionic specific detection. J. Chromatogr. 1981, 224, 25–32. [Google Scholar] [CrossRef]
- Provatas, A.A.; Yeudakimau, A.V.; Stuart, J.D.; Perkins, C.R. Rapid QuEChERS Approach Using Novel Solid Phase Extraction for Insecticides in Lobster and Shellfish Tissue with Gas Chromatography–Tandem Mass Spectrometry. Anal. Lett. 2014, 47, 2461–2474. [Google Scholar] [CrossRef]
- Kang, Y.P.; Yu, J.; Huh, Y.; Oh, J.H.; Kwon, C.H.; Lee, S.J.; Ee, J.W.; Kim, G.T.; Lee, J.G.; Lee, J.; et al. Development of high performance liquid chromatography-ultraviolet detection method for screening mebendazole, clorsulon, diaveridine, and tolfenamic acid in animal-based food samples. Drug Test. Anal. 2014, 6, 246–256. [Google Scholar] [CrossRef]
- Canton, C.; Ceballos, L.; Domínguez, M.P.; Moreno, L.; Fiel, C.; Bernat, G.; Farías, C.; Lanusse, C.; Alvarez, L. Pharmaco-parasitological evaluation of the ricobendazole plus levamisole nematodicidal combination in cattle. J. Vet. Pharmacol. Ther. 2018, 41, 83–91. [Google Scholar] [CrossRef]
- Sakamoto, M.; Take Ba, K.; Fujinuma, K.; Jimbo, K.; Miyazaki, T. Determination of levamisole in livestock products using high performance liquid chromatography. Shokuhinseigaku Zasshi J. Food Hyg. Soc. Jpn. 2002, 43, 6–9. [Google Scholar] [CrossRef][Green Version]
- Pawar, R.P.; Mishra, P.; Durgbanshi, A.; Bose, D.; Albiol-Chiva, J.; Peris-Vicente, J.; García-Ferrer, D.; Esteve-Romero, J. Use of Micellar Liquid Chromatography to Determine Mebendazole in Dairy Products and Breeding Waste from Bovine Animals. Antibiotics 2020, 9, 86. [Google Scholar] [CrossRef]
- Kinsella, B.; Whelan, M.; Cantwell, H.; Mccormack, M.; Furey, A.; Lehotay, S.J.; Danaher, M. A dual validation approach to detect anthelmintic residues in bovine liver over an extended concentration range. Talanta 2011, 83, 14–24. [Google Scholar] [CrossRef]
- Xu, N.; Dong, J.; Yang, Y.; Liu, Y.; Yang, Q.; Ai, X. Development of a liquid chromatography-tandem mass spectrometry method with modified QuEChERS extraction for the quantification of mebendazole and its metabolites, albendazole and its metabolites, and levamisole in edible tissues of aquatic animals. Food Chem. 2018, 269, 442–449. [Google Scholar] [CrossRef]
- GB 29685-2013; National Food Safety Standard: Determination of Lincomycin, Clindamycin and Spectinomycin in Animal Derived Food by Gas Chroma-tography-Mass Spectrometry Method. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2013. Available online: http://down.foodmate.net/standard/yulan.php?itemid=38182 (accessed on 15 February 2022).
- The European Communities. Commission decision 2002/657/EC of 12 August 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Commun. 2002, 221, 8–36. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002D0657&from=ES (accessed on 15 June 2020).
