Development of a High-Throughput Screening Analysis for 195 Pesticides in Raw Milk by Modified QuEChERS Sample Preparation and Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Reagents and Materials
2.3. Preparation of Standard Solutions
2.4. Sample Preparation
2.5. Validation of the Method
3. Results
3.1. Optimization of the QuEChERS Procedure
3.1.1. Optimization of the Extraction Solvent Volume
3.1.2. Optimization of the Type of Extraction Salt
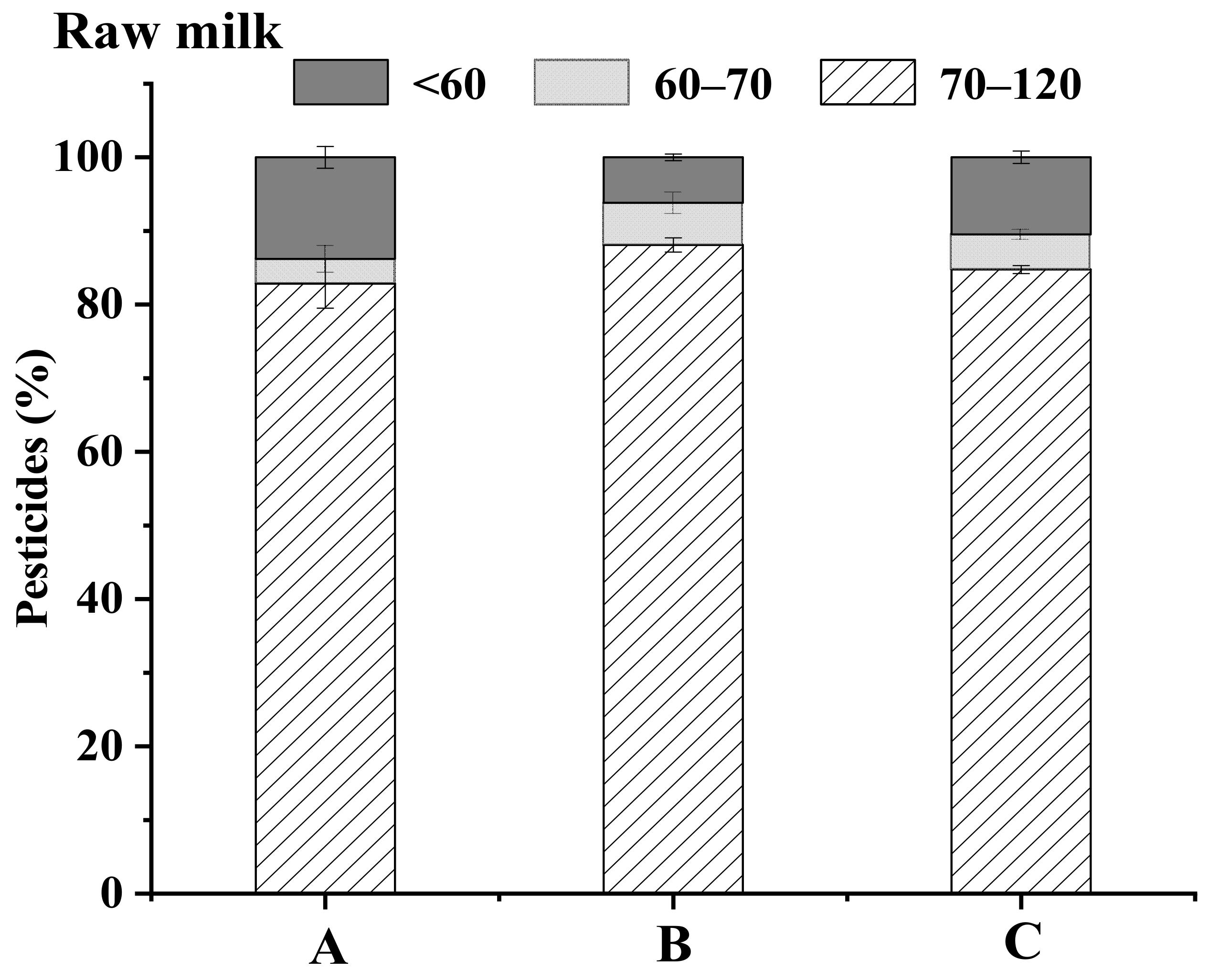
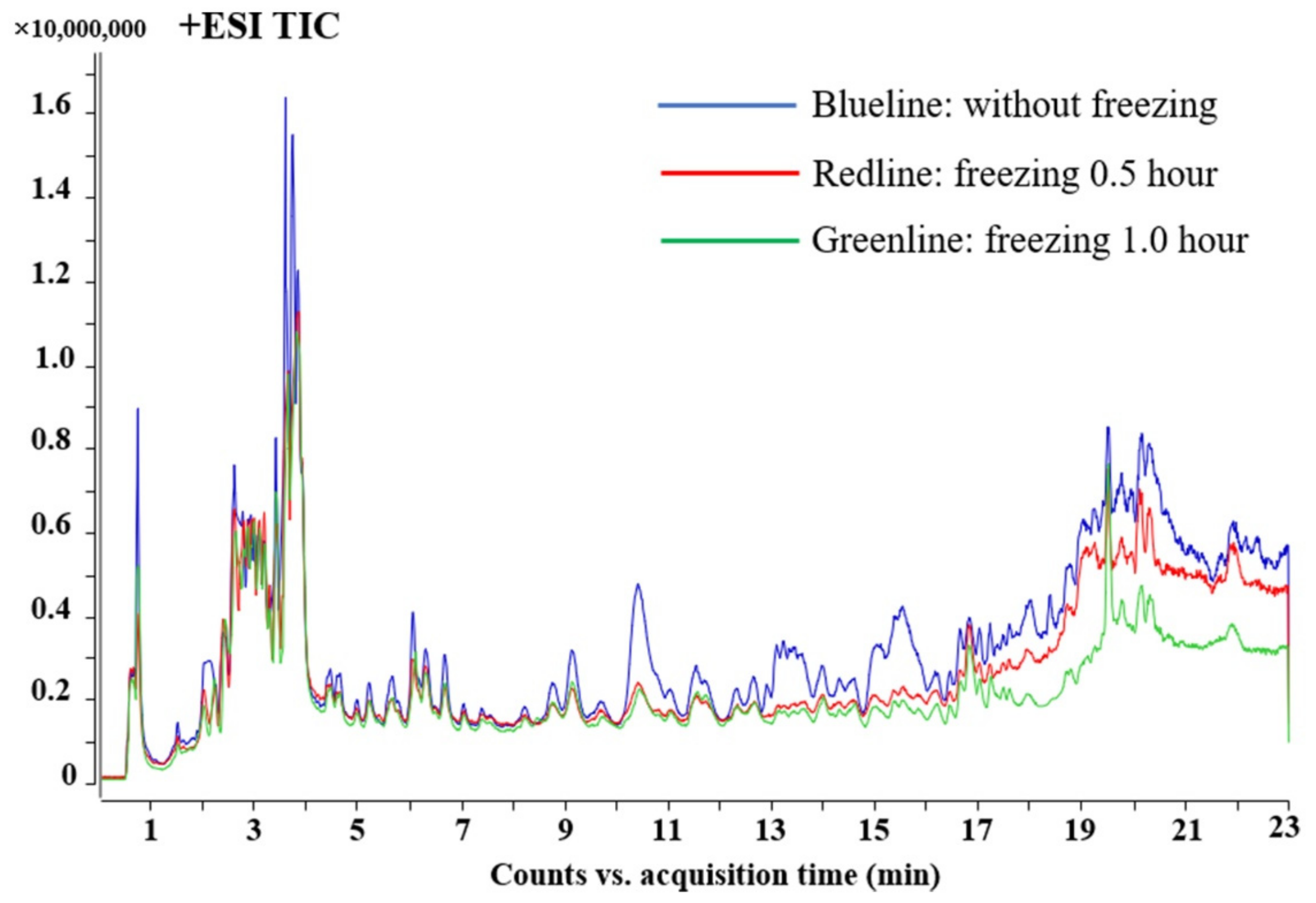
3.1.3. Optimization of the Freezing Temperature
3.1.4. Optimization of the Purification Adsorbent
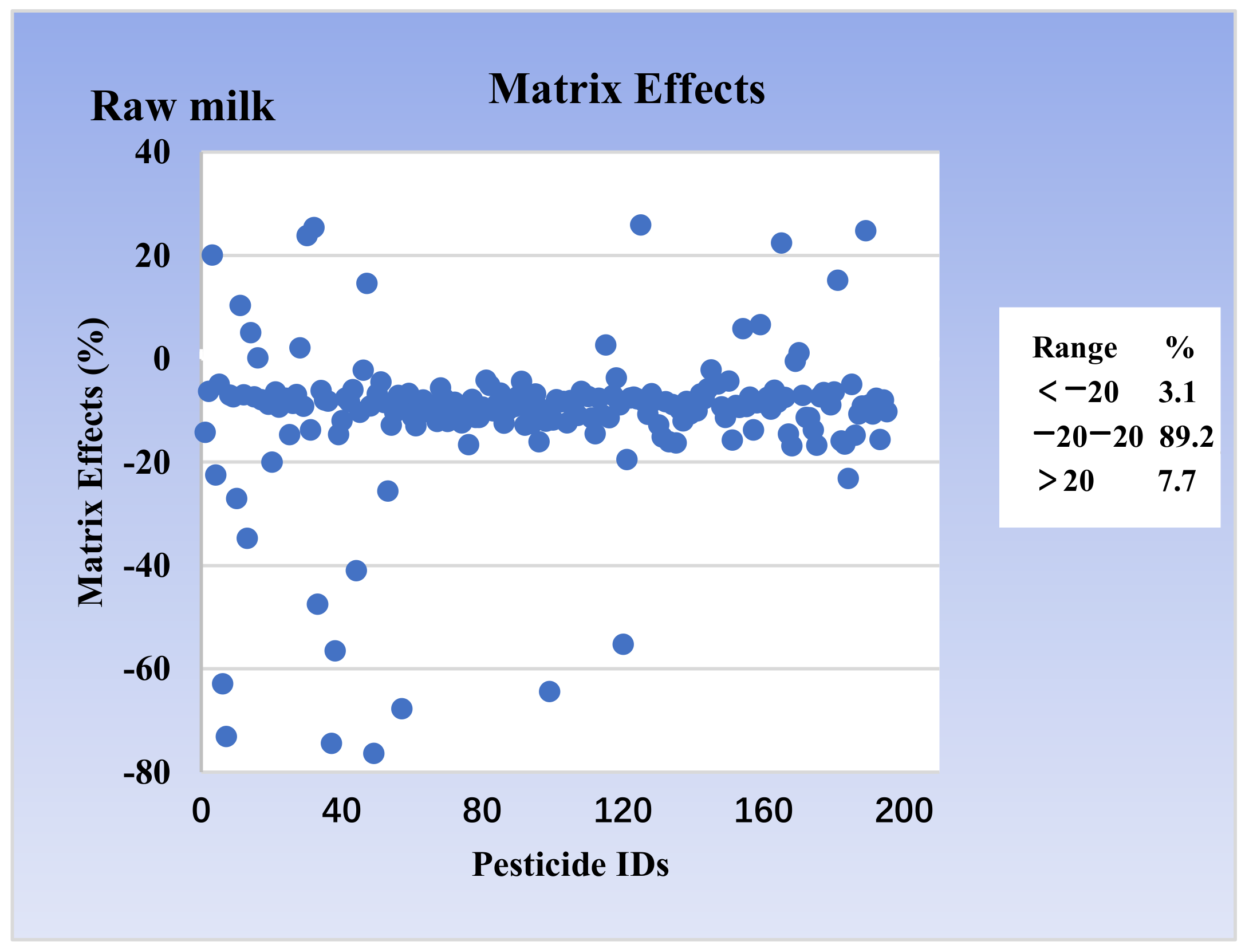
3.2. Matrix Effect
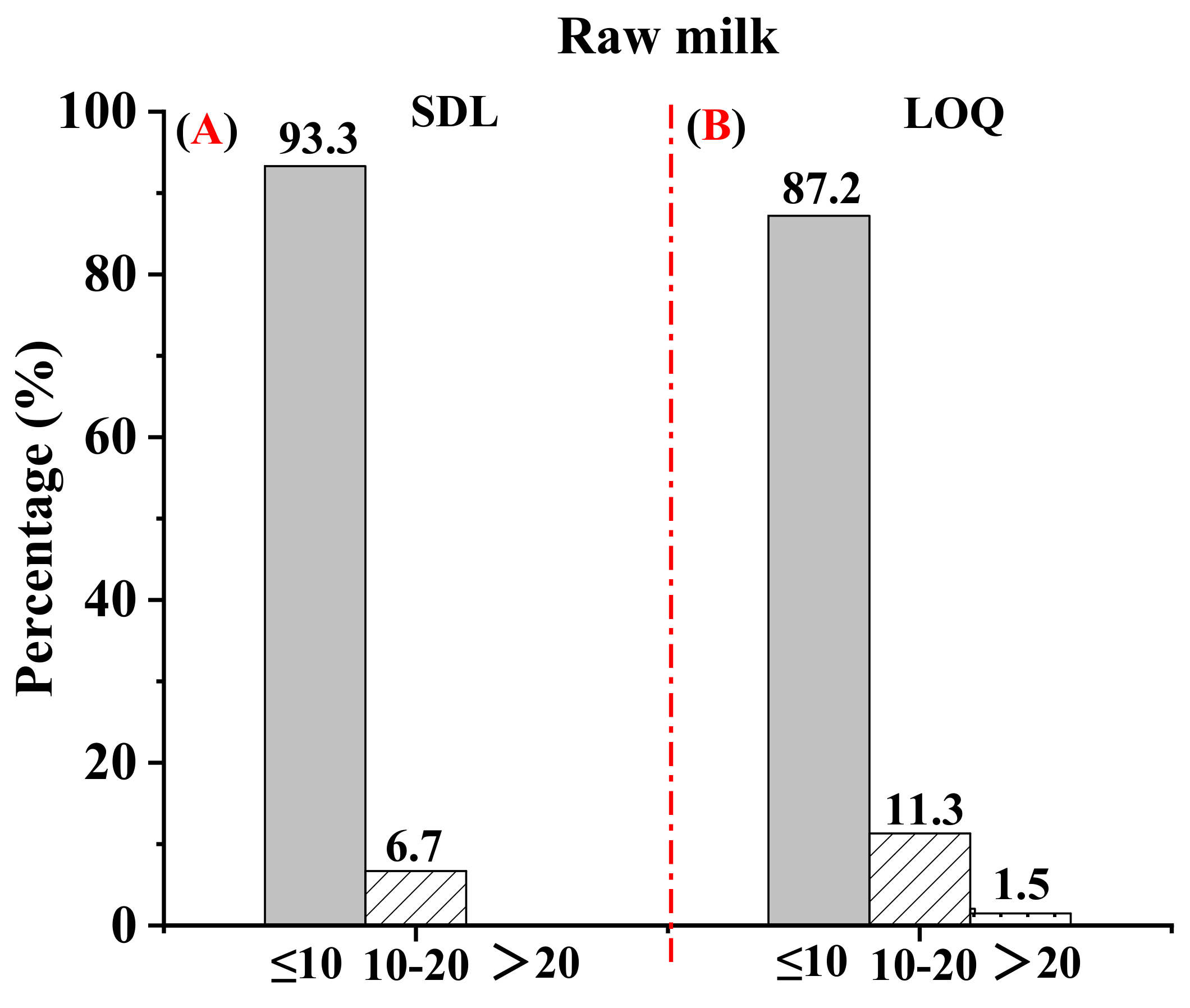
3.3. Method Validation
3.4. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Givens, D. MILK Symposium review: The importance of milk and dairy foods in the diets of infants, adolescents, pregnant women, adults, and the elderly. J. Dairy Sci. 2020, 103, 9681–9699. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Wang, J.; Chen, K.Z.; Fan, S.; Gao, H. Changing Chinese Diets to Achieve a Win–Win Solution for Health and the Environment. China World Econ. 2021, 29, 34–52. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Ariyawardana, A. Rebuilding milk safety trust in China: What do we learn and the way forward. J. Chin. Gov. 2021, 6, 1–23. [Google Scholar] [CrossRef]
