Enhanced Sample Throughput Capillary Zone Electrophoresis with UV Detection in Hydrodynamically Closed System for Determination of Ibuprofen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
2.2. Instrumentation
2.3. Procedures for Standard Solution and Sample Preparation
3. Results and Discussion
3.1. Optimization of the CZE-UV Method
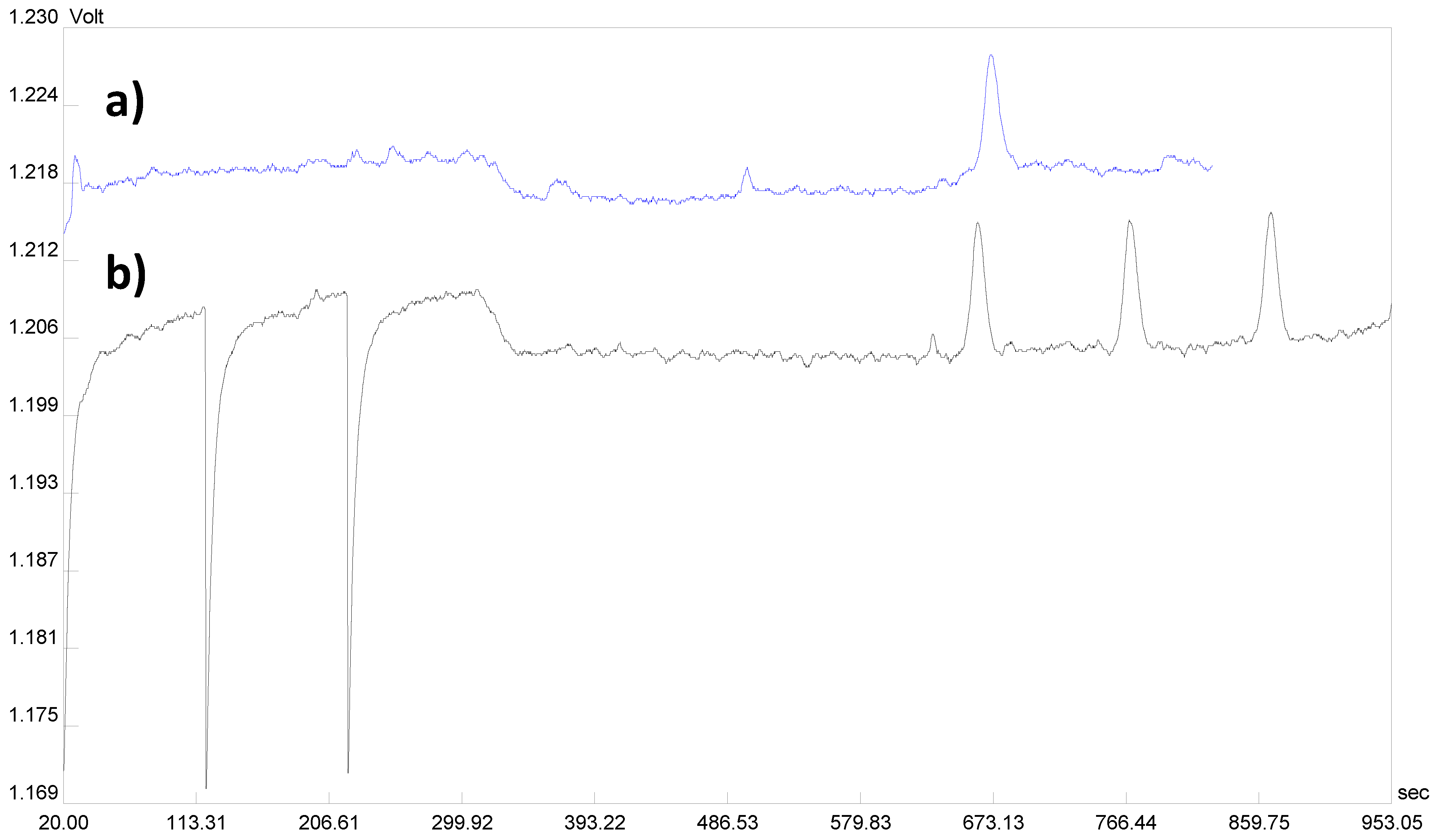
3.2. Repeated Injection Procedure
3.3. Validation
3.4. Application and Evaluation of the Analytical Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Plotka-Wasylka, J. Green analytical chemistry metrics: A review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, N.; Goossens, K.; Baeten, M.; Chen, Q. Recent advances in analytical techniques for high throughput experimentation. Anal. Sci. Adv. 2021, 2, 109–127. [Google Scholar] [CrossRef]
- Nowak, P.M.; Kościelniek, P. What color is your method? Adaptation of the RGB Additive Color Model to analytical method evaluation. Anal. Chem. 2019, 91, 10343–10352. [Google Scholar] [CrossRef]
- Nowak, P.M.; Sekula, E.; Kościelniak, P. Assessment and comparison of the overall analytical potential of capillary electrophoresis and high-performance liquid chromatography using RGB model: How much can we find out? Chromatographia 2020, 83, 1133–1144. [Google Scholar] [CrossRef]
- El Deeb, S.; Wätzig, H.; Abd El-Hady, D.; Sänger-van de Griend, C.; Scriba, G.K. Recent advances in capillary electrophoretic migration techniques for pharmaceutical analysis (2013–2015). Electrophoresis 2016, 37, 1591–1608. [Google Scholar] [CrossRef]
- Masár, M.; Hradski, J.; Schmid, M.G.; Szucs, R. Advantages and pitfalls of capillary electrophoresis of pharmaceutical compounds and their enantiomers in complex samples: Comparison of hydrodynamically opened and closed systems. Int. J. Mol. Sci. 2020, 21, 6852. [Google Scholar] [CrossRef]
- Lodén, H.; Amini, A. Quantification of buserelin in a pharmaceutical product by multiple-injection CZE. Electrophoresis 2007, 28, 1548–1556. [Google Scholar] [CrossRef]
- Zhang, Y.; Gomez, F.A. Multiple-step ligand injection affinity capillary electrophoresis for determining binding constants of ligands to receptors. J. Chromatogr. A 2000, 897, 339–347. [Google Scholar] [CrossRef]
- Chinchilla, D.; Zavaleta, J.; Martinez, K.; Gomez, F.A. Multiple-injection affinity capillary electrophoresis to estimate binding constants of receptors to ligands. Anal. Bioanal. Chem. 2005, 383, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, J.; Chinchilla, D.; Martinez, K.; Gomez, F.A. Multiple-injection affinity capillary electrophoresis to examine binding constants between glycopeptide antibiotics and peptides. J. Chromatogr. A 2006, 1105, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lodén, H.; Pettersson, C.; Arvidsson, T.; Amini, A. Quantitative determination of salbutamol in tablets by multiple-injection capillary zone electrophoresis. J. Chromatogr. A 2008, 1207, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Lodén, H.; Pettersson, C.; Arvidsson, T. Principles for different modes of multiple-injection CZE. Electrophoresis 2008, 29, 3952–3958. [Google Scholar] [CrossRef] [PubMed]
