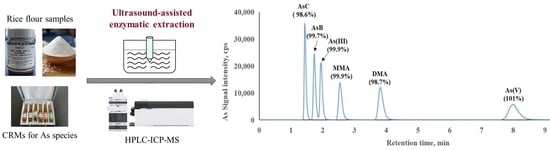
Green and Simple Extraction of Arsenic Species from Rice Flour Using a Novel Ultrasound-Assisted Enzymatic Hydrolysis Method
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Standards
2.2. Intrumentation
2.3. Samples
2.4. Total Arsenic Determination
2.5. Evaluation of Extraction Methods
2.6. Determination of Arsenic Species
2.7. Analyte Quantification
3. Results and Discussion
3.1. Determination of Total Arsenic by Microwave Digestion
3.2. Determination of Arsenic Species
3.3. Optimization of Extraction Methods
3.3.1. Extraction by Acidic Solvent
3.3.2. Enzymatic Extraction by Shaking
3.3.3. Ultrasound-Assisted Enzymatic Extraction
3.3.4. Stability of Arsenic Species
3.3.5. Limits of Detection and Quantification
3.4. Speciation Analysis of Arsenicals in Rice Flour Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Volume 100C: Arsenic, Metals, Fibers, and Dusts. Available online: http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C-6.pdf (accessed on 13 December 2016).
- Yim, S.R.; Park, G.Y.; Lee, K.W.; Chung, M.S.; Shim, S.M. Determination of total arsenic content and arsenic speciation in different types of rice. Food Sci. Biotechnol. 2017, 26, 293–298. [Google Scholar] [CrossRef]
- Akter, K.F.; Owens, G.; Davey, D.E.; Naidu, R. Arsenic Speciation and Toxicity in Biological Systems. Rev. Environ. Contam. Toxicol. 2005, 184, 97–149. [Google Scholar] [CrossRef]
- WHO Evaluation of Certain Food Additives and Contaminants (1989) 33rd Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 776; WHO: Geneva, Switzerland, 1988.
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.N.; Price, A.H.; Raab, A.; Hossain, S.A.; Feldmann, J.; Meharg, A.A. Variation in Arsenic Speciation and Concentration in Paddy Rice Related to Dietary Exposure. Environ. Sci. Technol. 2005, 39, 5531–5540. [Google Scholar] [CrossRef]
- Lombi, E.; Scheckel, K.G.; Pallon, J.; Carey, A.M.; Zhu, Y.G.; Meharg, A.A. Speciation and distribution of arsenic and localization of nutrients in rice grains. New Phytol. 2009, 184, 193–201. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Sun, G.X.; Lei, M.; Teng, M.; Liu, Y.X.; Chen, N.C.; Wang, L.H.; Carey, A.M.; Deacon, C.; Raab, A.; et al. High percentage inorganic arsenic content of mining impact and monimnacted chinese rice. Environ. Sci. Technol. 2008, 42, 5008–5013. [Google Scholar] [CrossRef]
- Williams, P.N.; Raab, A.; Feldmann, J.; Meharg, A.A. Market Basket Survey Shows Elevated Levels of As in South Central, U.S. Processed Rice Compared to California: Consequences for Human Dietary Exposure. Environ. Sci. Technol. 2007, 41, 2178–2183. [Google Scholar] [CrossRef]
- Li, X.; Xie, K.; Yue, B.; Gong, Y.Y.; Shao, Y.; Shang, X.; Wu, Y. Inorganic arsenic contamination of rice from Chinese major rice-producing areas and exposure assessment in Chinese population. Sci. China Chem. 2015, 58, 1898–1905. [Google Scholar] [CrossRef]
- GB2762–2017; National Food Safety Standard-Limit of Pollutants in Food. National Health and Family Planning Commission of China: Beijing, China, 2017.