- Gill, J.P.S.; Bedi, J.S.; Singh, R.; Fairoze, M.N.; Hazarika, R.A.; Gaurav, A.; Satpathy, S.K.; Chauhan, A.S.; Lindahl, J.; Grace, D.; et al. Pesticide Residues in Peri-Urban Bovine Milk from India and Risk Assessment: A Multicenter Study. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Tsakiris, I.N.; Goumenou, M.; Tzatzarakis, M.N.; Alegakis, A.K.; Tsitsimpikou, C.; Ozcagli, E.; Tsatsakis, A.M. Risk assessment for children exposed to DDT residues in various milk types from the Greek market. Food. Chem. Toxicol. 2015, 75, 156–165. [Google Scholar] [CrossRef]
- Lachat, L.; Glauser, G. Development and Validation of an Ultra-Sensitive UHPLC–MS/MS Method for Neonicotinoid Analysis in Milk. J. Agric. Food Chem. 2018, 66, 8639–8646. [Google Scholar] [CrossRef]
- LeDoux, M. Analytical methods applied to the determination of pesticide residues in foods of animal origin. A review of the past two decades. J. Chromatogr. A 2011, 1218, 1021–1036. [Google Scholar] [CrossRef]
- Năstăsescu, V.; Mititelu, M.; Goumenou, M.; Docea, A.O.; Renieri, E.; Udeanu, D.I.; Oprea, E.; Arsene, A.L.; Dinu-Pîrvu, C.E.; Ghica, M. Heavy metal and pesticide levels in dairy products: Evaluation of human health risk. Food Chem. Toxicol. 2020, 146, 111844. [Google Scholar] [CrossRef]
- Ramezani, S.; Mahdavi, V.; Gordan, H.; Rezadoost, H.; Conti, G.O.; Khaneghah, A.M. Determination of multi-class pesticides residues of cow and human milk samples from Iran using UHPLC-MS/MS and GC-ECD: A probabilistic health risk assessment. Environ. Res. 2022, 208, 112730. [Google Scholar] [CrossRef]
- European Commission. Pesticide Residue Online Database in/on Milk. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/?event=search.pr (accessed on 15 March 2022).
- GB 2763-2021; National Food Safety Standard-In Maximum Residue Limits for Pesticides in Food. China Agriculture Press: Beijing, China, 2021.
