Thenoyltrifluoroacetone: Preferable Molecule for Solvent Extraction of Metals—Ancient Twists to New Approaches
Abstract
1. Introduction
2. Presentation of Equilibrium Data and Evaluation Criteria
- ✓
- The extent to which essential reaction conditions have been specified. These include the purity of the ligand(s), temperature, ionic strength, nature of the supporting electrolyte, reaction kinetics, ligand:metal ratios, etc. The extractant concentration range must be specified absolutely as well. Attention must be paid to the achievement of a clean separation, so that neither of the liquid phases are contaminated by the other, i.e., to prevent entrainment of less than 0.1% of the wrong liquid phase [11];
- ✓
- The soundness of apparatus calibration and the specification of whether concentration or mixed constants were calculated;
- ✓
- The maintenance of constant temperature and ionic strength during the experimental work-up;
- ✓
- Reliable mathematical treatment of the experimental data;
- ✓
- Correct selection of auxiliary data from the literature;
- ✓
- Details of the calculation method applied. As a whole, the experimental method applied and the numerical analysis chosen by authors are considered to have minimal systematic errors.
3. Liquid–Liquid Extraction of Metal Ions with 1-(2-Thienyl)-4,4,4-Trifluoro-1,3-Butanedione (HTTA) Alone
4. Synergism in Liquid–Liquid Extraction of Metal Ions with a Chelating Ligand HTTA
- (i)
- HTTA metal chelate extraction systems show a much larger synergistic effect than most metal chelate extraction systems;
- (ii)
- The synergistic effect is larger when the neutral adduct-forming ligand is an organic phosphate ester than when it is any other neutral ligand;
- (iii)
- The synergistic effect is strongly dependent on the central metal ion; HTTA chelates of trivalent lanthanoids and actinoids or uranyl ions show larger synergistic effects than many other metals’ HTTA chelates;
- (iv)
- The synergistic effect also depends on the molecular organic diluents and it usually increases when water has a smaller solubility in the diluent.
5. Ionic Liquid Media for Solvent Extraction of Metal Ions with HTTA Alone as Well as in Mixed-Solvent Systems
5.1. IL’s Anion and Cation Solubility in the Upper Aqueous Phase
5.2. Solvent Extraction of Ln (III) Ion with Thenoyltrifluoroacetone in an IL Media, [C1Cnim+][Tf2N−]
- ⮚
- neutral species, n = Z+ [156]:
- ⮚
- ⮚
- cationic species, n < Z+:
5.3. Synergistic Solvent Extraction of Ln (III) Ions with Mixtures including Thenoyltrifluoroacetone
6. Conclusions and Remarks on Metal Solvent Extraction: Yesterday–Today–Tomorrow
Funding
Conflicts of Interest
References
- Binnemans, K. Rare Earth beta-diketones. ch. 225. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.-C.G., Gschneider, K.A., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 35. [Google Scholar]
- De, A.K.; Khopkar, S. Analytical application of thenoyltrifluoroacetone. J. Sci. Indust. Des. 1962, 21A, 131–135. [Google Scholar]
- Komatsu, Y.; Fujiki, Y.; Michine, Y.; Yajima, Y.; Sasaki, T. Solvent extraction separation of alkaline earth metal ions with thenoyltrifluoroacetone and trioctylphosphine oxide in carbon tetrachloride. Solvent Extr. Ion Exch. 1991, 9, 471–479. [Google Scholar] [CrossRef]
- Akki, S.B.; Khopkar, J.M. Solvent extraction of selenium(IV) with 2-thenoyltrifluoroacetone. Sep. Sci. Technol. 1971, 6, 455–460. [Google Scholar] [CrossRef]
- De, A.; Rahaman, M. Extraction and spectroscopic determination of platinum(IV) and palladium(II) with 2-thenoyltrifluoroacetone. Analyst 1964, 89, 795–799. [Google Scholar] [CrossRef]
- Poskanzer, A.M.; Foreman, B.M. A summary of TTA extraction coefficients. J. Inorg. Nucl. Chem. 1961, 16, 323–336. [Google Scholar] [CrossRef]
- Alstad, J.; Augustson, L.H.; Farbu, L.H. Solvent extraction of rare earth metal ions with thenoyltrifluoroacetone in carbon tetrachloride. J. Inorg. Nucl. Chem. 1974, 36, 899–903. [Google Scholar] [CrossRef]