- Barreca, S.; Busetto, M.; Vitelli, M.; Colzani, L.; Clerici, L.; Dellavedova, P. Online Solid-Phase Extraction LC-MS/MS: A Rapid and Valid Method for the Determination of Perfluorinated Compounds at Sub ng·L 1 Level in Natural Water. J. Chem. 2018, 2018, 3780825. [Google Scholar] [CrossRef]
- Barreca, S.; Orecchio, S.; Pace, A. Photochemical sample treatment: A greener approach to chlorobenzene determination in sediments. Talanta 2014, 129, 263–269. [Google Scholar] [CrossRef]
- Lopes, R.P.; Reyes, R.C.; Romero-González, R.; Vidal, J.; Frenich, A.G. Multiresidue determination of veterinary drugs in aquaculture fish samples by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life 2012, 895–896, 39–47. [Google Scholar] [CrossRef]
- Yoo, K.H.; Park, D.H.; El-Aty, A.; Kim, S.K.; Jung, H.N.; Jeong, D.H.; Cho, H.J.; Hacimuftuoglu, A.; Shim, J.H.; Ji, H.J. Development of an analytical method for multi-residue quantification of 18 anthelmintics in various animal-based food products using liquid chromatography-tandem mass spectrometry. J. Pharm. Anal. 2021, 11, 68–76. [Google Scholar] [CrossRef]
- Wei, H.; Tao, Y.; Chen, D.; Xie, S.; Pan, Y.; Liu, Z.; Huang, L.; Yuan, Z. Development and validation of a multi-residue screening method for veterinary drugs, their metabolites and pesticides in meat using liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 686–701. [Google Scholar] [CrossRef]
- Shea; Jennifer, L. Bioanalytical methods for quantitation of levamisole, a widespread cocaine adulterant. Clin. Chem. Lab. Med. 2013, 51, 205–212. [Google Scholar] [CrossRef]
- Baeza-Baeza, J.J.; García-Alvarez-Coque, M. Peak dispersion in gradient elution: An insight based on the plate model. J. Chromatogr. A 2019, 1613, 460670. [Google Scholar] [CrossRef]
- Soliman, M.; Khorshid, M.A.; Abo-Aly, M.M. Combination of Analyte Protectants and Sandwich Injection to Compensate for Matrix Effect of Pesticides Residue in GC-MS/MS. Microchem. J. 2020, 156, 104852. [Google Scholar] [CrossRef]
- Krupčík, J.; Májek, P.; Gorovenko, R.; Blaško, J.; Kubinec, R.; Sandra, P. Considerations on the determination of the limit of detection and the limit of quantification in one-dimensional and comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2015, 1396, 117–130. [Google Scholar] [CrossRef]
- Zhu, W.X.; Yang, J.Z.; Wang, Z.X.; Wang, C.J.; Liu, Y.F.; Zhang, L. Rapid determination of 88 veterinary drug residues in milk using automated TurborFlow online clean-up mode coupled to liquid chromatography-tandem mass spectrometry. Talanta 2016, 148, 401–411. [Google Scholar] [CrossRef]