- Rejczak, T.; Tuzimski, T. QuEChERS-based extraction with dispersive solid phase extraction clean-up using PSA and ZrO2-based sorbents for determination of pesticides in bovine milk samples by HPLC-DAD. Food Chem. 2017, 217, 225–233. [Google Scholar] [CrossRef]
- Tripathy, V.; Sharma, K.K.; Yadav, R.; Devi, S.; Tayade, A.; Sharma, K.; Shakil, N.A. Development, validation of QuEChERS-based method for simultaneous determination of multiclass pesticide residue in milk, and evaluation of the matrix effect. J. Environ. Sci. Health B 2019, 54, 394–406. [Google Scholar] [CrossRef]
- Manav, Ö.G.; Dinç-Zor, Ş.; Alpdoğan, G. Optimization of a modified QuEChERS method by means of experimental design for multiresidue determination of pesticides in milk and dairy products by GC–MS. Microchem. J. 2019, 144, 124–129. [Google Scholar] [CrossRef]
- Zheng, G.; Han, C.; Liu, Y.; Wang, J.; Zhu, M.; Wang, C.; Shen, Y. Multiresidue analysis of 30 organochlorine pesticides in milk and milk powder by gel permeation chromatography-solid phase extraction-gas chromatography-tandem mass spectrometry. J. Dairy Sci. 2014, 97, 6016–6026. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.S.; Kim, M.; Kim, E.J.; Choe, W.-J. Determination of 66 pesticide residues in livestock products using QuEChERS and GC–MS/MS. Food Sci. Biotechnol. 2020, 29, 1573–1586. [Google Scholar] [CrossRef]
- Imamoglu, H.; Oktem Olgun, E. Analysis of veterinary drug and pesticide residues using the ethyl acetate multiclass/multiresidue method in milk by liquid chromatography-tandem mass spectrometry. J. Anal. Method Chem. 2016, 2016, 2170165. [Google Scholar] [CrossRef] [Green Version]
- Görel-Manav, Ö.; Dinç-Zor, Ş.; Akyildiz, E.; Alpdoğan, G. Multivariate optimization of a new LC–MS/MS method for the determination of 156 pesticide residues in milk and dairy products. J. Sci. Food Agric. 2020, 100, 4808–4817. [Google Scholar] [CrossRef]
- Jadhav, M.R.; Pudale, A.; Raut, P.; Utture, S.; Shabeer, T.A.; Banerjee, K. A unified approach for high-throughput quantitative analysis of the residues of multi-class veterinary drugs and pesticides in bovine milk using LC-MS/MS and GC–MS/MS. Food Chem. 2019, 272, 292–305. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, R.; Shi, L.; Zhang, F.; Xu, X.; Chu, X. Construction of Non-Target Screening Method for Pesticides in Milk and Dairy Products Based on Mass Spectrometry Fracture Mechanism. Chin. J. Anal. Chem. 2019, 47, 1098–1149. [Google Scholar] [CrossRef]
- Aydoğan, C.; El Rassi, Z. MWCNT based monolith for the analysis of antibiotics and pesticides in milk and honey by integrated nano-liquid chromatography-high resolution orbitrap mass spectrometry. Anal. Methods UK 2019, 11, 21–28. [Google Scholar] [CrossRef]
- López-Ruiz, R.; Romero-González, R.; Frenich, A.G. Ultrahigh-pressure liquid chromatography-mass spectrometry: An overview of the last decade. TrAC Trends Anal. Chem. 2019, 118, 170–181. [Google Scholar] [CrossRef]
- Hajeb, P.; Zhu, L.; Bossi, R.; Vorkamp, K. Sample preparation techniques for suspect and non-target screening of emerging contaminants. Chemosphere 2022, 287, 132306. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.H.; Dias, J.; Mol, H.; de Kok, A. Selective multiresidue determination of highly polar anionic pesticides in plant-based milk, wine and beer using hydrophilic interaction liquid chromatography combined with tandem mass spectrometry. J. Chromatogr. A 2020, 1625, 461226. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yu, H.; He, Y.; Wang, M.; Liu, G.; Hong, S.; She, Y. A dummy molecularly imprinted solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry for selective determination of four pyridine carboxylic acid herbicides in milk. J. Chromatogr. B 2019, 1108, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS-Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- SANTE/12682/2019; Analytical Quality Control and Method Validation Procedures for Pesticides Residues and Analysis in Food and Feed. Directorate General for Health and Food Safety. European Union: Brussels, Belgium, 2020.
- González-Curbelo, M.Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; González-Sálamo, J.; Hernández-Borges, J.; Rodriguez-Delgado, M.A. Evolution and applications of the QuEChERS method. TrAC Trends Anal. Chem. 2015, 71, 169–185. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.; Bourmpopoulou, A.; Miliadis, G. Development and validation of a dispersive solid phase extraction liquid chromatography mass spectrometry method with electrospray ionization for the determination of multiclass pesticides and metabolites in meat and milk. Anal. Lett. 2013, 46, 2526–2541. [Google Scholar] [CrossRef]

| NO. | Compound | Formula | RT/Min | Quantitative Ion (m/z) | Production (m/z) | SDL (mg/kg) | LOQ (mg/kg) | MRL (mg/kg; European Union, China) | R2 | 1 × LOQ | 2 × LOQ | 10 × LOQ | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rec. (%) | RSD (%) | Rec. (%) | RSD (%) | Rec. (%) | RSD (%) | ||||||||||
| 1 | 1-(2-chloro-4-(4-chlorophenoxy)phenyl)-2-(1H-1,2,4-triazol-1-yl)ethanol | C16H13Cl2N3O2 | 10.16 | 350.0458 | 70.0400 | 20.0 | 20.0 | —, — | 0.9988 | 100.2 | 1.0 | 98.1 | 0.9 | 86.2 | 1.1 |
| 2 | 1-(2-Chloro-pyridin-5-yl-methyl)-2-imino-imidazolidine hydrochloride | C9H12Cl2N4 | 2.28 | 211.0745 | 90.0338 | 0.5 | 1.0 | —, — | 0.9990 | 94.4 | 18.7 | 82.2 | 6.7 | 101.0 | 16.8 |
| 3 | 1-methyl-3-(tetrahydro-3-furylmethyl) urea | C7H14N2O2 | 1.87 | 159.1128 | 58.0287 | 0.2 | 1.0 | —, — | 0.9926 | 96.2 | 7.6 | 98.7 | 14.3 | 104.2 | 11.7 |
