A High-Performance Thin-Layer Chromatographic Method for the Simultaneous Determination of Curcumin I, Curcumin II and Curcumin III in Curcuma longa and Herbal Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
2.2. Purification of Individual Curcumins
2.3. Charaterization of Curcumins
2.4. Extraction Procedure
2.5. HPTLC Conditions
2.6. Method Validation
2.6.1. Accuracy
2.6.2. Precision
2.6.3. Robustness
2.6.4. Limit of Detection and Quantification (Sensitivity)
2.6.5. Specificity
2.7. Quantification of Curcumins I–III in C. longa and Herbal Formulation
3. Results
3.1. Method Validation
3.1.1. Linearity
3.1.2. Accuracy
3.1.3. Precision
3.1.4. Robustness
3.1.5. Sensitivity
3.1.6. Specificity
3.2. Quantification of Curcumins I–III in C. longa and Herbal Formulation
4. Discussion
4.1. Characterization of Curcumins
4.2. HPTLC Analysis
4.3. Quantification of Curcumins I–III in C. longa and Herbal Formulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Am. J. Bot. 2002, 89, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K. A preliminary checklist of the Zingiberaceae of Thailand. Thai For. Bull. 1996, 24, 35–49. [Google Scholar]
- Xia, Q.; Zhao, K.J.; Huang, Z.G.; Zhang, P.; Dong, T.T.; Li, S.P.; Tsim, K.W. Molecular genetic and chemical assessment of Rhizoma Curcumae in China. J. Agric. Food Chem. 2005, 53, 6019–6026. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Shaw, B.P.; Mukherjee, A. A new glycosidic flavonoid from Jwarhar mahakashay (antipyretic) Ayurvedic preparation. Int. J. Ayurveda Res. 2010, 1, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, A.; Nigam, S.S. Antifungal activity of the essential oil of Curcuma angustifolia. Indian J. Pharmacol. 1977, 39, 143–145. [Google Scholar]
- Warrier, P.K.; Nambiar, V.P.; Ramankutty, C. Indian Medicinal Plants; Orient Longman Ltd.: Madras, India, 1994; pp. 1–5. [Google Scholar]
- Thakur, R.S.; Puri, H.S.; Husain, A. Major Medicinal Plants of India; CIMAP: Lucknow, India, 1989; pp. 50–52. [Google Scholar]
- Hanif, R.; Qiao, L.; Shiff, S.J. Curcumin., a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J. Laborat. Clin. Med. 1997, 130, 576–584. [Google Scholar] [CrossRef]
- Kolammal, M. Pharmacognosy of Ayurvedic Drugs; Ayurveda College: Trivandrum, India, 1979. [Google Scholar]