- Sanz, E.; Muñoz-Olivas, R.; Dietz, C.; Sanz, J.; Cámara, C. Alternative extraction methods for arsenic speciation in hair using ultrasound probe sonication and pressurised liquid extraction. J. Anal. At. Spectrom. 2007, 22, 131–139. [Google Scholar] [CrossRef]
- Kubachka, K.M.; Conklin, S.D.; Smith, C.C.; Castro, C. Quantitative Determination of Arsenic Species from Fruit Juices Using Acidic Extraction with HPLC-ICPMS. Food Anal. Methods 2019, 12, 2845–2856. [Google Scholar] [CrossRef]
- Wolle, M.M.; Conklin, S.D. Speciation analysis of arsenic in seafood and seaweed: Part I—Evaluation and optimization of methods. Anal. Bioanal. Chem. 2018, 410, 5675–5687. [Google Scholar] [CrossRef]
- Maher, W.A.; Ellwood, M.J.; Krikowa, F.; Raber, G.; Foster, S. Measurement of arsenic species in environmental, biological fluids and food samples by HPLC-ICPMS and HPLC-HG-AFS. J. Anal. At. Spectrom. 2015, 30, 2129–2183. [Google Scholar] [CrossRef]
- Wolle, M.M.; Conklin, S.D. Speciation analysis of arsenic in seafood and seaweed: Part II—Single laboratory validation of method. Anal. Bioanal. Chem. 2018, 410, 5689–5702. [Google Scholar] [CrossRef]
- Quiroz, W. Speciation analysis in chemistry. Chem Texts 2021, 7, 1–17. [Google Scholar] [CrossRef]
- Narukawa, T.; Chiba, K. Heat-Assisted Aqueous Extraction of Rice Flour for Arsenic Speciation Analysis. J. Agric. Food Chem. 2010, 58, 8183–8188. [Google Scholar] [CrossRef]
- Narukawa, T.; Suzuki, T.; Inagaki, K.; Hioki, A. Extraction techniques for arsenic species in rice flour and their speciation by HPLC-ICP-MS. Talanta 2014, 130, 213–220. [Google Scholar] [CrossRef]
- Maher, W.A.; Eggins, S.; Krikowa, F.; Jagtap, R.; Foster, S. Measurement of As species in rice by HPLC-ICPMS after extraction with sub-critical water and hydrogen peroxide. J. Anal. At. Spectrom. 2017, 32, 1129–1134. [Google Scholar] [CrossRef]
- Huang, J.H.; Fecher, P.; Ilgen, G.; Hu, K.N.; Yang, J. Speciation of arsenite and arsenate in rice grain—Verification of nitric acid based extraction method and mass sample survey. Food Chem. 2012, 130, 453–459. [Google Scholar] [CrossRef]
- Heitkemper, D.T.; Vela, N.P.; Stewart, K.R.; Westphal, C.S. Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2001, 16, 299–306. [Google Scholar] [CrossRef]
- Chaney, R.L.; Green, C.E.; Lehotay, S.J. Inter-laboratory validation of an inexpensive streamlined method to measure inorganic arsenic in rice grain. Anal. Bioanal. Chem. 2018, 410, 5703–5710. [Google Scholar] [CrossRef]
- López, R.; D’Amato, R.; Trabalza-Marinucci, M.; Regni, L.; Proietti, P.; Maratta, A.; Cerutti, S.; Pacheco, P. Green and simple extraction of free seleno-amino acids from powdered and lyophilized milk samples with natural deep eutectic solvents. Food Chem. 2020, 326, 126965. [Google Scholar] [CrossRef]
- GB 5009.11-2014; National Food Safety Standard-Determination of Total Arsenic and Inorganic Arsenic in Food. Food and Medical Products Administration of China: Beijing, China, 2016.
- Narukawa, T.; Inagaki, K.; Kuroiwa, T.; Chiba, K. The extraction and speciation of arsenic in rice flour by HPLC-ICP-MS. Talanta 2008, 77, 427–432. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Salvini, S.; Parpinel, M. Food Composition Database for Epidemiological Studies in Italy, Version 1; IEO European Institute of Oncology: Milan, Italy, 2008; Available online: https://www.ieo.it/bda (accessed on 10 March 2022).