- Kolanovic, B.S.; Bilandzic, N.; Kos, B.; Suskovic, J.; Cvetnic, L.; Varenina, I.; Luburic, D.B.; Varga, I.; Pavlicek, D.; Lugomer, M.D. Distribution and elimination of levamisole in eggs and tissues after oral administration to laying hens, determined by LC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 729–739. [Google Scholar] [CrossRef]
- Whelan, M.; Kinsella, B.; Furey, A.; Moloney, M.; Cantwell, H.; Lehotay, S.J.; Danaher, M. Determination of anthelmintic drug residues in milk using ultra high performance liquid chromatography–tandem mass spectrometry with rapid polarity switching. J. Chromatogr. A 2010, 1217, 4612–4622. [Google Scholar] [CrossRef]















| Time (min) | Flow Rate (mL/min) | Mobile Phase A (%) | Mobile Phase B (%) | Curve |
|---|---|---|---|---|
| 0 | 0.6 | 10 | 90 | Inception |
| 1 | 0.6 | 10 | 90 | 6 |
| 2 | 0.6 | 48 | 52 | 6 |
| 8 | 0.6 | 48 | 52 | 6 |
| 9 | 0.6 | 10 | 90 | 6 |
| 10 | 0.6 | 10 | 90 | 6 |
| Compound | Molecular Weight | Retention Time (min) | Mass Transitions (m/z) | Declustering Potential (V) | Collision Energy (eV) |
|---|---|---|---|---|---|
| LMS | 205 | 5.91 | 205 > 178.0 * 205 > 123.0 | 110 | 29 |
| 38 | |||||
| MBZ | 296 | 7.68 | 296 > 264.0 * 296 > 104.8 | 115 | 28 |
| 23 | |||||
| HMBZ | 298 | 6.37 | 298 > 265.8 * 298 > 160.0 | 121 | 24 |
| 35 | |||||
| AMBZ | 238 | 6.38 | 238 > 105.0 * 238 > 76.9 | 155 | 33 |
| 45 |
| Compound | Molecular Weight | Retention Time (min) | Mass Transition (m/z) | Declustering Potential (V) | Collision Energy (eV) |
|---|---|---|---|---|---|
| LMS | 205 | 5.91 | 205 > 178.0 * 205 > 123.0 | 110 | 29 |
| 38 | |||||
| MBZ | 296 | 7.68 | 296 > 264.0 * 296 > 104.8 | 115 | 28 |
| 23 | |||||
| HMBZ | 298 | 6.37 | 298 > 265.8 * 298 > 160.0 | 121 | 24 |
| 35 | |||||
| AMBZ | 238 | 6.38 | 238 > 105.0 * 238 > 76.9 | 155 | 33 |
| 45 |
| Matrix | Analyte | Regression Equation | Determination Coefficient (R2) | Linear Range (μg/kg) |
|---|---|---|---|---|
| Hen whole egg | LMS | y = 957,963x − 127,586 | 0.9994 | 0.22–25 |
| MBZ | y = 380,097x + 36,275 | 0.9996 | 0.20–150 | |
| HMBZ | y = 79,362x + 27,380 | 0.9996 | 0.80–150 | |
| AMBZ | y = 29,480x + 11,372 | 0.9997 | 1.00–150 | |
| Hen albumen | LMS | y = 1,013,364x + 13,536 | 0.9996 | 0.10–25 |
| MBZ | y = 411,595x + 85,604 | 0.9997 | 0.10–150 | |
| HMBZ | y = 87,374x + 50,885 | 0.9996 | 0.60–150 | |
| AMBZ | y = 30,001x + 6980 | 0.9996 | 1.00–150 | |
| Hen yolk | LMS | y = 1,010,486x − 134,389 | 0.9997 | 0.20–25 |
| MBZ | y =431,745x + 53,780 | 0.9995 | 0.13–150 | |
| HMBZ | y = 84,672x + 21,968 | 0.9997 | 0.50–150 | |
| AMBZ | y = 27,639x + 12,385 | 0.9996 | 0.80–150 | |