| 4 | 3-(Trifluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide | C6H6F3N3O | 2.63 | 194.0536 | 134.0349 | 10.0 | 10.0 | —, — | 0.9932 | 94.8 | 14.7 | 106.4 | 8.5 | 85.8 | 6.6 |
| 5 | 5-hydroxy Imidacloprid | C9H10ClN5O3 | 3.05 | 272.0545 | 225.0538 | 2.0 | 5.0 | —, — | 0.9992 | 109.6 | 11.3 | 112.9 | 18.0 | 101.4 | 18.0 |
| 6 | Acetamiprid | C10H11ClN4 | 3.97 | 223.0745 | 126.0105 | 0.5 | 0.5 | 0.2, — | 0.9994 | 77.9 | 5.8 | 84.6 | 10.4 | 103.9 | 7.5 |
| 7 | Acetamiprid-N-desmethyl | C9H9ClN4 | 3.62 | 209.0589 | 126.0105 | 0.2 | 1.0 | —, — | 0.9976 | 119.0 | 12.3 | 94.6 | 15.3 | 97.1 | 15.7 |
| 8 | Acetochlor | C14H20ClNO2 | 12.62 | 270.1255 | 133.0886 | 1.0 | 1.0 | 0.01, — | 0.9989 | 83.4 | 18.5 | 119.8 | 6.8 | 101.7 | 10.5 |
| 9 | Alachlor | C14H20ClNO2 | 12.58 | 270.1255 | 238.0993 | 1.0 | 2.0 | 0.01, — | 0.9989 | 118.6 | 7.4 | 98.4 | 3.2 | 94.7 | 2.2 |
| 10 | Aldicarb-sulfone | C7H14N2O4S | 2.66 | 223.0747 | 62.9899 | 10.0 | 20.0 | —, — | 0.9980 | 99.3 | 7.5 | 95.7 | 3.6 | 87.6 | 2.4 |
| 11 | Allidochlor | C8H12ClNO | 5.00 | 174.0680 | 98.0964 | 10.0 | 10.0 | —, — | 0.9968 | 71.4 | 16.8 | 85.9 | 6.5 | 72.1 | 17.6 |
| 12 | Ametryn | C9H17N5S | 6.71 | 228.1277 | 68.0243 | 0.1 | 0.5 | —, — | 0.9973 | 96.2 | 2.8 | 98.0 | 1.7 | 100.2 | 1.4 |
| 13 | Aminocyclopyrachlor | C8H8ClN3O2 | 0.76 | 214.0378 | 68.0495 | 10.0 | 10.0 | —, — | 0.9976 | 72.9 | 9.9 | 75.7 | 8.8 | 86.4 | 11.6 |
| 14 | Aminopyralid | C6H4Cl2N2O2 | 1.70 | 206.9723 | 160.9668 | 20.0 | 50.0 | 0.02, — | 0.9973 | 70.0 | 8.9 | 76.0 | 6.7 | 83.0 | 5.3 |
| 15 | Atrazine | C8H14ClN5 | 6.44 | 216.1010 | 174.0541 | 0.1 | 0.1 | —, — | 0.9976 | 87.3 | 13.6 | 105.9 | 3.9 | 101.7 | 4.4 |
| 16 | Avermectin | C48H72O14 | 18.72 | 895.4814 | 751.4052 | 0.5 | 0.5 | —, — | 0.9993 | 87.4 | 7.4 | 108.6 | 3.8 | 92.7 | 4.7 |
| 17 | Azoxystrobin | C22H17N3O5 | 11.17 | 404.1241 | 329.0795 | 0.1 | 0.1 | 0.01, — | 0.9973 | 86.8 | 19.3 | 97.2 | 12.6 | 100.7 | 3.9 |
| 18 | Benalaxyl | C20H23NO3 | 14.11 | 326.1751 | 91.0542 | 0.2 | 0.5 | 0.02, — | 0.9981 | 110.5 | 9.8 | 92.5 | 2.7 | 101.2 | 1.5 |
| 19 | Benzovindiflupyr | C18H15Cl2F2N3O | 14.43 | 398.0640 | 159.0364 | 0.5 | 0.5 | 0.01, — | 0.9985 | 93.6 | 7.0 | 107.6 | 4.2 | 100.7 | 2.0 |
| 20 | Bioresmethrin | C22H26O3 | 19.09 | 339.1955 | 143.0855 | 10.0 | 20.0 | —, — | 0.9905 | 103.3 | 17.6 | 80.5 | 11.7 | 82.1 | 9.8 |
| 21 | Bitertanol | C20H23N3O2 | 12.77 | 338.1863 | 70.0400 | 10.0 | 10.0 | 0.01, — | 0.9964 | 101.6 | 16.1 | 83.5 | 5.8 | 90.0 | 3.5 |
| 22 | Boscalid | C18H12Cl2N2O | 11.30 | 343.0399 | 271.0866 | 1.0 | 1.0 | 0.02, — | 0.9989 | 116.4 | 8.3 | 105.6 | 8.9 | 104.1 | 13.6 |
| 23 | Bromobutide | C15H22BrNO | 13.80 | 312.0958 | 119.0855 | 1.0 | 2.0 | —, — | 0.9999 | 90.9 | 18.1 | 104.3 | 8.5 | 101.0 | 3.4 |
| 24 | Bupirimate | C13H24N4O3S | 12.61 | 317.1642 | 44.0495 | 0.5 | 0.5 | 0.01, — | 0.9993 | 110.5 | 5.7 | 103.8 | 4.6 | 100.0 | 1.1 |
| 25 | Buprofezin | C16H23N3OS | 17.42 | 306.1635 | 57.0699 | 0.5 | 0.5 | 0.01, — | 0.9978 | 104.4 | 12.1 | 106.6 | 18.6 | 102.4 | 3.7 |
| 26 | Butachlor | C17H26ClNO2 | 17.52 | 312.1725 | 57.0699 | 0.5 | 1.0 | —, — | 0.9988 | 86.8 | 17.0 | 84.9 | 9.8 | 102.8 | 12.1 |
| 27 | Butamifos | C13H21N2O4PS | 16.50 | 333.1035 | 95.9668 | 0.5 | 1.0 | —, — | 0.9984 | 107.1 | 10.5 | 86.0 | 14.5 | 106.0 | 8.8 |
| 28 | Butylate | C11H23NOS | 16.72 | 218.1573 | 57.0699 | 10.0 | 20.0 | 0.01, — | 0.9985 | 92.2 | 14.2 | 72.3 | 17.8 | 77.0 | 6.7 |
| 29 | Cadusafos | C10H23O2PS2 | 14.78 | 271.0950 | 96.9508 | 0.2 | 0.5 | 0.01, — | 0.9995 | 73.5 | 17.7 | 75.0 | 11.5 | 96.0 | 2.9 |
| 30 | Carbaryl | C12H11NO2 | 6.29 | 202.0863 | 127.0542 | 20.0 | 50.0 | 0.05, — | 0.9952 | 72.0 | 13.5 | 83.0 | 7.2 | 88.0 | 6.2 |
| 31 | Carbendazim | C9H9N3O2 | 2.65 | 192.0768 | 160.0505 | 0.1 | 0.2 | 0.05, — | 0.9992 | 70.9 | 12.6 | 102.9 | 3.9 | 107.6 | 4.6 |
| 32 | Carbofuran | C12H15NO3 | 5.87 | 222.1125 | 123.0441 | 0.5 | 1.0 | 0.001, — | 0.9974 | 102.3 | 12.3 | 115.8 | 6.1 | 96.7 | 11.2 |
| 33 | Carbofuran-3-Hydroxy | C12H15NO4 | 3.60 | 238.1074 | 107.0491 | 1.0 | 1.0 | —, — | 0.9924 | 71.0 | 13.5 | 99.2 | 8.5 | 110.0 | 13.8 |
| 34 | Carfentrazone-ethyl | C15H14Cl2F3N3O3 | 14.29 | 412.0435 | 345.9956 | 1.0 | 1.0 | 0.01, — | 0.9997 | 115.7 | 12.5 | 92.1 | 4.0 | 107.4 | 15.5 |
| 35 | Chlorantraniliprole | C18H14BrCl2N5O2 | 8.36 | 481.9781 | 283.9216 | 1.0 | 1.0 | 0.05, — | 0.9987 | 97.3 | 14.9 | 76.1 | 11.5 | 103.3 | 15.7 |
| 36 | Chlorfenvinphos | C12H14Cl3O4P | 13.78 | 358.9768 | 98.9843 | 0.5 | 0.5 | 0.01, — | 0.9990 | 74.3 | 19.6 | 97.8 | 11.4 | 90.1 | 5.0 |
| 37 | Chloridazon | C10H8ClN3O | 3.67 | 222.0429 | 77.0386 | 0.5 | 5.0 | 0.3, — | 0.9951 | 112.5 | 5.7 | 105.6 | 13.2 | 92.2 | 13.2 |
| 38 | Chlormequat | C5H12ClN | 0.75 | 122.0731 | 58.0651 | 0.1 | 0.1 | 0.5, 0.5 | 0.9990 | 118.2 | 4.9 | 108.0 | 3.0 | 119.3 | 6.0 |
| 39 | Chlorotoluron | C10H13ClN2O | 6.15 | 213.0789 | 72.0449 | 0.5 | 0.5 | 0.01, — | 0.9995 | 98.0 | 9.7 | 104.7 | 5.0 | 100.2 | 3.6 |
| 40 | Chlorpyrifos | C9H11Cl3NO3PS | 17.76 | 349.9336 | 96.9508 | 5.0 | 5.0 | 0.01, — | 0.9924 | 115.4 | 8.8 | 95.2 | 19.9 | 90.9 | 19.9 |
| 41 | Clodinafop-propargyl | C17H13ClFNO4 | 15.12 | 350.0590 | 91.0542 | 0.5 | 0.5 | —, — | 0.9998 | 116.4 | 15.1 | 117.3 | 8.3 | 104.1 | 2.8 |
| 42 | Clofentezine | C14H8Cl2N4 | 15.40 | 303.0199 | 102.0338 | 10.0 | 10.0 | 0.05, — | 0.9955 | 91.8 | 11.1 | 83.5 | 2.5 | 93.0 | 4.4 |
| 43 | Clomazone | C12H14ClNO2 | 8.00 | 240.0786 | 125.0153 | 2.0 | 5.0 | 0.01, — | 0.9979 | 96.6 | 8.9 | 94.6 | 8.7 | 92.7 | 8.7 |