- Staub, A.; Rudaz, S.; Veuthey, J.-L.; Schappler, J. Multiple injection technique for the determination and quantitation of insulin formulations by capillary electrophoresis and time-of-flight mass spectrometry. J. Chromatogr. A 2010, 1217, 8041–8047. [Google Scholar] [CrossRef]
- Amini, A. Separation of somatropin charge variants by multiple-injection CZE with polybrene/chondroitin sulfate A double-coated capillaries. J. Sep. Sci. 2013, 36, 2686–2690. [Google Scholar] [CrossRef]
- Valese, A.C.; Spudeit, D.A.; Dolzan, M.D.; Bretanha, L.C.; Vitali, L.; Micke, G.A. High-throughput analysis of lidocaine in pharmaceutical formulation by capillary zone electrophoresis using multiple injections in a single run. J. Anal. Methods Chem. 2016, 2016, 4126810. [Google Scholar] [CrossRef] [Green Version]
- Kovács, Z.; Szarka, M.; Szigeti, M.; Guttman, A. Separation window dependent multiple injection (SWDMI) for large scale analysis of therapeutic antibody N-glycans. J. Pharm. Biomed. Anal. 2016, 128, 367–370. [Google Scholar] [CrossRef] [Green Version]
- Siebert, D.A.; Campos, J.S.; Alberton, M.D.; Vitali, L.; Micke, G.A. Dual electrophoretically-mediated microanalysis in multiple injection mode for the simultaneous determination of acetylcholinesterase and α-glucosidase activity applied to selected polyphenols. Talanta 2021, 224, 121773. [Google Scholar] [CrossRef]
- do Nascimento, M.P.; Marques, R.; Pereira, P.; de Souza Martins, R.; Bombonato, F.I.; de Oliveira, M.A.L. Determination of purity and anionic exchange efficiency of amino acid ionic liquids synthesis by multiple-injection capillary zone electrophoresis. Talanta 2022, 237, 122945. [Google Scholar] [CrossRef] [PubMed]
- Kuehnbaum, N.L.; Kormendi, A.; Britz-McKibbin, P. Multisegment injection-capillary electrophoresis-mass spectrometry: A high-throughput platform for metabolomics with high data fidelity. Anal. Chem. 2013, 85, 10664–10669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, B.; Zhao, Y.; Yang, J.; Pei, P.; Ji, X.; Quan, Y. High-throughput and high-sensitivity capillary electrophoresis-mass spectrometry method for sulfur-containing amino acids. J. Anal. Sci. Technol. 2021, 12, 43. [Google Scholar] [CrossRef]
- DiBattista, A.; Rampersaud, D.; Lee, H.; Kim, M.; Britz-McKibbin, P. High throughput screening method for systematic surveillance of drugs of abuse by multisegment injection–capillary electrophoresis–mass spectrometry. Anal. Chem. 2017, 89, 11853–11861. [Google Scholar] [CrossRef] [PubMed]
- Boley, D.A.; Zhang, Z.; Dovichi, N.J. Multisegment injections improve peptide identification rates in capillary zone electrophoresis-based bottom-up proteomics. J. Chromatogr. A 2017, 1523, 123–126. [Google Scholar] [CrossRef]