| Duck whole egg | LMS | y = 1,019,591x − 84,584 | 0.9996 | 0.14–25 |
| MBZ | y = 467,308x + 85,913 | 0.9997 | 0.11–150 | |
| HMBZ | y = 83,203x + 23,249 | 0.9995 | 0.65–150 | |
| AMBZ | y = 28,911x + 7386 | 0.9996 | 1.00–150 | |
| Duck albumen | LMS | y = 1,064,528x − 58,623 | 0.9997 | 0.11–25 |
| MBZ | y = 472,492x + 68,317 | 0.9995 | 0.12–150 | |
| HMBZ | y =91,233x + 18,843 | 0.9993 | 0.62–150 | |
| AMBZ | y = 29,262x + 6591 | 0.9995 | 0.95–150 | |
| Duck yolk | LMS | y = 602,175x − 2468 | 0.9995 | 0.13–25 |
| MBZ | y =329,946x + 57,029 | 0.9996 | 0.13–150 | |
| HMBZ | y = 42,095x + 46,793 | 0.9997 | 0.67–150 | |
| AMBZ | y = 27,095x + 7711 | 0.9995 | 0.9–150 | |
| Goose whole egg | LMS | y = 1,055,130x-50794 | 0.9998 | 0.11–25 |
| MBZ | y = 390,529x + 58,837 | 0.9995 | 0.10–150 | |
| HMBZ | y = 82,563x-16,126 | 0.9998 | 0.60–150 | |
| AMBZ | y = 30,066x + 10,552 | 0.9999 | 0.95–150 | |
| Goose albumen | LMS | y = 980,422x − 61,695 | 0.9997 | 0.12–25 |
| MBZ | y = 379,055x + 10,0976 | 0.9995 | 0.09–150 | |
| HMBZ | y = 84,992x + 26,072 | 0.9997 | 0.61–150 | |
| AMBZ | y = 29,520x + 3389 | 0.9998 | 0.90–150 | |
| Goose yolk | LMS | y = 955,941x − 30,613 | 0.9996 | 0.10–25 |
| MBZ | y =352,099x + 77,681 | 0.9997 | 0.09–150 | |
| HMBZ | y = 80,227x + 21,379 | 0.9998 | 0.62–150 | |
| AMBZ | y = 29,487x + 2960 | 0.9997 | 0.97–150 |
| Matrix | Analyte | Addition Level (µg/kg) | Recovery (%) | RSD (%) | Intraday RSD (%) | Interday RSD (%) |
|---|---|---|---|---|---|---|
| Whole egg | LMS | 0.22 | 88.10 ± 2.68 | 3.04 | 1.67 | 3.00 |
| 5 | 90.06 ± 3.11 | 3.46 | 2.28 | 3.62 | ||
| 10α | 91.17 ± 1.87 | 2.06 | 2.69 | 3.11 | ||
| 20 | 94.30 ± 2.03 | 2.16 | 2.72 | 3.15 | ||
| MBZ | 0.20 | 91.93 ± 2.83 | 3.08 | 4.61 | 5.06 | |
| 30 | 89.81 ± 3.79 | 4.22 | 3.88 | 5.09 | ||
| 60α | 94.25 ± 2.09 | 2.22 | 4.34 | 4.51 | ||
| 120 | 95.77 ± 2.40 | 2.50 | 3.75 | 4.11 | ||
| HMBZ | 0.80 | 91.06 ± 3.45 | 3.79 | 3.60 | 4.68 | |
| 30 | 92.05 ± 3.17 | 3.44 | 3.27 | 4.25 | ||
| 60α | 92.41 ± 2.22 | 2.41 | 3.35 | 3.74 | ||
| 120 | 95.84 ± 2.38 | 2.48 | 3.24 | 3.73 | ||
| AMBZ | 1.00 | 90.71 ± 2.94 | 3.24 | 3.79 | 4.48 | |
| 30 | 91.35 ± 3.57 | 3.91 | 1.53 | 3.59 | ||
| 60α | 94.60 ± 2.07 | 2.19 | 4.81 | 4.88 | ||
| 120 | 95.38 ± 3.65 | 3.83 | 2.20 | 3.84 | ||
| Albumen | LMS | 0.10 | 88.14 ± 3.06 | 3.47 | 3.55 | 4.43 |
| 5 | 89.30 ± 2.37 | 2.65 | 2.47 | 3.25 | ||
| 10α | 94.95 ± 1.99 | 2.10 | 2.01 | 2.59 | ||
| 20 | 95.55 ± 1.96 | 2.05 | 3.07 | 3.37 | ||
| MBZ | 0.10 | 94.91 ± 2.73 | 2.88 | 2.50 | 3.40 | |
| 30 | 91.29 ± 2.24 | 2.45 | 2.80 | 3.35 | ||
| 60α | 92.89 ± 2.18 | 2.35 | 2.92 | 3.48 | ||