- Kurup, P.N.; Ramdas, V.N.; Joshi, P. Handbook of Medicinal Plants; New Delhi, Oxford and IBH Publishing Co pvt Ltd.: Delhi, India, 1979. [Google Scholar]
- Nadkarni, A.K. Indian Materia Medica; Popular Prakashay: Bombay, India, 1954; Volume 408, p. 1476. [Google Scholar]
- Toda, S.; Miyase, T.; Arich, H. Natural antioxidants. Antioxidative compounds isolated from rhizome of Curcuma longa. Laborat Chem. Pharmacol. Bullet. 1985, 33, 1725–1728. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Jeon, C.H.; Ko, G. Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J. Pharm. Pharmacol. 2000, 52, 437–440. [Google Scholar] [CrossRef]
- Ammon, H.P.T.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef]
- Goh, C.L.; Ng, S.K. Allergic contact dermatitis to Curcuma longa (turmeric). Cont. Dermat. 1987, 17, 186. [Google Scholar]
- Chempakam, B.; Parthasarathy, V.A.; Zachariah, T.J. Chemistry of Spices; Chempakam, B., Parthasarathy, V.A., Eds.; Turmeric; CABI Publishing: Oxford, UK, 2008. [Google Scholar]
- Asher, G.N.; Spelman, K. Clinical utility of curcumin extract. Altern. Ther. Health Med. 2013, 19, 20–22. [Google Scholar]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Samad, T.; Abdi, S. Cyclooxygenase-2 and antagonists in pain management. Curr. Opin. Anaesthesiol. 2001, 14, 527–532. [Google Scholar] [CrossRef]
- Tajik, H.; Tamaddonfard, E.; Hamzeh-Gooshchi, N. The effect of curcumin (active substance of turmeric) on the acetic acidinduced visceral nociception in rats. Pak. J. Biol. Sci. 2008, 11, 312–314. [Google Scholar] [CrossRef] [Green Version]
- De Paz-Campos, M.A.; Chavez-Pina, A.E.; Ortiz, M.I.; Castaneda-Hernandez, G. Evidence for the participation of ATP sensitive potassium channels in the antinociceptive effect of curcumin. Korean J. Pain. 2012, 25, 221–227. [Google Scholar] [CrossRef]
- Chandran, B.; Goel, A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin therapy in inflammatory bowel disease: A pilot study. Digest Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef] [Green Version]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef]