- Saoi, M.; Sasaki, K.; Sagawa, H.; Abe, K.; Kogiso, T.; Tokushige, K.; Hashimoto, E.; Ohashi, Y.; Britz-McKibbin, P. High throughput screening of serum γ-glutamyl dipeptides for risk assessment of nonalcoholic steatohepatitis with impaired glutathione salvage pathway. J. Proteome Res. 2020, 19, 2689–2699. [Google Scholar] [CrossRef]
- Shanmuganathan, M.; Kroezen, Z.; Gill, B.; Azab, S.; de Souza, R.J.; Teo, K.K.; Atkinson, S.; Subbaro, P.; Desai, D.; Anand, S.S.; et al. The maternal serum metabolome by multisegment injection-capillary electrophoresis-mass spectrometry: A high-throughput platform and standardized data workflow for large-scale epidemiological studies. Nat. Protoc. 2021, 16, 1966–1994. [Google Scholar] [CrossRef]
- Igarashi, K.; Ota, S.; Kaneko, M.; Hirayama, A.; Enomoto, M.; Katumata, K.; Sugimoto, M.; Soga, T. High-throughput screening of salivary polyamine markers for discrimination of colorectal cancer by multisegment injection capillary electrophoresis tandem mass spectrometry. J. Chromatogr. A 2021, 1652, 462355. [Google Scholar] [CrossRef]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Immunopharmacol 2009, 17, 275–342. [Google Scholar] [CrossRef]
- World Health Organization. Model List of Essential Medicines, 22nd ed.; World Health Organization: Geneva, Switzerland, 2021; p. 66. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed on 14 March 2022).
- Ritter, J.; Flower, R.; Henderson, G.; Loke, Y.K.; MacEwan, D.; Rang, H. Rang & Dale´s Pharmacology, 9th ed.; Elsevier: London, UK, 2018; pp. 343–361. [Google Scholar]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/ibuprofen-dexibuprofen-containing-medicines (accessed on 14 March 2022).
- Hamoudova, R.; Pospisilova, M. Determination of ibuprofen and flurbiprofen in pharmaceuticals by capillary zone electrophoresis. J. Pharm. Biomed. Anal. 2006, 41, 1463–1467. [Google Scholar] [CrossRef]
- Amini, A. Double-injection capillary electrophoresis for the identification of analytes. Electrophoresis 2014, 35, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Ragab, M.A.A.; Abdel-Hay, M.H.; Ahmed, H.M.; Mohyeldin, S.M. Application of capillary zone electrophoresis coupled with a diode array detector (CZE-DAD) for simultaneous analysis of ibuprofen and phenylephrine. J. AOAC Int. 2019, 102, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.R.; Chaves, S.C.; Ribeiro, M.M.A.C.; Torres, L.M.F.C.; Munoz, R.A.A.; Dos Santos, W.T.P.; Richter, E.M. Simultaneous determination of caffeine, paracetamol, and ibuprofen in pharmaceutical formulations by high-performance liquid chromatography with UV detection and by capillary electrophoresis with conductivity detection. J. Sep. Sci. 2015, 38, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- do Prado, A.A.; Ribeiro, M.M.A.C.; Richter, E.M. Ultra-rapid capillary zone electrophoresis method for simultaneous determination of arginine and ibuprofen. J. Sep. Sci. 2021, 44, 2596–2601. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology, 4th ed.; European Medicines Agency: Amsterdam, The Netherlands, 2005. Available online: http://www.ich.org/cache/compo/276-254-1.html (accessed on 14 March 2022).