| 44 | Clothianidin | C6H8ClN5O2S | 3.54 | 250.0160 | 131.9669 | 2.0 | 5.0 | 0.02, — | 0.9917 | 109.8 | 8.8 | 101.4 | 17.2 | 103.2 | 17.2 |
| 45 | Cyanazine | C9H13ClN6 | 5.22 | 241.0963 | 214.0854 | 0.5 | 5.0 | —, — | 0.9976 | 106.2 | 2.6 | 106.7 | 16.4 | 99.2 | 16.4 |
| 46 | Cycloate | C11H21NOS | 15.41 | 216.1417 | 55.0542 | 10.0 | 20.0 | —, — | 0.9981 | 89.2 | 7.9 | 84.5 | 4.0 | 75.3 | 4.7 |
| 47 | Cycloxydim | C17H27NO3S | 16.37 | 326.1784 | 107.0491 | 1.0 | 1.0 | 0.05, — | 0.9994 | 87.1 | 15.0 | 84.6 | 20.0 | 91.6 | 9.6 |
| 48 | Cyprodinil | C14H15N3 | 11.76 | 226.1339 | 93.0573 | 0.1 | 0.5 | 0.02, — | 0.9982 | 103.3 | 9.2 | 104.9 | 1.7 | 96.7 | 2.6 |
| 49 | Cyromazine | C6H10N6 | 0.80 | 167.1040 | 85.0509 | 2.0 | 2.0 | 0.01, — | 0.9989 | 73.5 | 10.6 | 74.0 | 9.8 | 93.1 | 6.7 |
| 50 | Desmetryn | C8H15N5S | 5.23 | 214.1121 | 172.0651 | 0.2 | 0.2 | —, — | 0.9978 | 99.7 | 13.3 | 90.8 | 8.9 | 101.0 | 3.7 |
| 51 | Diallate | C10H17Cl2NOS | 16.72 | 270.0481 | 86.0600 | 10.0 | 20.0 | —, — | 0.9972 | 93.4 | 12.7 | 74.5 | 3.8 | 78.1 | 2.3 |
| 52 | Diazinon | C12H21N2O3PS | 15.09 | 305.1083 | 96.9508 | 0.2 | 0.5 | 0.02, — | 0.9984 | 94.0 | 7.9 | 94.0 | 6.5 | 94.7 | 0.9 |
| 53 | Dichlorvos | C4H7Cl2O4P | 5.24 | 220.9532 | 109.0049 | 20.0 | 20.0 | —, — | 0.9908 | 110.3 | 20.0 | 81.5 | 12.2 | 71.5 | 14.0 |
| 54 | Difenoconazole | C19H17Cl2N3O3 | 14.63 | 406.0720 | 251.0025 | 0.5 | 1.0 | 0.005, — | 0.9979 | 98.4 | 6.4 | 100.1 | 6.0 | 101.4 | 14.3 |
| 55 | Diflubenzuron | C14H9ClF2N2O2 | 12.19 | 311.0393 | 141.0146 | 20.0 | 20.0 | 0.01, — | 0.9954 | 116.1 | 14.9 | 91.0 | 10.4 | 90.9 | 2.1 |
| 56 | Dimethenamid | C12H18ClNO2S | 9.77 | 276.0820 | 244.0557 | 0.2 | 0.5 | 0.01, — | 0.9970 | 114.3 | 13.4 | 96.9 | 11.9 | 91.2 | 12.7 |
| 57 | Dimethoate | C5H12NO3PS2 | 3.83 | 230.0069 | 198.9647 | 5.0 | 5.0 | 0.01, 0.05 | 0.9924 | 94.3 | 15.2 | 100.4 | 18.1 | 88.1 | 18.1 |
| 58 | Dimethylvinphos (E) | C10H10Cl3O4P | 11.58 | 330.9455 | 127.0155 | 10.0 | 20.0 | —, — | 0.9918 | 105.9 | 18.9 | 93.3 | 15.0 | 89.5 | 4.5 |
| 59 | Dimethylvinphos (Z) | C10H10Cl3O4P | 10.59 | 330.9455 | 127.0155 | 5.0 | 5.0 | —, — | 0.9984 | 98.8 | 10.6 | 94.3 | 13.4 | 93.5 | 13.4 |
| 60 | Diniconazole | C15H17Cl2N3O | 13.05 | 326.0821 | 70.0400 | 5.0 | 5.0 | 0.01, — | 0.9980 | 100.7 | 3.3 | 104.6 | 14.7 | 94.8 | 14.7 |
| 61 | Dinotefuran | C7H14N4O3 | 2.33 | 203.1139 | 58.0526 | 5.0 | 10.0 | 0.1, — | 0.9975 | 81.0 | 19.3 | 106.5 | 3.5 | 96.2 | 6.5 |
| 62 | Dioxabenzofos | C8H9O3PS | 9.19 | 217.0083 | 77.0386 | 2.0 | 5.0 | —, — | 0.9990 | 101.7 | 2.7 | 98.2 | 11.8 | 97.2 | 11.8 |
| 63 | Dipropetryn | C11H21N5S | 11.42 | 256.1590 | 102.0120 | 0.1 | 0.5 | —, — | 0.9995 | 96.0 | 5.6 | 103.4 | 2.8 | 98.0 | 0.5 |
| 64 | Diuron | C9H10Cl2N2O | 6.72 | 233.0243 | 72.0449 | 0.5 | 0.5 | 0.05, — | 1.0000 | 97.4 | 8.8 | 92.4 | 5.3 | 103.3 | 2.2 |
| 65 | Edifenphos | C14H15O2PS2 | 13.54 | 311.0324 | 109.0107 | 0.5 | 0.5 | —, — | 0.9981 | 104.8 | 7.0 | 101.9 | 1.9 | 104.4 | 1.4 |
| 66 | Emamectin B1a | C49H75NO13 | 15.63 | 886.5311 | 158.1176 | 0.2 | 0.5 | 0.01, — | 0.9980 | 92.6 | 9.3 | 113.7 | 14.2 | 93.9 | 3.5 |
| 67 | Ethion | C9H22O4P2S4 | 17.97 | 384.9949 | 199.0011 | 1.0 | 1.0 | 0.01, — | 0.9970 | 111.4 | 14.9 | 107.3 | 16.2 | 101.1 | 11.7 |
| 68 | Ethoprophos | C8H19O2PS2 | 10.96 | 243.0637 | 96.9508 | 0.5 | 0.5 | 0.01, — | 0.9991 | 91.6 | 17.4 | 88.0 | 6.0 | 93.4 | 2.8 |
| 69 | Etrimfos | C10H17N2O4PS | 14.61 | 293.0719 | 124.9821 | 0.5 | 1.0 | —, — | 0.9986 | 114.6 | 5.3 | 107.6 | 7.6 | 96.4 | 7.7 |
| 70 | Fenamidone | C17H17N3OS | 10.94 | 312.1165 | 92.0495 | 0.5 | 0.5 | 0.01, — | 0.9957 | 82.7 | 16.2 | 110.9 | 8.1 | 103.7 | 3.2 |
| 71 | Fenamiphos | C13H22NO3PS | 10.60 | 304.1131 | 201.9848 | 0.5 | 0.5 | 0.005, — | 0.9979 | 100.2 | 7.4 | 91.1 | 5.7 | 100.2 | 2.2 |
| 72 | Fenamiphos-sulfone | C13H22NO5PS | 5.65 | 336.1029 | 266.0247 | 0.2 | 0.5 | —, — | 0.9988 | 111.4 | 5.2 | 93.3 | 5.6 | 100.8 | 3.5 |
| 73 | Fenamiphos-sulfoxide | C13H22NO4PS | 4.65 | 320.1080 | 108.0573 | 0.1 | 0.5 | —, — | 0.9988 | 94.9 | 7.4 | 97.4 | 2.5 | 101.0 | 1.4 |
| 74 | Fenarimol | C17H12Cl2N2O | 10.69 | 331.0399 | 81.0447 | 1.0 | 5.0 | 0.02, — | 0.9980 | 97.2 | 2.0 | 104.1 | 11.9 | 101.2 | 11.9 |
| 75 | Fenbuconazole | C19H17ClN4 | 12.50 | 337.1215 | 70.0400 | 1.0 | 1.0 | 0.05, — | 0.9992 | 77.7 | 4.9 | 86.2 | 11.5 | 107.7 | 10.3 |
| 76 | Fenobucarb | C12H17NO2 | 8.91 | 208.1332 | 77.0386 | 20.0 | 20.0 | —, — | 0.9906 | 88.2 | 16.7 | 87.5 | 11.2 | 89.9 | 1.0 |
| 77 | Fensulfothion | C11H17O4PS2 | 7.53 | 309.0379 | 140.0290 | 0.5 | 0.5 | —, — | 0.9986 | 99.8 | 4.1 | 115.2 | 6.9 | 101.1 | 1.6 |
| 78 | Fenthion-sulfoxide | C10H15O4PS2 | 6.06 | 295.0222 | 109.0049 | 0.2 | 0.5 | —, — | 0.9982 | 103.6 | 8.1 | 100.5 | 4.3 | 98.5 | 1.6 |
| 79 | Fluacrypyrim | C20H21F3N2O5 | 16.71 | 427.1475 | 145.0648 | 0.5 | 0.5 | —, — | 0.9992 | 92.6 | 15.9 | 104.3 | 6.9 | 101.5 | 3.1 |
| 80 | Fluazifop-butyl | C19H20F3NO4 | 17.73 | 384.1417 | 91.0542 | 0.1 | 0.1 | —, — | 0.9974 | 113.1 | 11.1 | 107.3 | 9.5 | 117.5 | 16.4 |
| 81 | Flubendiamide | C23H22F7IN2O4S | 14.68 | 705.0125 | 530.9799 | 0.2 | 0.5 | 0.1, — | 0.9987 | 106.8 | 2.8 | 97.7 | 5.6 | 99.6 | 2.8 |
| 82 | Flumiclorac-pentyl | C21H23ClFNO5 | 17.51 | 441.1593 | 308.0484 | 0.5 | 1.0 | —, — | 0.9963 | 109.9 | 11.3 | 97.6 | 13.7 | 81.7 | 16.8 |
| 83 | Fluopicolide | C14H8Cl3F3N2O | 11.97 | 382.9727 | 172.9556 | 1.0 | 1.0 | 0.02, — | 0.9991 | 90.2 | 10.1 | 101.6 | 4.8 | 104.8 | 12.3 |
| 84 | Fluquinconazole | C16H8Cl2FN5O | 11.52 | 376.0163 | 306.9836 | 10.0 | 10.0 | 0.01, — | 0.9988 | 94.2 | 14.9 | 95.3 | 4.5 | 95.0 | 1.8 |