- Srivastava, R.; Puri, V.; Srimal, R.C.; Dhawan, B.N. Effect of curcumin on platelet aggregation and vascular prostacyclin synthesis. Arzneimittelforschung 1986, 36, 715–717. [Google Scholar]
- Limtrakul, P.; Lipigorngoson, S.; Namwong, O. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett. 1997, 116, 197203. [Google Scholar] [CrossRef]
- Wagner, H. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Shewiyo, D.H.; Kaaleb, E.; Rishab, P.G.; Dejaegherc, B.; Verbekec, J.S.; Heydenc, Y.V. HPTLC methods to assay active ingredients in pharmaceutical formulations: A review of the method development and validation steps. J. Pharm. Biomed. Anal. 2012, 66, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Shuijun, L.; Gangyi, L.; Jingying, J.; Xiaochuan, L.; Chen, Y. Liquid chromatography-negative ion electrospray tandem mass spectrometry method for the quantification of ezetimibe in human. Plasma J. Pharm. Biomed. Anal. 2006, 40, 987–992. [Google Scholar]
- ICH. Q2 (R2): Validation of Analytical Procedures–Text and Methodology. In Proceedings of the International Conference on Harmonization (ICH), Geneva, Switzerland, 31 March 2022. [Google Scholar]
- Chearwae, W.; Wu, C.P.; Chu, H.Y.; Lee, T.R.; Ambudkar, S.V.; Limtrakul, P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1). Cancer Chemother. Pharmacol. 2006, 57, 376–388. [Google Scholar] [CrossRef]
- Ahmed, M.; Abdul, Q.M.; Imtiaz, S.M.; Muddassar, M.; Hameed, A.; Nadeem, A.M.; Asiri, A.M. Curcumin: Synthesis optimization and in silico interaction with cyclin dependent kinase. Acta Pharm. 2017, 67, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidu, M.M.; Shyamala, B.N.; Manjunatha, J.R.; Sulochanamma, G.; Srinivas, P. Simple HPLC method for resolution of curcuminoids with antioxidant potential. J. Food Sci. 2009, 74, C312–C318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]

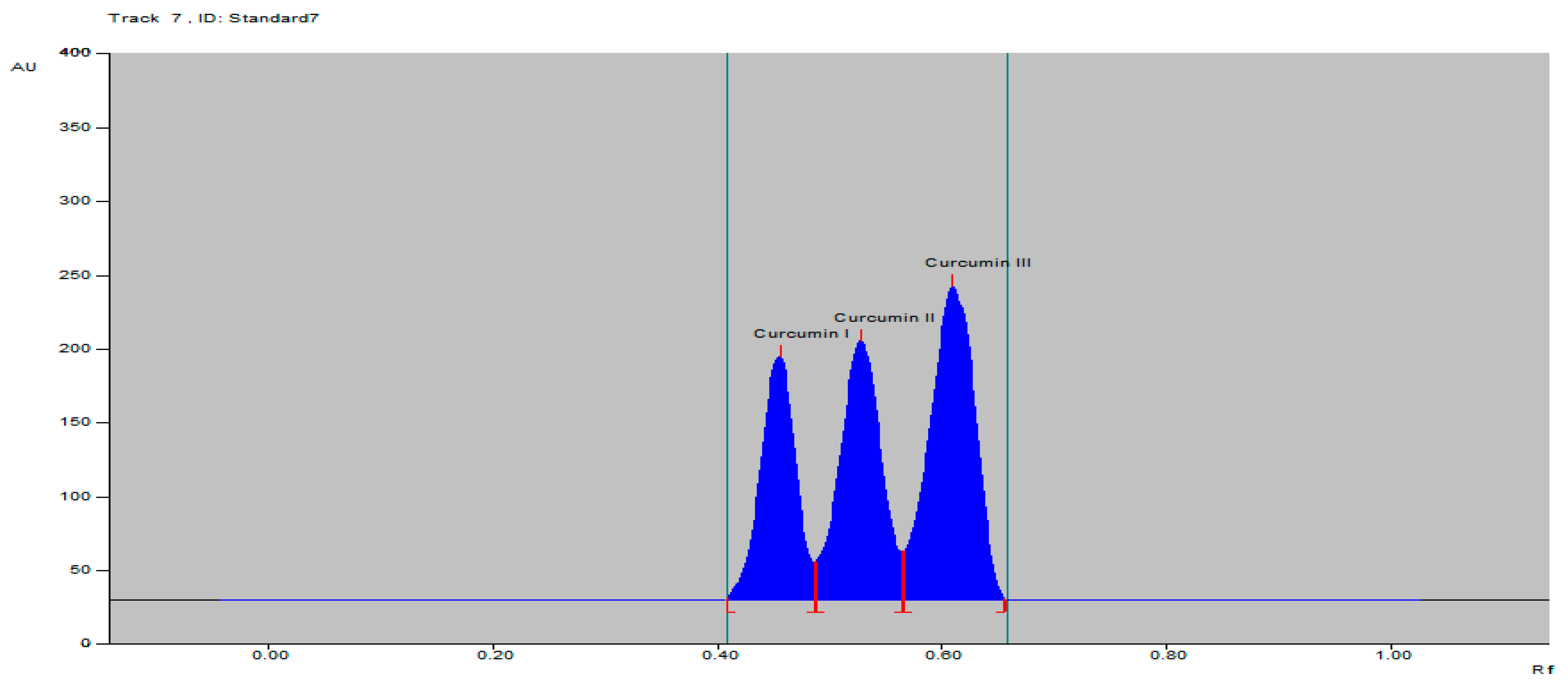

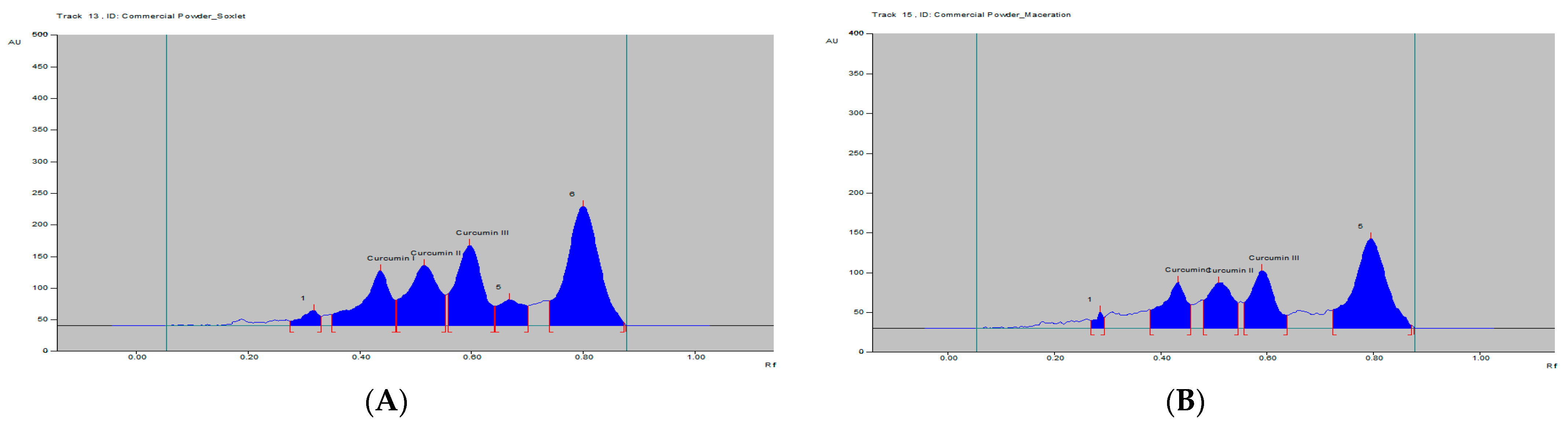

| Parameters | Curcumin I | Curcumin II | Curcumin III |
|---|---|---|---|
| Linearity range (ng/spot) | 50–500 | 50–500 | 50–500 |
| Regression equation | Y = 4.3931x + 424.66 | Y = 8.1074x + 254.62 | Y = 13.66x + 2.858 |
| Correlation coefficient | 0.998 | 0.999 | 0.997 |
| Slope ± SD | 4.3931 ± 0.1123 | 8.1074 ± 0.1342 | 13.66 ± 0.1391 |