| Electrolyte | pH | tm (min) | Peak Height | RSDpeak height (%) | N |
|---|---|---|---|---|---|
| 10 mM (NH4)2CO3 | 9.21 | 18.16 | 70.6 | 3.5 | 10,152 |
| 10 mM NH4HCO3 | 7.80 | 19.53 | 80.8 | 1.2 | 11,949 |
| 10 mM NH4OH + 20 mM MES | 5.76 | 12.26 | 41.9 | 2.5 | 4894 |
| 5 mM EACA + 10 mM NH4OH | 9.75 | 6.56 | 25.2 | 7.9 | 1707 |
| 10 mM MOPS + 20 mM TRIS | 8.29 | 11.24 | 186.1 | 1.6 | 20,775 |
| 25 mM MOPS + 50 mM TRIS | 8.44 | 15.04 | 80.1 | 3.4 | 9070 |
| Parameter | Ibuprofen |
|---|---|
| tm [min] | 11.61 |
| RSDtm [%], n = 6 | 0.54 |
| RSDarea [%], n = 6 | 5.51 |
| Regression equation | y = 139.23x − 22.09 |
| r2 | 0.9984 |
| Linear range [µg/mL] | 1.25–50 |
| LOD [µg/mL] | 0.31 |
| LOQ [µg/mL] | 1.25 |
| N | 20,944 |
| QC Sample | Intra-Day, n = 3 | Inter-Day, n = 9 | ||||
|---|---|---|---|---|---|---|
| Nominal (µg/mL) | Found (µg/mL) | RSD (%) | RE (%) | Found (µg/mL) | RSD (%) | RE (%) |
| 1.25 | 1.09 | 3.4 | −12.9 | 1.25 | 15.0 | 0.3 |
| 2.5 | 2.35 | 2.1 | −6.0 | 2.46 | 9.9 | −1.4 |
| 5 | 4.60 | 1.9 | −7.9 | 4.84 | 5.6 | −3.2 |
| 10 | 9.15 | 5.6 | −8.5 | 9.49 | 6.6 | −5.1 |
| 20 | 21.30 | 3.4 | 6.5 | 20.53 | 4.9 | 2.7 |
| 50 | 49.68 | 1.9 | −0.7 | 49.89 | 2.6 | −0.3 |
| Preparation | Parameter | |||
|---|---|---|---|---|
| Found ± SD (µg/mL) | RSD (%), n = 3 | Declared (µg/mL) | RE (%) | |
| Ibalgin tbl. | 9.32 ± 0.27 | 2.9 | 10 | −6.8 |
| Nurofen tbl. | 18.19 ± 0.27 | 3.6 | 20 | −9.1 |
| Nurofen susp. | 9.31 ± 0.31 | 3.3 | 10 | −6.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanik, O.; Horniakova, A.; Cizmarova, I.; Matuskova, M.; Mikusova, V.; Mikus, P.; Piestansky, J. Enhanced Sample Throughput Capillary Zone Electrophoresis with UV Detection in Hydrodynamically Closed System for Determination of Ibuprofen. Separations 2022, 9, 118. https://doi.org/10.3390/separations9050118
Stefanik O, Horniakova A, Cizmarova I, Matuskova M, Mikusova V, Mikus P, Piestansky J. Enhanced Sample Throughput Capillary Zone Electrophoresis with UV Detection in Hydrodynamically Closed System for Determination of Ibuprofen. Separations. 2022; 9(5):118. https://doi.org/10.3390/separations9050118
Chicago/Turabian StyleStefanik, Ondrej, Andrea Horniakova, Ivana Cizmarova, Michaela Matuskova, Veronika Mikusova, Peter Mikus, and Juraj Piestansky. 2022. "Enhanced Sample Throughput Capillary Zone Electrophoresis with UV Detection in Hydrodynamically Closed System for Determination of Ibuprofen" Separations 9, no. 5: 118. https://doi.org/10.3390/separations9050118
APA StyleStefanik, O., Horniakova, A., Cizmarova, I., Matuskova, M., Mikusova, V., Mikus, P., & Piestansky, J. (2022). Enhanced Sample Throughput Capillary Zone Electrophoresis with UV Detection in Hydrodynamically Closed System for Determination of Ibuprofen. Separations, 9(5), 118. https://doi.org/10.3390/separations9050118