| ICP-MS |
|---|
| RF powder: 1500 W |
| Carrier gas: 1.0 mL/min |
| Reaction gas: O2, 25% |
| Isotope monitored: 91AsO+ |
| Integration time: 0.1 s (spectrum) per point |
| Points per peak: 3 |
| HPLC |
| Column: PRP-X100 anion exchange |
| Dimensions: 250 mm × 4.1 mm, particle size: 10 μm |
| Mobile phase: 20 mM (NH4)2HPO4, pH 6.0 |
| Injection volume: 20 μL |
| Flow rate: 1.2 mL/min |
| Mode: Isocratic |
| Extraction | Procedure | Solvent/Solution | Extraction Temperature and Time | Heating Device |
|---|---|---|---|---|
| Acidic | A-1 | 10 mL 1% (v/v) HNO3 | stand overnight; 80 °C, 2.5 h | Oven |
| A-2 | 10 mL 1% (v/v) HNO3 | stand overnight; 90 °C, 2.5 h | Oven | |
| A-3 | 10 mL 1% (v/v) HNO3 | stand overnight; 100 °C, 2.5 h | Oven | |
| A-4 | 10 mL 1% (v/v) HNO3 | stand overnight; 100 °C, 0.5 h | Oven | |
| A-5 | 10 mL 1% (v/v) HNO3 | stand overnight; 100 °C, 1.5 h | Oven | |
| A-6 | 10 mL 1% (v/v) HNO3 | stand overnight; 100 °C, 2.5 h | Oven | |
| A-7 | 10 mL 1% (v/v) HNO3 | stand overnight; 100 °C, 3.5 h | Oven | |
| Enzymatic | B-1 | 5 mL 10 mg/mL α-amylase | 60 °C, overnight | Shaking incubator |
| B-2 | 5 mL 10 mg/mL α-amylase | 60 °C, overnight; 100 °C, 2.5 h | Shaking incubator Oven | |
| Ultrasound-assisted enzymatic | C-1 | 5 mL 10 mg/mL α-amylase | 60 °C, 1 h | Ultrasonic bath |
| C-2 | 5 mL 10 mg/mL α-amylase | 60 °C, 1 h; 100 °C, 2.5 h | Ultrasonic bath Oven |
| Code | Description | Total As (mg kg−1) | Certified Value (mg kg−1) |
|---|---|---|---|
| NMIJ 7532a | Rice flour from NMIJ | 0.316 ± 0.012 a | 0.320 ± 0.010 |
| NMIJ 7502a | Rice flour from NMIJ | 0.112 ± 0.003 | 0.109 ± 0.005 |
| NIST 1568b | Rice flour from NIST | 0.288 ± 0.006 | 0.285 ± 0.014 |
| S1 | Chinese brown rice flour | 0.157 ± 0.003 | - |
| S2 | Chinese brown rice flour | 0.482 ± 0.008 | - |
| S3 | Chinese brown rice flour | 0.378 ± 0.008 | - |
| Procedure | iAs (mg kg−1) | DMA (mg kg−1) | Total (mg kg−1) |
|---|---|---|---|
| A-1 | 0.253 ± 0.005 (84.8%) a | 0.0163 ± 0.0003 (87.9%) | 0.269 ± 0.005 (84.1%) |
| A-2 | 0.284 ± 0.006 (95.3%) | 0.0171 ± 0.0003 (92.1%) | 0.301 ± 0.006 (94.1%) |
| A-3 | 0.299 ± 0.006 (100.3%) | 0.0182 ± 0.0004 (97.8%) | 0.317 ± 0.006 (99.1%) |
| A-4 | 0.264 ± 0.006 (88.6%) | 0.0170 ± 0.0003 (91.4%) | 0.281 ± 0.006 (87.8%) |
| A-5 | 0.288 ± 0.006 (96.6%) | 0.0190 ± 0.0004 (102.2%) | 0.307 ± 0.006 (95.9%) |
| A-6 | 0.300 ± 0.006 (100.7%) | 0.0190 ± 0.0004 (102.2%) | 0.319 ± 0.006 (99.7%) |
| A-7 | 0.298 ± 0.006 (100.0%) | 0.0180 ± 0.0004 (96.8%) | 0.316 ± 0.006 (98.8%) |