| 120 | 94.05 ± 2.99 | 3.18 | 3.29 | 4.11 | ||
| HMBZ | 0.60 | 91.86 ± 2.90 | 3.16 | 3.88 | 4.54 | |
| 30 | 90.20 ± 1.88 | 2.09 | 3.70 | 4.05 | ||
| 60α | 93.28 ± 2.58 | 2.77 | 3.55 | 4.17 | ||
| 120 | 95.03 ± 2.85 | 3.00 | 3.47 | 4.19 | ||
| AMBZ | 1.00 | 87.89 ± 3.35 | 3.81 | 4.28 | 5.27 | |
| 30 | 88.06 ± 1.92 | 2.18 | 2.10 | 2.73 | ||
| 60α | 96.93 ± 2.73 | 2.81 | 3.51 | 4.10 | ||
| 120 | 93.54 ± 2.72 | 2.91 | 3.82 | 4.41 | ||
| Yolk | LMS | 0.20 | 87.28 ± 2.24 | 2.57 | 1.72 | 2.74 |
| 5 | 91.30 ± 1.89 | 2.07 | 3.88 | 4.05 | ||
| 10α | 94.23 ± 4.29 | 4.55 | 2.21 | 4.38 | ||
| 20 | 94.56 ± 3.15 | 3.34 | 3.14 | 4.07 | ||
| MBZ | 0.13 | 90.48 ± 2.56 | 2.82 | 2.65 | 3.45 | |
| 30 | 91.38 ± 3.07 | 3.36 | 2.90 | 3.93 | ||
| 60α | 94.67 ± 3.47 | 3.66 | 3.65 | 4.62 | ||
| 120 | 95.42 ± 2.87 | 3.01 | 2.97 | 3.79 | ||
| HMBZ | 0.50 | 93.19 ± 2.74 | 2.94 | 3.55 | 4.17 | |
| 30 | 90.84 ± 1.85 | 2.04 | 4.04 | 4.18 | ||
| 60α | 93.45 ± 2.14 | 2.29 | 1.40 | 2.34 | ||
| 120 | 96.46 ± 2.91 | 3.02 | 3.61 | 4.26 | ||
| AMBZ | 0.80 | 91.26 ± 3.58 | 3.92 | 4.14 | 5.10 | |
| 30 | 91.13 ± 1.90 | 2.09 | 2.50 | 3.02 | ||
| 60α | 94.91 ± 1.99 | 2.10 | 3.10 | 3.49 | ||
| 120 | 97.38 ± 1.99 | 2.04 | 4.13 | 4.26 |
| Matrix | Analyte | Addition Level (µg/kg) | Recovery (%) | RSD (%) | Intraday RSD (%) | Interday RSD (%) |
|---|---|---|---|---|---|---|
| Whole egg | LMS | 0.14 | 89.25 ± 2.18 | 2.44 | 1.92 | 2.77 |
| 5 | 91.40 ± 1.99 | 2.18 | 2.01 | 2.66 | ||
| 10α | 94.27 ± 3.14 | 3.33 | 2.97 | 4.02 | ||
| 20 | 95.20 ± 1.92 | 2.02 | 3.16 | 3.57 | ||
| MBZ | 0.11 | 89.42 ± 3.10 | 3.47 | 5.85 | 6.32 | |
| 30 | 89.12 ± 2.54 | 2.85 | 4.01 | 4.52 | ||
| 60α | 96.26 ± 3.31 | 3.44 | 3.63 | 4.49 | ||
| 120 | 93.18 ± 2.01 | 2.16 | 2.46 | 3.03 | ||
| HMBZ | 0.65 | 88.44 ± 3.39 | 3.84 | 2.21 | 3.85 | |
| 30 | 89.70 ± 3.19 | 3.56 | 3.29 | 4.33 | ||
| 60α | 94.13 ± 2.98 | 3.16 | 2.66 | 3.68 | ||
| 120 | 96.29 ± 2.91 | 3.02 | 5.00 | 5.35 | ||
| AMBZ | 1.00 | 92.48 ± 3.54 | 3.83 | 2.03 | 3.77 | |
| 30 | 91.69 ± 1.91 | 2.08 | 4.27 | 4.38 | ||
| 60α | 94.00 ± 3.42 | 3.64 | 2.80 | 4.07 | ||
| 120 | 94.11 ± 2.45 | 2.60 | 2.53 | 3.25 | ||
| Albumen | LMS | 0.11 | 89.38 ± 1.85 | 2.07 | 1.96 | 2.54 |
| 5 | 93.04 ± 2.53 | 2.72 | 2.81 | 3.55 | ||
| 10α | 93.66 ± 3.36 | 3.59 | 3.06 | 4.26 | ||
| 20 | 94.55 ± 2.27 | 2.40 | 4.25 | 4.47 | ||
| MBZ | 0.12 | 90.01 ± 3.70 | 4.11 | 3.12 | 4.61 | |
| 30 | 87.92 ± 2.36 | 2.69 | 2.59 | 3.32 | ||
| 60α | 93.81 ± 2.24 | 2.39 | 4.03 | 4.36 | ||
| 120 | 96.55 ± 3.75 | 3.88 | 1.41 | 3.52 | ||
| HMBZ | 0.62 | 89.37 ± 2.71 | 3.03 | 2.88 | 3.75 | |
| 30 | 93.76 ± 2.13 | 2.27 | 1.90 | 2.72 | ||