| 85 | Fluridone | C19H14F3NO | 9.35 | 330.1100 | 309.0960 | 0.1 | 0.1 | —, — | 0.9988 | 114.7 | 11.4 | 95.3 | 5.9 | 102.1 | 1.9 |
| 86 | Flusilazole | C16H15F2N3Si | 12.45 | 316.1076 | 247.0749 | 0.5 | 1.0 | 0.02, — | 0.9974 | 114.7 | 7.7 | 93.4 | 2.6 | 102.6 | 12.7 |
| 87 | Flutriafol | C16H13F2N3O | 6.46 | 302.1099 | 70.0400 | 0.5 | 1.0 | 0.01, — | 0.9979 | 99.2 | 3.9 | 100.4 | 3.2 | 102.6 | 15.4 |
| 88 | Fluxapyroxad | C18H12F5N3O | 11.58 | 382.0973 | 342.0849 | 0.5 | 0.5 | 0.02, — | 0.9995 | 119.7 | 11.5 | 110.1 | 3.3 | 95.5 | 2.5 |
| 89 | Fonofos | C10H15OPS2 | 15.40 | 247.0375 | 80.9558 | 5.0 | 10.0 | —, — | 0.9960 | 116.8 | 7.3 | 103.8 | 6.7 | 89.9 | 3.7 |
| 90 | Fosthiazate | C9H18NO3PS2 | 6.44 | 284.0538 | 104.0165 | 0.5 | 0.5 | —, — | 0.9992 | 118.5 | 6.3 | 98.8 | 7.1 | 94.4 | 4.1 |
| 91 | Furathiocarb | C18H26N2O5S | 17.31 | 383.1635 | 195.0474 | 0.1 | 0.5 | 0.001, — | 0.9987 | 95.2 | 9.1 | 100.6 | 4.1 | 103.9 | 2.1 |
| 92 | Haloxyfop | C15H11ClF3NO4 | 12.37 | 362.0401 | 316.0347 | 20.0 | 20.0 | 0.015, — | 0.9972 | 79.2 | 10.0 | 103.2 | 4.5 | 86.8 | 3.3 |
| 93 | Haloxyfop-2-ethoxyethyl | C19H19ClF3NO5 | 17.12 | 434.0977 | 91.0542 | 0.5 | 0.5 | —, — | 0.9986 | 116.0 | 12.2 | 117.6 | 8.1 | 101.0 | 2.1 |
| 94 | Haloxyfop-methyl | C16H13ClF3NO4 | 16.30 | 376.0546 | 272.0085 | 0.5 | 0.5 | —, — | 0.9993 | 93.1 | 17.6 | 111.8 | 8.1 | 100.4 | 2.5 |
| 95 | Hexaconazole | C14H17Cl2N3O | 12.29 | 314.0825 | 70.0400 | 1.0 | 5.0 | —, — | 0.9972 | 91.6 | 3.3 | 103.8 | 12.3 | 97.3 | 12.3 |
| 96 | Hexythiazox | C17H21ClN2O2S | 17.76 | 353.1085 | 168.0570 | 5.0 | 5.0 | 0.05, — | 0.9987 | 117.6 | 8.2 | 99.6 | 10.3 | 90.7 | 10.3 |
| 97 | Imazalil | C14H14Cl2N2O | 5.78 | 297.0550 | 69.0447 | 0.2 | 0.5 | 0.02, — | 0.9979 | 98.6 | 15.5 | 112.1 | 11.9 | 99.2 | 1.8 |
| 98 | Imazapyr | C13H15N3O3 | 3.11 | 262.1186 | 69.0699 | 1.0 | 5.0 | 0.01, — | 0.9987 | 102.0 | 2.4 | 98.6 | 17.2 | 93.2 | 17.2 |
| 99 | Imidacloprid | C9H10ClN5O2 | 3.73 | 256.0596 | 209.0589 | 10.0 | 10.0 | 0.01, — | 0.9908 | 105.4 | 17.2 | 101.5 | 7.9 | 88.4 | 7.5 |
| 100 | Imidacloprid-Olefin | C9H8ClN5O2 | 3.07 | 254.0439 | 171.0665 | 5.0 | 5.0 | —, — | 0.9948 | 115.6 | 10.6 | 113.0 | 12.9 | 98.7 | 12.9 |
| 101 | Iprobenfos | C13H21O3PS | 12.40 | 289.1022 | 91.0542 | 5.0 | 5.0 | —, — | 0.9985 | 108.2 | 16.2 | 100.3 | 11.6 | 88.9 | 11.6 |
| 102 | Iprovalicarb | C18H28N2O3 | 10.60 | 321.2173 | 119.0855 | 1.0 | 1.0 | 0.01, — | 0.9987 | 118.7 | 12.8 | 95.6 | 7.4 | 101.5 | 13.5 |
| 103 | Isazofos | C9H17ClN3O3PS | 13.69 | 314.0490 | 119.9957 | 0.1 | 0.5 | —, — | 0.9976 | 108.4 | 5.5 | 106.6 | 3.9 | 99.3 | 2.8 |
| 104 | Isofenphos | C15H24NO4PS | 16.54 | 346.1236 | 121.0287 | 20.0 | 20.0 | —, — | 0.9973 | 113.5 | 10.4 | 107.3 | 15.7 | 94.7 | 5.0 |
| 105 | Isoproturon | C12H18N2O | 6.73 | 207.1492 | 72.0444 | 0.2 | 0.5 | 0.01, — | 0.9995 | 100.0 | 9.3 | 100.4 | 3.5 | 103.2 | 1.5 |
| 106 | Isopyrazam | C20H23F2N3O | 15.74 | 360.1895 | 320.1758 | 0.5 | 0.5 | 0.01, — | 0.9979 | 105.6 | 9.5 | 105.0 | 3.4 | 97.9 | 0.9 |
| 107 | Kresoxim-methyl | C18H19NO4 | 14.39 | 314.1387 | 116.0495 | 5.0 | 5.0 | 0.02, — | 0.9991 | 82.8 | 12.7 | 105.8 | 7.1 | 98.1 | 7.1 |
| 108 | Linuron | C9H10Cl2N2O2 | 9.22 | 249.0192 | 132.9606 | 5.0 | 5.0 | 0.01, — | 0.9986 | 102.3 | 14.3 | 97.5 | 11.1 | 95.0 | 11.1 |
| 109 | Malaoxon | C10H19O7PS | 5.77 | 315.0662 | 99.0077 | 0.1 | 0.5 | 0.02, — | 0.9984 | 116.8 | 7.6 | 97.2 | 4.6 | 97.9 | 1.8 |
| 110 | Malathion | C10H19O6PS2 | 12.60 | 331.0433 | 99.0077 | 1.0 | 1.0 | 0.02, — | 0.9995 | 119.3 | 16.3 | 104.4 | 7.1 | 103.0 | 12.0 |
| 111 | Mepanipyrim | C14H13N3 | 11.59 | 224.1182 | 77.0386 | 0.5 | 5.0 | 0.01, — | 0.9984 | 98.6 | 4.1 | 109.0 | 11.9 | 98.1 | 11.9 |
| 112 | Metaflumizone | C24H16F6N4O2 | 17.44 | 507.1250 | 178.0463 | 10.0 | 10.0 | 0.01, — | 0.9973 | 105.7 | 18.1 | 95.4 | 15.6 | 91.9 | 6.6 |
| 113 | Metalaxyl | C15H21NO4 | 6.76 | 280.1543 | 45.0335 | 0.1 | 0.2 | 0.01, — | 0.9995 | 105.1 | 10.5 | 118.3 | 12.0 | 103.6 | 3.3 |
| 114 | Metconazole | C17H22ClN3O | 12.54 | 320.1524 | 70.0400 | 5.0 | 5.0 | 0.02, — | 0.9974 | 102.2 | 2.1 | 101.4 | 15.0 | 98.7 | 15.0 |
| 115 | Methiocarb | C11H15NO2S | 8.96 | 226.0896 | 121.0648 | 10.0 | 50.0 | 0.03, — | 0.9943 | 72.0 | 6.6 | 78.0 | 5.8 | 89.0 | 5.1 |
| 116 | Methiocarb-sulfoxide | C11H15NO3S | 3.51 | 242.0845 | 122.0726 | 0.5 | 0.5 | 0.03, — | 0.9945 | 98.8 | 13.7 | 94.0 | 6.3 | 114.4 | 6.4 |
| 117 | Metolachlor | C15H22ClNO2 | 12.41 | 284.1412 | 252.1150 | 0.2 | 0.5 | 0.01, — | 0.9987 | 87.7 | 10.6 | 116.9 | 7.4 | 97.4 | 3.0 |
| 118 | Metrafenone | C19H21BrO5 | 16.32 | 409.0645 | 209.0808 | 0.2 | 0.5 | 0.01, — | 0.9989 | 111.0 | 13.2 | 112.2 | 13.5 | 99.1 | 2.7 |
| 119 | Metribuzin | C8H14N4OS | 5.33 | 215.0961 | 49.0106 | 1.0 | 5.0 | 0.1, — | 0.9975 | 99.4 | 2.4 | 99.0 | 11.9 | 98.8 | 11.9 |
| 120 | Mevinphos | C7H13O6P | 3.43 | 225.0523 | 127.0155 | 2.0 | 5.0 | —, — | 0.9919 | 74.4 | 19.6 | 112.2 | 16.5 | 74.5 | 16.5 |
| 121 | Monocrotophos | C7H14NO5P | 2.81 | 224.0682 | 58.0287 | 0.5 | 0.5 | —, — | 0.9986 | 74.0 | 17.7 | 80.6 | 18.3 | 105.2 | 8.3 |
| 122 | Myclobutanil | C15H17ClN4 | 10.67 | 289.1215 | 70.0400 | 5.0 | 5.0 | 0.01, — | 0.9993 | 103.9 | 7.8 | 105.0 | 14.4 | 94.1 | 14.4 |
| 123 | Napropamide | C17H21NO2 | 11.72 | 272.1645 | 171.0804 | 0.2 | 0.5 | 0.01, — | 0.9985 | 105.3 | 12.7 | 113.0 | 5.5 | 98.0 | 1.2 |
| 124 | Norflurazon | C12H9ClF3N3O | 7.15 | 304.0459 | 140.0306 | 0.1 | 0.2 | —, — | 0.9977 | 92.7 | 8.1 | 94.2 | 4.7 | 96.4 | 1.1 |
| 125 | Omethoate | C5H12NO4PS | 2.10 | 214.0297 | 182.9875 | 0.5 | 0.5 | 0.01, — | 0.9993 | 101.0 | 8.6 | 104.0 | 5.1 | 99.1 | 3.2 |