| B-1 | 0.220 ± 0.005 (73.7%) | 0.0133 ± 0.0003 (71.4%) | 0.233 ± 0.005 (72.8%) |
| B-2 | 0.240 ± 0.005 (80.4%) | 0.0145 ± 0.0003 (78.2%) | 0.254 ± 0.005 (79.4%) |
| C-1 | 0.278 ± 0.006 (93.2%) | 0.0163 ± 0.0003 (87.5%) | 0.294 ± 0.006 (91.9%) |
| C-2 | 0.297 ± 0.006 (99.7%) | 0.0182 ± 0.0004 (98.1%) | 0.315 ± 0.006 (98.5%) |
| Certified value | 0.298 ± 0.008 | 0.0186 ± 0.0008 | 0.320 ± 0.010 |
| Concentration of Spiked Standards | Recovery (%) | |||||
|---|---|---|---|---|---|---|
| As(III) | As(V) | MMA | DMA | AsB | AsC | |
| 10 ng g−1 | 99.6 | 100.7 | 99.5 | 98.5 | 99.4 | 98.0 |
| 100 ng g−1 | 100.2 | 101.3 | 100.3 | 98.9 | 100.1 | 99.2 |
| Species | As(III) | As(V) | MMA | DMA | AsB | AsC |
|---|---|---|---|---|---|---|
| LOD | 0.47 | 1.67 | 0.71 | 0.80 | 0.32 | 0.21 |
| LOQ | 1.51 | 5.34 | 2.26 | 2.57 | 1.01 | 0.66 |
| Code | iAs (mg kg−1) | MMA (mg kg−1) | DMA (mg kg−1) | Sum (mg kg−1) |
|---|---|---|---|---|
| NMIJ 7502a | 0.096 ± 0.003 (98.0%) a | - b | 0.0130 ± 0.0004 (100.7%) | 0.109 (100.0%) |
| NIST 1568b | 0.089 ± 0.003 (96.7%) | 0.0118 ± 0.004 (101.7%) | 0.181 ± 0.006 (100.5%) | 0.282 (98.9%) |
| S1 | 0.149 ± 0.005 | - | 0.0061 ± 0.0002 | 0.155 (98.7%) |
| S2 | 0.440 ± 0.015 | 0.0032 ± 0.0002 | 0.041 ± 0.001 | 0.484 (100.4%) |
| S3 | 0.365 ± 0.012 | - | 0.0092 ± 0.0003 | 0.374 (98.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ma, Q.; Wei, C.; Cai, W.; Chen, H.; Xing, R.; Song, P. Green and Simple Extraction of Arsenic Species from Rice Flour Using a Novel Ultrasound-Assisted Enzymatic Hydrolysis Method. Separations 2022, 9, 105. https://doi.org/10.3390/separations9050105
Li X, Ma Q, Wei C, Cai W, Chen H, Xing R, Song P. Green and Simple Extraction of Arsenic Species from Rice Flour Using a Novel Ultrasound-Assisted Enzymatic Hydrolysis Method. Separations. 2022; 9(5):105. https://doi.org/10.3390/separations9050105
Chicago/Turabian StyleLi, Xiao, Qian Ma, Chao Wei, Wei Cai, Huanhuan Chen, Rui Xing, and Panshu Song. 2022. "Green and Simple Extraction of Arsenic Species from Rice Flour Using a Novel Ultrasound-Assisted Enzymatic Hydrolysis Method" Separations 9, no. 5: 105. https://doi.org/10.3390/separations9050105
APA StyleLi, X., Ma, Q., Wei, C., Cai, W., Chen, H., Xing, R., & Song, P. (2022). Green and Simple Extraction of Arsenic Species from Rice Flour Using a Novel Ultrasound-Assisted Enzymatic Hydrolysis Method. Separations, 9(5), 105. https://doi.org/10.3390/separations9050105