| 60α | 94.65 ± 2.41 | 2.55 | 2.39 | 3.24 | ||
| 120 | 95.57 ± 1.94 | 2.03 | 4.94 | 4.95 | ||
| AMBZ | 0.95 | 91.98 ± 1.84 | 2.00 | 4.26 | 4.34 | |
| 30 | 89.30 ± 3.42 | 3.83 | 4.25 | 5.13 | ||
| 60α | 92.40 ± 2.26 | 2.45 | 1.99 | 2.82 | ||
| 120 | 91.27 ± 3.50 | 3.84 | 2.67 | 4.11 | ||
| Yolk | LMS | 0.13 | 91.38 ± 2.21 | 2.42 | 4.20 | 4.49 |
| 5 | 88.94 ± 1.80 | 2.02 | 2.20 | 2.70 | ||
| 10α | 96.28 ± 3.04 | 3.16 | 2.13 | 3.35 | ||
| 20 | 94.10 ± 2.00 | 2.12 | 3.95 | 4.16 | ||
| MBZ | 0.13 | 93.92 ± 3.30 | 3.52 | 3.15 | 4.22 | |
| 30 | 88.03 ± 1.81 | 2.05 | 2.53 | 2.96 | ||
| 60α | 92.73 ± 3.07 | 3.31 | 2.27 | 3.51 | ||
| 120 | 93.93 ± 2.13 | 2.27 | 2.38 | 2.94 | ||
| HMBZ | 0.67 | 88.99 ± 1.79 | 2.01 | 3.95 | 4.08 | |
| 30 | 91.75 ± 2.18 | 2.38 | 3.38 | 3.88 | ||
| 60α | 95.11 ± 2.14 | 2.25 | 2.99 | 3.40 | ||
| 120 | 93.66 ± 1.97 | 2.10 | 2.64 | 3.08 | ||
| AMBZ | 0.90 | 89.92 ± 3.03 | 3.37 | 1.70 | 3.26 | |
| 30 | 89.77 ± 1.92 | 2.14 | 2.33 | 2.85 | ||
| 60α | 94.90 ± 2.65 | 2.79 | 5.09 | 5.34 | ||
| 120 | 93.99 ± 2.16 | 2.30 | 3.14 | 3.59 |
| Matrix | Analyte | Addition Level (µg/kg) | Recovery (%) | RSD (%) | Intraday RSD (%) | Interday RSD (%) |
|---|---|---|---|---|---|---|
| Whole egg | LMS | 0.11 | 87.82 ± 1.82 | 2.08 | 2.14 | 2.73 |
| 5 | 86.04 ± 3.24 | 3.76 | 3.22 | 4.40 | ||
| 10α | 93.95 ± 2.47 | 2.62 | 3.54 | 4.05 | ||
| 20 | 94.57 ± 3.72 | 3.93 | 3.91 | 5.02 | ||
| MBZ | 0.10 | 85.98 ± 1.91 | 2.22 | 3.28 | 3.61 | |
| 30 | 96.27 ± 3.29 | 3.42 | 2.60 | 3.79 | ||
| 60α | 88.60 ± 2.46 | 2.46 | 2.56 | 3.38 | ||
| 120 | 91.32 ± 2.42 | 2.42 | 2.41 | 3.18 | ||
| HMBZ | 0.60 | 91.00 ± 3.13 | 3.44 | 2.12 | 3.51 | |
| 30 | 95.22 ± 2.06 | 2.17 | 2.75 | 3.18 | ||
| 60α | 96.28 ± 4.41 | 4.58 | 3.05 | 4.84 | ||
| 120 | 95.86 ± 2.21 | 2.30 | 1.75 | 2.57 | ||
| AMBZ | 0.95 | 92.10 ± 2.19 | 2.37 | 4.74 | 4.89 | |
| 30 | 88.37 ± 3.36 | 3.80 | 2.97 | 4.26 | ||
| 60α | 94.32 ± 2.38 | 2.53 | 4.41 | 4.72 | ||
| 120 | 96.12 ± 2.00 | 2.08 | 3.59 | 3.81 | ||
| Albumen | LMS | 0.12 | 87.36 ± 1.75 | 2.02 | 1.93 | 2.49 |
| 5 | 91.57 ± 3.92 | 4.29 | 3.00 | 4.63 | ||
| 10α | 96.64 ± 2.76 | 2.85 | 3.86 | 4.46 | ||
| 20 | 95.83 ± 2.81 | 2.93 | 5.15 | 5.44 | ||
| MBZ | 0.09 | 86.75 ± 2.04 | 2.35 | 3.39 | 3.76 | |
| 30 | 90.82 ± 2.83 | 3.12 | 2.92 | 3.80 | ||
| 60α | 95.09 ± 4.30 | 4.52 | 4.83 | 5.92 | ||
| 120 | 94.52 ± 1.97 | 2.09 | 3.46 | 3.72 | ||
| HMBZ | 0.61 | 92.61 ± 2.34 | 2.52 | 3.45 | 3.92 | |
| 30 | 89.44 ± 2.47 | 2.76 | 4.45 | 4.84 | ||
| 60α | 96.48 ± 2.49 | 2.59 | 2.93 | 3.61 | ||
| 120 | 94.73 ± 2.97 | 3.14 | 3.56 | 4.43 | ||