- Stary, J.; Freiser, H. Equilibrium Constants of Liquid Distribution Reactions: Part IV. Chelating Extractants; IUPAC Chemical Data Series, 18; Pergamon: Oxford, UK, 1975; pp. 131–178. [Google Scholar]
- Ensor, D.D.; Nicks, M.; Pruett, D. Extraction of uranyl ions by synergistic mixtures of thenoyltrifluoroacetone and macrocyclic donors. Solvent Extr. Ion Exch. 1988, 6, 621–629. [Google Scholar] [CrossRef]
- Reddy, A.S.; Reddy, L.K. Solvent extraction separation of some actinides and lanthanides with 2-thenoyltrifluoroacetone. Sep. Sci. Technol. 1980, 15, 1263–1269. [Google Scholar] [CrossRef]
- Marcus, Y. Development and publication of solvent extraction methods. Talanta 1976, 23, 203–209. [Google Scholar] [CrossRef]
- Dukov, I.; Atanassova, M. High molecular weight amines and quaternary ammonium salts as synergistic agents in the solvent extraction of metal ions with chelating extractants. chap. 7. In Handbook of Inorganic Chemistry Researc; Morrison, D.A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 245–267. [Google Scholar]
- Atanassova, M.; Lachkova, V.; Vassilev, N.; Shivachev, B.; Varbanov, S.; Dukov, I. Effect of p-tert-butylcalix[4]arene fitted with phosphinoyl pendant arms as synergistic agent in the solvent extraction of trivalent lanthanoids with 4,4,4-trifluoro-1-(2-thienyl)1,3-butanedione and structural study of solid complexes by IR, NMR and X-ray. Polyhedron 2008, 27, 3306–3312. [Google Scholar] [CrossRef]
- Dukov, I.; Atanassova, M. Some aspects of synergistic metal extractions with chelating extractants and amines or quaternary ammonium salts. J. Univ. Chem. Technol. Met. 2003, 38, 7–22. [Google Scholar]
- Beck, M.T. Critical evaluation of equilibrium constants in solution. Stability constants of metal complexes. Pure Appl. Chem. 1977, 49, 127–135. [Google Scholar] [CrossRef]
- Mathur, J. Synergism of trivalent actinides and lanthanides. Solvent Extr. Ion Exch. 1983, 1, 349–412. [Google Scholar] [CrossRef]
- Nash, K. Aqueous complexes in separations of f-elements: Options and strategies for future development. Sep. Sci. Technol. 1999, 34, 911–929. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Dukov, I. The interaction of extractants during synergistic solvent extraction of metals. Is it an important reaction? RSC Adv. 2016, 6, 81250–81265. [Google Scholar] [CrossRef]
- Quinn, J.; Soldenhoff, K.; Stevens, G.; Lengkeek, N. Solvent extraction of rare earth elements using phosphonic/phosphinic acid mixtures. Hydrometallurgy 2015, 157, 298–305. [Google Scholar] [CrossRef]
- Hokura, A.; Sekine, T. Gradual destruction of synergistic enhancement of copper(II) extraction into carbon tetrachloride with 2-thenoyltrifluoroacetone and trioctylphosphine oxide. Solvent Extr. Res. Dev. Jpn. 1995, 2, 102–111. [Google Scholar]
- Kiss, T.; Sovago, I.; Gergely, A. Critical survey of stability constants of complexes of glycine. Pure Appl. Chem. 1991, 63, 597–638. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E.; Chen, Y. Critical evaluation of stability constants for nucleotide complexes with protons and metal ions and the accompanying enthalpy changes. Pure Appl. Chem. 1991, 63, 1015–1080. [Google Scholar] [CrossRef]
- Yamauchi, O.; Odani, A. Stability constants of metal complexes of amino acids with charged side chains—Part I: Positively charged side chains (Technical Report). Pure Appl. Chem. 1996, 68, 469–496. [Google Scholar] [CrossRef]
- Lajunen, L.; Portanova, R.; Piispanen, J.; Tolazzi, M. Critical evaluation of stability constants for alpha-hydroxycarboxylic acid complexes with protons and metal ions and the accompanying enthalpy changes. Part I: Aromatic ortho-hydroxycarboxylic acids (Technical Report). Pure Appl. Chem. 1997, 69, 329–382. [Google Scholar] [CrossRef]
- Popov, K.I.; Rönkkömäki, H.; Lajunen, L.H.J. Critical evaluation of stability constants of phosphonic acids (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 1641–1677. [Google Scholar] [CrossRef]
- Anderegg, G.; Arnaud-Neu, F.; Delgado, R.; Felcman, J.; Popov, K. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1445–1495. [Google Scholar] [CrossRef]