| 126 | Oxadixyl | C14H18N2O4 | 5.06 | 279.1339 | 132.0808 | 1.0 | 1.0 | 0.01, — | 0.9968 | 101.5 | 12.7 | 98.6 | 8.7 | 103.1 | 12.9 |
| 127 | Paclobutrazol | C15H20ClN3O | 8.77 | 294.1368 | 70.0400 | 1.0 | 1.0 | 0.01, — | 0.9993 | 94.4 | 7.4 | 93.5 | 3.4 | 106.5 | 13.8 |
| 128 | Pendimethalin | C13H19N3O4 | 17.75 | 282.1448 | 92.0495 | 10.0 | 20.0 | 0.02, — | 0.9963 | 102.6 | 10.8 | 108.9 | 9.2 | 81.1 | 5.6 |
| 129 | Penthiopyrad | C16H20F3N3OS | 14.57 | 360.1362 | 256.0351 | 0.2 | 0.5 | 0.01, — | 0.9979 | 113.8 | 9.1 | 101.5 | 5.7 | 100.2 | 3.0 |
| 130 | Phenthoate | C12H17O4PS2 | 15.02 | 321.0379 | 79.0542 | 5.0 | 20.0 | —, — | 0.9938 | 97.1 | 11.2 | 88.6 | 4.9 | 82.2 | 2.8 |
| 131 | Phorate-Sulfone | C7H17O4PS3 | 8.65 | 293.0097 | 96.9508 | 20.0 | 20.0 | 0.01, — | 0.9982 | 96.0 | 13.2 | 113.8 | 5.7 | 82.5 | 4.0 |
| 132 | Phorate-sulfoxide | C7H17O3PS3 | 6.37 | 277.0150 | 96.9508 | 0.5 | 0.5 | 0.01, — | 0.9992 | 105.4 | 9.4 | 97.6 | 6.5 | 109.1 | 3.4 |
| 133 | Phosalone | C12H15ClNO4PS2 | 16.04 | 367.9941 | 110.9996 | 20.0 | 20.0 | 0.01, — | 0.9990 | 119.9 | 15.3 | 109.9 | 5.7 | 86.9 | 5.6 |
| 134 | Phosphamidon | C10H19ClNO5P | 4.73 | 300.0762 | 127.0155 | 0.2 | 0.5 | —, — | 0.9978 | 95.1 | 4.2 | 95.5 | 4.3 | 104.1 | 2.2 |
| 135 | Phoxim | C12H15N2O3PS | 16.05 | 299.0614 | 77.0389 | 10.0 | 20.0 | 0.02, — | 0.9933 | 90.9 | 19.6 | 97.3 | 4.5 | 108.2 | 13.4 |
| 136 | Picoxystrobin | C18H16F3NO4 | 14.80 | 368.1104 | 145.0648 | 0.5 | 1.0 | 0.01, — | 0.9995 | 114.8 | 19.0 | 71.2 | 10.8 | 99.1 | 16.5 |
| 137 | Piperonyl Butoxide | C19H30O5 | 17.12 | 356.2423 | 119.0855 | 0.2 | 0.5 | —, — | 0.9993 | 115.1 | 16.2 | 108.4 | 8.6 | 100.4 | 4.6 |
| 138 | Pirimicarb | C11H18N4O2 | 4.42 | 239.1503 | 72.0444 | 0.5 | 1.0 | 0.05, — | 0.9982 | 105.8 | 12.0 | 95.1 | 6.5 | 102.7 | 14.5 |
| 139 | Pirimiphos-methyl | C11H20N3O3PS | 15.91 | 306.1036 | 164.1182 | 0.5 | 0.5 | 0.01, — | 0.9971 | 104.2 | 9.3 | 101.5 | 5.6 | 99.7 | 2.4 |
| 140 | Pretilachlor | C17H26ClNO2 | 16.25 | 312.1725 | 252.1150 | 0.2 | 0.5 | —, — | 0.9977 | 98.8 | 10.0 | 116.7 | 6.6 | 101.2 | 3.3 |
| 141 | Prochloraz | C15H16Cl3N3O2 | 13.12 | 376.0381 | 70.0287 | 0.5 | 0.5 | 0.03, — | 0.9977 | 115.1 | 8.7 | 101.3 | 8.8 | 93.7 | 2.4 |
| 142 | Profenofos | C11H15BrClO3PS | 16.19 | 372.9424 | 96.9509 | 5.0 | 5.0 | 0.01, — | 0.9989 | 105.5 | 13.6 | 106.3 | 7.5 | 97.9 | 7.5 |
| 143 | Prometryn | C10H19N5S | 8.68 | 242.1434 | 68.0243 | 0.2 | 0.5 | —, — | 0.9975 | 104.2 | 2.3 | 101.0 | 4.1 | 100.0 | 1.2 |
| 144 | Propamocarb | C9H20N2O2 | 2.16 | 189.1598 | 74.0237 | 1.0 | 1.0 | 0.01, — | 0.9990 | 88.6 | 6.1 | 72.3 | 6.3 | 98.0 | 13.7 |
| 145 | Propanil | C9H9Cl2NO | 8.21 | 218.0134 | 127.0178 | 5.0 | 5.0 | 0.01, — | 0.9954 | 95.9 | 13.0 | 116.3 | 10.5 | 91.6 | 10.5 |
| 146 | Propaphos | C13H21O4PS | 13.19 | 305.0971 | 221.0032 | 0.2 | 0.5 | —, — | 0.9987 | 108.8 | 9.1 | 102.8 | 8.4 | 96.2 | 3.4 |
| 147 | Propargite | C19H26O4S | 18.36 | 368.1886 | 57.0699 | 20.0 | 20.0 | 0.01, — | 0.9906 | 99.4 | 10.7 | 91.2 | 4.1 | 82.9 | 2.8 |
| 148 | Propazine | C9H16ClN5 | 8.22 | 230.1167 | 146.0228 | 0.1 | 0.1 | —, — | 0.9972 | 111.4 | 6.6 | 114.9 | 3.9 | 96.8 | 7.4 |
| 149 | Propiconazole | C15H17Cl2N3O2 | 13.16 | 342.0771 | 69.0699 | 0.1 | 1.0 | 0.01, — | 0.9982 | 102.8 | 6.3 | 100.0 | 4.2 | 102.0 | 11.7 |
| 150 | Propyzamide | C12H11Cl2NO | 11.12 | 256.0290 | 189.9821 | 5.0 | 5.0 | 0.01, — | 0.9953 | 115.0 | 14.2 | 98.2 | 12.5 | 94.8 | 12.5 |
| 151 | Prothioconazole-desthio | C14H15Cl2N3O | 10.55 | 312.0664 | 70.0400 | 0.5 | 0.5 | 0.01, — | 0.9994 | 119.4 | 3.9 | 108.1 | 11.1 | 111.4 | 1.8 |
| 152 | Prothiofos | C11H15Cl2O2PS2 | 19.11 | 344.9701 | 240.9041 | 20.0 | 20.0 | —, — | 0.9917 | 118.6 | 10.6 | 83.6 | 10.1 | 82.0 | 9.6 |
| 153 | Pyraclostrobin | C19H18ClN3O4 | 15.47 | 388.1059 | 194.0812 | 0.5 | 0.5 | 0.01, — | 0.9981 | 119.8 | 4.3 | 108.5 | 6.0 | 103.0 | 0.5 |
| 154 | Pyridaben | C19H25ClN2OS | 18.85 | 365.1449 | 147.1168 | 0.5 | 0.5 | 0.01, — | 0.9969 | 86.6 | 3.9 | 118.6 | 18.3 | 104.4 | 11.6 |
| 155 | Pyridaphenthion | C14H17N2O4PS | 11.69 | 341.0719 | 92.0498 | 0.5 | 0.5 | —, — | 0.9992 | 100.6 | 13.8 | 98.8 | 19.7 | 103.5 | 4.0 |
| 156 | Pyrimethanil | C12H13N3 | 7.56 | 200.1182 | 77.0386 | 0.5 | 0.5 | 0.05, — | 0.9972 | 102.4 | 7.0 | 96.2 | 4.2 | 100.1 | 2.8 |
| 157 | Pyriproxyfen | C20H19NO3 | 17.56 | 322.1438 | 96.0444 | 0.5 | 0.5 | 0.05, — | 0.9977 | 114.8 | 13.3 | 118.8 | 19.0 | 108.9 | 8.2 |
| 158 | Quinalphos | C12H15N2O3PS | 14.06 | 299.0614 | 96.9508 | 0.5 | 0.5 | —, — | 0.9986 | 113.0 | 8.7 | 110.8 | 3.6 | 99.1 | 3.3 |
| 159 | Quinoxyfen | C15H8Cl2FNO | 16.82 | 308.0040 | 196.9789 | 1.0 | 1.0 | 0.05, — | 0.9963 | 117.7 | 14.5 | 104.5 | 15.3 | 92.9 | 7.8 |
| 160 | Quizalofop-ethyl | C19H17ClN2O4 | 16.68 | 373.0950 | 91.0542 | 0.5 | 1.0 | —, — | 0.9995 | 99.8 | 12.5 | 98.2 | 11.7 | 102.0 | 11.7 |
| 161 | Saflufenacil | C17H17ClF4N4O5S | 11.03 | 501.0617 | 348.9998 | 2.0 | 5.0 | 0.01, — | 0.9982 | 109.8 | 8.8 | 101.4 | 17.2 | 103.2 | 17.2 |
| 162 | Simazine | C7H12ClN5 | 5.04 | 202.0854 | 132.0323 | 0.5 | 0.5 | 0.01, — | 0.9974 | 100.8 | 2.2 | 99.3 | 1.7 | 101.7 | 1.4 |
| 163 | Spinosyn A | C41H65NO10 | 12.82 | 732.4681 | 142.1226 | 0.2 | 0.5 | 0.2, — | 0.9990 | 100.9 | 3.5 | 110.2 | 6.3 | 97.6 | 1.0 |
| 164 | Spinosyn D | C42H67NO10 | 14.44 | 746.4838 | 142.1226 | 1.0 | 1.0 | 0.2, — | 0.9991 | 110.3 | 13.1 | 94.1 | 5.5 | 99.7 | 16.4 |
| 165 | Spirodiclofen | C21H24Cl2O4 | 19.01 | 411.1124 | 71.0855 | 1.0 | 5.0 | 0.004, — | 0.9997 | 87.0 | 13.1 | 106.5 | 16.8 | 92.2 | 16.8 |