| AMBZ | 0.90 | 89.90 ± 2.18 | 2.42 | 3.60 | 3.98 | |
| 30 | 90.56 ± 1.84 | 2.03 | 2.74 | 3.17 | ||
| 60α | 94.18 ± 2.13 | 2.26 | 3.79 | 4.11 | ||
| 120 | 94.91 ± 2.04 | 2.15 | 2.59 | 3.14 | ||
| Yolk | LMS | 0.10 | 86.21 ± 1.85 | 2.15 | 2.98 | 3.38 |
| 5 | 91.63 ± 3.61 | 3.95 | 2.69 | 4.20 | ||
| 10α | 93.32 ± 1.91 | 2.05 | 2.43 | 2.92 | ||
| 20 | 95.55 ± 2.14 | 2.24 | 4.05 | 4.28 | ||
| MBZ | 0.09 | 93.43 ± 2.50 | 2.68 | 3.23 | 3.55 | |
| 30 | 89.94 ± 2.18 | 2.43 | 1.64 | 2.60 | ||
| 60α | 95.33 ± 2.69 | 2.82 | 2.88 | 3.61 | ||
| 120 | 93.85 ± 1.88 | 2.00 | 2.13 | 2.65 | ||
| HMBZ | 0.62 | 89.13 ± 3.02 | 3.39 | 2.26 | 3.59 | |
| 30 | 88.66 ± 2.93 | 3.31 | 3.51 | 4.36 | ||
| 60α | 96.13 ± 2.94 | 3.06 | 2.54 | 3.61 | ||
| 120 | 92.74 ± 1.98 | 2.14 | 2.19 | 2.77 | ||
| AMBZ | 0.97 | 89.81 ± 2.63 | 2.93 | 1.80 | 2.99 | |
| 30 | 91.33 ± 2.46 | 2.69 | 2.43 | 3.30 | ||
| 60α | 95.00 ± 3.02 | 3.18 | 3.55 | 4.34 | ||
| 120 | 95.77 ± 2.15 | 2.25 | 4.01 | 4.27 |
| Analyte | Matrix | LOD (µg/kg) | LOQ (µg/kg) |
|---|---|---|---|
| LMS | Hen whole egg | 0.07 | 0.22 |
| Hen albumen | 0.05 | 0.10 | |
| Hen yolk | 0.07 | 0.20 | |
| Duck whole egg | 0.05 | 0.14 | |
| Duck albumen | 0.04 | 0.11 | |
| Duck yolk | 0.06 | 0.13 | |
| Goose whole egg | 0.04 | 0.11 | |
| Goose albumen | 0.05 | 0.12 | |
| Goose yolk | 0.03 | 0.10 | |
| MBZ | Hen whole egg | 0.05 | 0.20 |
| Hen albumen | 0.04 | 0.10 | |
| Hen yolk | 0.04 | 0.13 | |
| Duck whole egg | 0.04 | 0.11 | |
| Duck albumen | 0.04 | 0.12 | |
| Duck yolk | 0.05 | 0.13 | |
| Goose whole egg | 0.04 | 0.10 | |
| Goose albumen | 0.03 | 0.09 | |
| Goose yolk | 0.03 | 0.08 | |
| HMBZ | Hen whole egg | 0.23 | 0.80 |
| Hen albumen | 0.21 | 0.60 | |
| Hen yolk | 0.15 | 0.50 | |
| Duck whole egg | 0.22 | 0.65 | |
| Duck albumen | 0.20 | 0.62 | |
| Duck yolk | 0.23 | 0.67 | |
| Goose whole egg | 0.20 | 0.60 | |
| Goose albumen | 0.20 | 0.61 | |
| Goose yolk | 0.22 | 0.62 | |
| AMBZ | Hen whole egg | 0.25 | 1.00 |
| Hen albumen | 0.30 | 1.00 | |
| Hen yolk | 0.32 | 0.80 | |
| Duck whole egg | 0.32 | 1.00 | |
| Duck albumen | 0.30 | 0.95 | |
| Duck yolk | 0.28 | 0.90 | |
| Goose whole egg | 0.32 | 0.95 | |
| Goose albumen | 0.30 | 0.90 | |
| Goose yolk | 0.33 | 0.97 |
| Detection Method (Reference) | Extractant | Sample Preparation Method | Analyte | Animal-Derived Food(s) | Column | LOD (μg/kg) | LOQ (μg/kg) | Recovery (%) | Detection Time (min) |
|---|---|---|---|---|---|---|---|---|---|
| HPLC–MS [30] | A: 10 mM aqueous solution; B: methanol | QuEchERS | LMS | Fish | Gemini-NX C18 (100 × 2.0 mm, 3 μm) | 1.6 | 5.0 | 81.61–105.20 | 8.5 |