| Intercept ± SD | 424.66 ± 56.12 | 254.62 ± 53.23 | 2.858 ± 62.90 |
| Standard error of slope | 0.0310 | 0.0538 | 0.0457 |
| Standard error of intercept | 28.15 | 33.07 | 30.18 |
| 95% confidence interval of slope | 12.456–13.032 | 11.89–12.12 | 13.34–15.02 |
| 95% confidence interval of intercept | 6540–6712 | 4651–5478 | 5231–5365 |
| p value | <0.0001 | <0.0001 | <0.0001 |
| Excess Drug Added to Analyte (%) | Theoretical Content (ng) | Conc. Found (ng) ± SD | % Recovery | % RSD |
|---|---|---|---|---|
| Curcumin I | ||||
| 0 | 100 | 98.67 ± 0.52 | 98.67 | 0.52 |
| 50 | 150 | 145.83 ± 2.23 | 97.22 | 1.53 |
| 100 | 200 | 194.50 ± 2.88 | 97.25 | 1.48 |
| 150 | 300 | 293.67 ± 3.14 | 97.89 | 1.07 |
| Curcumin II | ||||
| 0 | 100 | 97.83 ± 1.17 | 97.83 | 1.19 |
| 50 | 150 | 147.33 ± 1.37 | 98.22 | 0.93 |
| 100 | 200 | 195.83 ± 2.04 | 97.92 | 1.04 |
| 150 | 300 | 295.67 ± 6.12 | 98.56 | 0.63 |
| Curcumin III | ||||
| 0 | 100 | 97.17 ± 0.98 | 97.17 | 1.01 |
| 50 | 150 | 145.83 ± 2.48 | 97.22 | 1.70 |
| 100 | 200 | 295.17 ± 1.72 | 98.39 | 0.58 |
| Repeatability (Intraday Precision) | Intermediate Precision (Interday) | |||||
|---|---|---|---|---|---|---|
| Conc. (ng/spot) | Avg Conc. ± SD (n = 6) | Standard Error | % RSD | Avg Conc. ± SD (n = 6) | Standard Error | % RSD |
| Curcumin I | ||||||
| 200 | 1360.00 ± 21.24 | 8.67 | 1.56 | 1354.50 ± 23.24 | 9.49 | 1.72 |
| 300 | 1747.50 ± 13.58 | 5.54 | 0.78 | 1750.17 ± 18.99 | 7.75 | 1.08 |
| 400 | 2149.83 ± 24.27 | 9.91 | 1.13 | 2156.33 ± 28.14 | 11.49 | 1.30 |
| Curcumin II | ||||||
| 200 | 1842.50 ± 16.53 | 6.75 | 0.90 | 1873.00 ± 22.72 | 9.28 | 1.21 |
| 300 | 2694.83 ± 28.53 | 11.65 | 1.06 | 2674.00 ± 32.43 | 13.24 | 1.21 |
| 400 | 3574.17 ± 17.36 | 7.09 | 0.49 | 3572.33 ± 26.52 | 10.83 | 0.74 |
| Curcumin III | ||||||
| 200 | 2617.83 ± 27.23 | 11.12 | 1.04 | 1589.49 ± 22.75 | 9.29 | 1.43 |
| 300 | 4217.00 ± 32.12 | 13.12 | 0.76 | 4214.17 ± 25.44 | 10.39 | 0.60 |
| 400 | 5627.33 ± 20.45 | 8.35 | 0.36 | 5631.17 ± 24.91 | 10.17 | 0.44 |
| Conc. (ng/spot) | Original | Used | Area ± SD (n = 3) | % RSD | Rf | |
|---|---|---|---|---|---|---|
| Curcumin I | ||||||
| 8.9:1.1 | −0.1, +0.1 | 2148 ± 28 | 1.33 | 0.43 | ||
| 400 | 9:1 | 9:1 | 0.0 | 2149 ± 23 | 1.09 | 0.45 |