- Arnaud-Neu, F.; Delgado, R.; Chaves, S. Critical evaluation of stability constants and thermodynamic functions of metal complexes of crown ethers (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 71–102. [Google Scholar] [CrossRef]
- Marcus, Y. Diluent effects in solvent extraction. Solvent Extr. Ion Exch. 1989, 7, 567–575. [Google Scholar] [CrossRef]
- Baes, C.F., Jr.; McDowell, W.J.; Byan, S.A. The interpretation of equilibrium data from synergistic solvent extraction systems. Solvent Extr. Ion Exch. 1987, 5, 1–28. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Separation of europium(III) and americium(III) by solvent extraction of their metal chelate complexes. Talanta 1964, 11, 864–873. [Google Scholar] [CrossRef]
- Dyrssen, D.; Heffez, M.; Sekine, T. Influence of the chelating agent on the separation of Eu3+ and Am3+ ions by solvent extraction. J. Inog. Nucl. Chem. 1961, 16, 367–370. [Google Scholar] [CrossRef]
- Sekine, T.; Koike, Y.; Hasegawa, Y. Studies of actinium (III) in various solutions. II. Distribution behavior of lanthanum (III) and actinium (III) in some chelate extraction systems. Bull. Chem. Soc. Jpn. 1969, 42, 432–436. [Google Scholar] [CrossRef]
- Albinsson, Y. Solvent extraction studies of lanthanide acetylacetonates. Part III. Complexes formed by Tb, Ho, Tm and Lu. Acta Chim. Scand. 1989, 43, 919–925. [Google Scholar] [CrossRef][Green Version]
- Albinsson, Y.; Mahmood, A.; Majdan, M.; Rydberg, J. Solvent extraction studies of lanthanide acetylacetonates. Complexes formed by La, Nd, Sm and Eu. Radiochim. Acta 1989, 48, 49–57. [Google Scholar] [CrossRef]
- Dyrssen, D. On the complex formation of strontium with oxine. Sven. Kem. Tidsk. 1955, 67, 311. [Google Scholar]
- Newman, L.; Klotz, P. Synergistic effect of tri-n-octylamine on the solvent extraction of americium by thenoyltrifluoroacetone. Inorg. Chem. 1966, 5, 461–466. [Google Scholar] [CrossRef]
- Moyer, B.A. Ion Exchange and Solvent Extraction: A Series of Advances; CRC Press: Boca Raton, FL, USA; Taylor & Francis group: Tokyo, Japan, 2010; Volume 19, pp. 1–673. [Google Scholar]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Varbanov, S.; Dukov, I. Behavior of mixed systems based on para-substituted 4-aroyl-5-pyrazolones in the presence of phosphorus containing calix[4]arene towards lanthanoids: Synergistic solvent extraction and separation. Sep. Purif. Technol. 2012, 95, 58–63. [Google Scholar] [CrossRef]
- Foust, A.; Wenzel, L.; Maus, L.; Clump, C.; Andesen, L. Solvent Extraction; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Atanassova, M.; Dukov, I. Synergistic solvent extraction of trivalent lanthanoids with mixtures of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone and crown ethers. Acta Chim. Slov. 2006, 53, 457–463. [Google Scholar]
- Schmidt, V.S. Amine Extraction; Kerter Press: Jerusalem, Israel, 1971. [Google Scholar]
- Atanassova, M.; Kaloyanova, S.; Deligeorgiev, T. Application of 4,4,4-trifluoro-1-(biphenyl-4-yl)butane-1,3-dione as a chelating extractant in the solvent extraction and separation of light lanthanoids in combination with phosphine oxides. Acta Chim. Slov. 2010, 57, 821–827. [Google Scholar] [PubMed]
- Okamura, H.; Takagi, H.; Isomura, T.; Morita, K.; Nagatani, H.; Imura, H. Highly selective synergism for the extraction of lanthanoid (III) ions with β-diketones and trioctylphosphine oxide in an ionic liquid. Anal. Sci. 2014, 30, 323–325. [Google Scholar] [CrossRef]
- Jordanov, V.; Atanassova, M.; Dukov, I. Solvent extraction of lanthanides with 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone. Sep. Sci. Technol. 2002, 37, 3349–3356. [Google Scholar] [CrossRef]
- Atanassova, M. Synergistic solvent extraction and separation of lanthanide (III) ions with 4-benzoyl-3-phenyl-5-isoxazolone and the quaternary ammonium salt. Solvent Extr. Ion Exch. 2009, 27, 159–171. [Google Scholar] [CrossRef]
- Atanassova, M.; Jordanov, V.; Dukov, I. Effect of the quaternary ammonium salt Aliquat 336 on the solvent extraction of lanthanoid(III) ions with thenoyltrifluoroacetone. Hydrometallurgy 2002, 63, 41–47. [Google Scholar] [CrossRef]