| 166 | Spirotetramat | C21H27NO5 | 10.19 | 374.1962 | 302.1751 | 5.0 | 5.0 | 0.01, — | 0.9981 | 73.0 | 14.5 | 96.1 | 13.1 | 79.3 | 13.1 |
| 167 | Spirotetramat-enol | C18H23NO3 | 5.33 | 302.1758 | 216.1019 | 0.5 | 0.5 | 0.01, — | 0.9943 | 95.1 | 7.6 | 118.6 | 2.5 | 91.9 | 5.6 |
| 168 | Spirotetramat-enol-glucoside | C24H33NO8 | 2.89 | 464.2279 | 302.1751 | 2.0 | 5.0 | —, — | 0.9979 | 109.8 | 8.8 | 101.4 | 17.2 | 103.2 | 17.2 |
| 169 | Spiroxamine | C18H35NO2 | 8.31 | 298.2741 | 100.1121 | 0.5 | 0.5 | 0.015, — | 0.9959 | 95.4 | 10.1 | 105.7 | 10.5 | 97.5 | 2.9 |
| 170 | Sulfentrazone | C11H10Cl2F2N4O3S | 6.43 | 386.9891 | 306.9944 | 5.0 | 10.0 | —, — | 0.9987 | 112.7 | 15.1 | 96.7 | 5.9 | 96.8 | 2.0 |
| 171 | Sulfotep | C8H20O5P2S2 | 15.80 | 323.0300 | 96.9508 | 1.0 | 1.0 | —, — | 0.9970 | 92.1 | 4.5 | 96.0 | 2.6 | 92.5 | 12.0 |
| 172 | Sulfoxaflor | C10H10F3N3OS | 4.57 | 278.0569 | 154.0463 | 1.0 | 10.0 | 0.2, — | 0.9989 | 99.0 | 7.2 | 101.5 | 3.6 | 92.9 | 1.3 |
| 173 | Sulprofos | C12H19O2PS3 | 18.03 | 323.0358 | 218.9698 | 5.0 | 5.0 | —, — | 0.9990 | 102.9 | 18.6 | 94.4 | 7.5 | 91.0 | 7.5 |
| 174 | Tebuconazole | C16H22ClN3O | 11.84 | 308.1524 | 70.0400 | 1.0 | 5.0 | 0.02, — | 0.9990 | 91.7 | 2.8 | 103.7 | 12.9 | 97.0 | 12.9 |
| 175 | Tebufenozide | C22H28N2O2 | 14.05 | 353.2224 | 133.0648 | 1.0 | 10.0 | 0.01, — | 0.9908 | 118.8 | 9.3 | 93.0 | 10.0 | 94.9 | 7.7 |
| 176 | Terbufos-Sulfone | C9H21O4PS3 | 11.80 | 321.0412 | 275.0535 | 5.0 | 5.0 | 0.01, — | 0.9992 | 102.3 | 9.4 | 100.0 | 12.0 | 101.7 | 12.0 |
| 177 | Terbufos-Sulfoxide | C9H21O3PS3 | 8.40 | 305.0465 | 130.9385 | 0.5 | 1.0 | 0.01, — | 0.9983 | 109.5 | 14.5 | 115.5 | 8.7 | 101.4 | 13.8 |
| 178 | Terbumeton | C10H19N5O | 5.61 | 226.1662 | 170.1036 | 0.2 | 0.5 | —, — | 0.9982 | 92.2 | 2.2 | 100.5 | 3.5 | 101.9 | 1.5 |
| 179 | Terbuthylazine | C9H16ClN5 | 8.90 | 230.1167 | 174.0541 | 0.5 | 0.5 | 0.02, — | 0.9972 | 111.0 | 10.5 | 106.3 | 11.4 | 96.4 | 4.5 |
| 180 | Terbutryn | C10H19N5S | 9.09 | 242.1434 | 186.0808 | 0.2 | 0.5 | —, — | 0.9983 | 99.3 | 9.0 | 94.3 | 6.2 | 101.9 | 2.2 |
| 181 | Tetramethrin | C19H25NO4 | 17.09 | 332.1856 | 164.0706 | 20.0 | 20.0 | —, — | 0.9934 | 90.9 | 5.2 | 89.8 | 9.4 | 84.7 | 4.0 |
| 182 | Thiabendazole | C10H7N3S | 2.90 | 202.0433 | 131.0604 | 0.2 | 0.2 | 0.2, 0.2 | 0.9996 | 81.2 | 16.6 | 107.1 | 16.1 | 72.9 | 4.3 |
| 183 | Thiacloprid | C10H9ClN4S | 4.55 | 253.0309 | 126.0087 | 0.2 | 0.5 | 0.05, — | 0.9999 | 106.3 | 5.7 | 89.5 | 7.0 | 104.8 | 2.0 |
| 184 | Thiamethoxam | C8H10ClN5O3S | 3.17 | 292.0266 | 131.9664 | 0.5 | 1.0 | 0.05, — | 0.9994 | 100.8 | 16.1 | 91.4 | 9.6 | 103.4 | 17.3 |
| 185 | Thiobencarb | C12H16ClNOS | 15.23 | 258.0714 | 125.0153 | 1.0 | 5.0 | 0.01, — | 0.9985 | 100.0 | 5.2 | 99.4 | 8.2 | 92.6 | 8.2 |
| 186 | Thiophanate-methyl | C12H14N4O4S2 | 5.50 | 343.0529 | 151.0324 | 2.0 | 20.0 | 0.05, — | 0.9995 | 78.2 | 13.8 | 81.7 | 4.4 | 77.7 | 14.2 |
| 187 | Tolfenpyrad | C21H22ClN3O2 | 16.96 | 384.1477 | 197.0961 | 0.5 | 0.5 | —, — | 0.9994 | 108.7 | 16.7 | 118.0 | 12.1 | 102.2 | 7.5 |
| 188 | Triadimefon | C14H16ClN3O2 | 11.26 | 294.1004 | 57.0699 | 1.0 | 5.0 | 0.01, — | 0.9993 | 101.6 | 3.5 | 102.4 | 14.5 | 97.1 | 14.5 |
| 189 | Trichlorfon | C4H8Cl3O4P | 3.36 | 256.9299 | 78.9945 | 10.0 | 10.0 | 0.01, — | 0.9981 | 105.9 | 17.6 | 102.6 | 13.3 | 95.8 | 3.4 |
| 190 | Trifloxystrobin | C20H19F3N2O4 | 16.78 | 409.1370 | 145.0260 | 0.2 | 0.5 | 0.02, — | 0.9982 | 106.0 | 19.8 | 109.7 | 9.2 | 102.3 | 1.3 |
| 191 | Triflumizole | C15H15ClF3N3O | 15.00 | 346.0929 | 69.0447 | 0.5 | 0.5 | 0.01, — | 0.9980 | 96.7 | 11.1 | 119.4 | 6.5 | 99.8 | 3.1 |
| 192 | Trinexapac-ethyl | C13H16O5 | 7.60 | 253.1071 | 69.0335 | 10.0 | 20.0 | —, — | 0.9976 | 73.1 | 12.7 | 100.9 | 4.9 | 83.2 | 0.7 |
| 193 | Uniconazole | C15H18ClN3O | 10.67 | 292.1213 | 70.0400 | 0.5 | 0.5 | —, — | 0.9980 | 108.2 | 11.8 | 107.6 | 4.9 | 101.6 | 1.3 |
| 194 | Warfarin | C19H16O4 | 9.15 | 309.1121 | 163.0390 | 0.5 | 0.5 | 0.01, — | 0.9991 | 79.4 | 19.0 | 76.6 | 10.5 | 92.6 | 2.3 |
| 195 | Zoxamide | C14H16Cl3NO2 | 15.00 | 336.0319 | 186.9712 | 0.5 | 0.5 | 0.01, — | 0.9994 | 95.5 | 17.6 | 96.2 | 6.3 | 99.8 | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Tong, K.; Yu, C.; Hou, S.; Xie, Y.; Fan, C.; Chen, H.; Lu, M.; Wang, W. Development of a High-Throughput Screening Analysis for 195 Pesticides in Raw Milk by Modified QuEChERS Sample Preparation and Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry. Separations 2022, 9, 98. https://doi.org/10.3390/separations9040098
Wu X, Tong K, Yu C, Hou S, Xie Y, Fan C, Chen H, Lu M, Wang W. Development of a High-Throughput Screening Analysis for 195 Pesticides in Raw Milk by Modified QuEChERS Sample Preparation and Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry. Separations. 2022; 9(4):98. https://doi.org/10.3390/separations9040098
Chicago/Turabian StyleWu, Xingqiang, Kaixuan Tong, Changyou Yu, Shuang Hou, Yujie Xie, Chunlin Fan, Hui Chen, Meiling Lu, and Wenwen Wang. 2022. "Development of a High-Throughput Screening Analysis for 195 Pesticides in Raw Milk by Modified QuEChERS Sample Preparation and Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry" Separations 9, no. 4: 98. https://doi.org/10.3390/separations9040098
APA StyleWu, X., Tong, K., Yu, C., Hou, S., Xie, Y., Fan, C., Chen, H., Lu, M., & Wang, W. (2022). Development of a High-Throughput Screening Analysis for 195 Pesticides in Raw Milk by Modified QuEChERS Sample Preparation and Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry. Separations, 9(4), 98. https://doi.org/10.3390/separations9040098