| 9.1:0.9 | +0.1, −0.1 | 2158 ± 22 | 1.01 | 0.46 | ||
| Curcumin II | ||||||
| 8.9:1.1 | −0.1, +0.1 | 3573 ± 27 | 0.75 | 0.51 | ||
| 400 | 9:1 | 9:1 | 0.0 | 3574 ± 26 | 0.73 | 0.52 |
| 9.1:0.9 | +0.1, −0.1 | 3574 ± 13 | 0.36 | 0.49 | ||
| Curcumin III | ||||||
| 8.9:1.1 | −0.1, +0.1 | 5645 ± 31 | 0.55 | 0.60 | ||
| 400 | 9:1 | 9:1 | 0.0 | 5650 ± 22 | 0.39 | 0.61 |
| 9.1:0.9 | +0.1, −0.1 | 5641 ± 25 | 0.45 | 0.63 | ||
| Samples | Cur. I | Cur. II | Cur. III |
|---|---|---|---|
| 1. Curcuma Ext_Fresh (Maceration) | 0.44 | 0.28 | 0.65 |
| 2. Curcuma Ext_Fresh (Soxhlet) | 0.53 | 0.37 | 0.76 |
| 3. Commercial Powder (Maceration) | 0.23 | 0.15 | 0.33 |
| 4. Commercial Powder (Soxhlet) | 0.34 | 0.25 | 0.47 |
| 5. Formulation (Maceration) | 0.26 | 0.19 | 0.41 |
| 6. Formulation (Soxhlet) | 0.28 | 0.22 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Kader, M.S.; Salkini, A.A.; Alam, P.; Alshahrani, K.A.; Foudah, A.I.; Alqarni, M.H. A High-Performance Thin-Layer Chromatographic Method for the Simultaneous Determination of Curcumin I, Curcumin II and Curcumin III in Curcuma longa and Herbal Formulation. Separations 2022, 9, 94. https://doi.org/10.3390/separations9040094
Abdel-Kader MS, Salkini AA, Alam P, Alshahrani KA, Foudah AI, Alqarni MH. A High-Performance Thin-Layer Chromatographic Method for the Simultaneous Determination of Curcumin I, Curcumin II and Curcumin III in Curcuma longa and Herbal Formulation. Separations. 2022; 9(4):94. https://doi.org/10.3390/separations9040094
Chicago/Turabian StyleAbdel-Kader, Maged S., Ayman A. Salkini, Prawez Alam, Khaled A. Alshahrani, Ahmed I. Foudah, and Mohammed H. Alqarni. 2022. "A High-Performance Thin-Layer Chromatographic Method for the Simultaneous Determination of Curcumin I, Curcumin II and Curcumin III in Curcuma longa and Herbal Formulation" Separations 9, no. 4: 94. https://doi.org/10.3390/separations9040094
APA StyleAbdel-Kader, M. S., Salkini, A. A., Alam, P., Alshahrani, K. A., Foudah, A. I., & Alqarni, M. H. (2022). A High-Performance Thin-Layer Chromatographic Method for the Simultaneous Determination of Curcumin I, Curcumin II and Curcumin III in Curcuma longa and Herbal Formulation. Separations, 9(4), 94. https://doi.org/10.3390/separations9040094