| HPLC–UVD [18] | A: 20 mM ammonium formate aqueous solution; B: 12.5 mM ammonium formate in methanol | LLE | MBZ | Beef, milk, pork, chicken, eggs | Phenomenex C18 (150 × 4.6 mm, 5 μm) | 1–14 | 4–41 | 80.0–92.0 | 20 |
| HPLC–DAD [19] | A: 0.02 M potassium dihydrogen phosphate aqueous solution; B: acetonitrile | LLE | LMS | Beef, pork, poultry muscle | TSkgel ODS-80Ts (4.6 × 150 mm, 5 μm) | 5 | - | 102–105 | 11 |
| HPLC–DAD [15] | 0.15 M sodium dodecyl sulfate:6% 1-pentanol:0.01 M disodium hydrogen phosphate aqueous solution | SPE | MBZ | Milk product | SPHER-100 C18 (250 × 4.6 mm, 5 μm) | 1–2 | 3–6 | 92.50–102.30 | 14 |
| HPLC–MS/MS [30] | A: 0.1 M ammonium acetate solution; B: acetonitrile (50:50, v/v) | LLE | LMS | Chicken, eggs | Poroshell 120 EC C18 (50 × 3 mm, 2.7 μm) | 0.03–0.04 | 0.13–0.15 | 98.7–106.8 | 22 |
| HPLC–MS/MS [27] | A: 12.5 mM ammonium formate aqueous solution:acetonitrile (50:50, v/v); B: 12.5 mM ammonium formate in methanol | SPE | MBZ | Beef, pork, chicken | Hypersil C18 (150 × 2.1 mm, 5 μm) | - | - | 76–112 | 22 |
| UHPLC–MS/MS [36] | A: water:methanol:formic acid (90:5:5, v/v); B: 12.5 mM ammonium formate in methanol:acetonitrile (50:50, v/v) | QuEChERS | MBZ HMBZ AMBZ | Milk | BEH C18 (50 × 2.1 mm, 1.7 μm) | - | - | 95–108 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; He, Z.; Zhang, P.; Guo, Y.; Lu, Y.; Tang, Y.; Chen, J.; Xie, K. Simultaneous Determination of Levamisole, Mebendazole, and the Two Metabolite Residues of Mebendazole in Poultry Eggs by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Separations 2022, 9, 83. https://doi.org/10.3390/separations9040083
Chen L, He Z, Zhang P, Guo Y, Lu Y, Tang Y, Chen J, Xie K. Simultaneous Determination of Levamisole, Mebendazole, and the Two Metabolite Residues of Mebendazole in Poultry Eggs by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Separations. 2022; 9(4):83. https://doi.org/10.3390/separations9040083
Chicago/Turabian StyleChen, Lan, Zhaoyuan He, Peiyang Zhang, Yawen Guo, Yang Lu, Yayun Tang, Jinyuan Chen, and Kaizhou Xie. 2022. "Simultaneous Determination of Levamisole, Mebendazole, and the Two Metabolite Residues of Mebendazole in Poultry Eggs by High-Performance Liquid Chromatography–Tandem Mass Spectrometry" Separations 9, no. 4: 83. https://doi.org/10.3390/separations9040083
APA StyleChen, L., He, Z., Zhang, P., Guo, Y., Lu, Y., Tang, Y., Chen, J., & Xie, K. (2022). Simultaneous Determination of Levamisole, Mebendazole, and the Two Metabolite Residues of Mebendazole in Poultry Eggs by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Separations, 9(4), 83. https://doi.org/10.3390/separations9040083