- Pavithran, R.; Reddy, M.L.P. Crown ethers as synergists in the extraction of trivalent lanthanoids with 3-phenyl-4-(4-fluorobenzoyl)-5-isoxazolone. Radiochim. Acta 2004, 92, 31–38. [Google Scholar] [CrossRef]
- Reddy, K.; Kumar, A.; Reddy, M. Synergistic extraction of zirconium(IV) and hafnium(IV) with 4-acylbis(1-phenyl-3-methyl-5-pyrazolones) in the presence of neutral organophosphorus extractants. Solvent Extr. Ion Exch. 2006, 24, 419–432. [Google Scholar] [CrossRef]
- Remya, P.; Raj, D.; Reddy, M.L.P. Para-substituted 1-phenyl-3-methyl-4-aroyl-5-pyrazolones as selective extractants for vanadium (V) from acidic chloride solutions. Solvent Extr. Ion Exch. 2006, 24, 877–892. [Google Scholar] [CrossRef]
- Messaodi, A.; Torkestani, K.; Goetz-Grandmont, G.; Brunette, J. Synergistic extraction of alkaline earth cations with 3-phenyl-4-benzoyl-isoxazol-5-one and tri-n-octylphosphine oxide in toluene. J. Radioanal. Nucl. Chem. 1996, 208, 123–132. [Google Scholar] [CrossRef]
- Kolarik, Z. The effect of diluent on the solvent extraction of Tb(III) and Eu(III) by di-n-octyl phosphoric acid. In Solvent Extraction Chemistry; Dyrssen, D., Liljenzin, J.-O., Rydberg, J., Eds.; John Wiley: Amsterdam, The Netherlands, 1967; pp. 250–255. [Google Scholar]
- Cunningham, J.G.; Scargill, D.; Willis, H. The Solvent Extraction of Praseodymium and Neodymium; (AERE-C/M 215); UKAEA-Research Group: Harwell, UK, 1954. [Google Scholar]
- Blake, C.A.; Baes, C.F.; Brown, K.B.; Coleman, C.F.; White, C. Proceedings of the Second United Nations International Conference on the Peaceful Uses of Atomic Energy, Geneva, Switzerland, 1–13 September 1958; IAEA: Vienna, Austria, 1958; Volume 28, p. 289. [Google Scholar]
- Duyckaerts, G.; Desreux, J. Recent developments and new combinations of extractants in synergic processes. In Proceedings of the International Solvent Extraction Conference (ISEC’77), Toronto, ON, Canada, 9–16 September 1977; CIM, special, Lucas, B., Ritcey, G., Smith, H., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montrea, QC, Canada, 1979; Volume 21, pp. 73–85. [Google Scholar]
- Madic, C. Phénomènes de synergismes lors de l’extraction par solvant. Chim. Anal. 1972, 54, 102–113. [Google Scholar]
- Irving, H.; Edgington, D.N. Synergic effects in the solvent extraction of the actinides I-uranium(VI). J. Inorg. Nucl. Chem. 1960, 15, 158–170. [Google Scholar] [CrossRef]
- Healy, T.V. Synergism with thenoyltrifluoroacetone in the solvent extraction of metallic species. Nucl. Sci. Eng. 1963, 16, 413–420. [Google Scholar] [CrossRef]
- Healy, T. Synergistic adducts as coordination compounds. In Proceedings of the Fifth International Solvent Extraction Conference, Jerusalem, Israel, 16–18 September 1969; Kerters, A.S., Marcus, Y., Eds.; Wiley Interscience: New York, NY, USA, 1969; pp. 257–277. [Google Scholar]
- Atanassova, M. Lanthanoid complexes with thenoyltrifluoroacetone and 4-tert-butylcalix[4]arene-tetraacetic acid tetraethyl ester: Synergistic extraction and separation. Microchim. Acta 2011, 174, 175–181. [Google Scholar] [CrossRef]
- Petrova, M.; Lachkova, V.; Vassilev, N.; Varbanov, S. Effect of the diluents on the synergistic solvent extraction and separation of trivalent lanthanoids with 4-benzoyl-3-phenyl-5-isoxazolone and tert-butylcalix[4]arene tetrakis(N,N-dimethyl acetamide) and structural study of Gd(III) solid complex by IR and NMR. Ind. Eng. Chem. Res. 2010, 49, 6189–6195. [Google Scholar]
- Atanassova, M.; Vassilev, N.; Dukov, I. p-tert-Butylcalix[4]arene tetrakis(N,N-dimethylacetamide) as a second ligand in the complexation of trivalent lanthanoids with thenoyltrifluoroacetone in solution and investigation of a solid Eu(III) complex. Sep. Purif. Technol. 2011, 78, 214–219. [Google Scholar] [CrossRef]
- Turanov, A.; Karandashev, V.; Baulin, D.; Baulin, V.; Tsivadze, A. Extraction of rare earth elements(III) with mixtures of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone and 2-phosphorylphenoxyacetamides. Russion J. Inorg. Chem. 2019, 64, 407–413. [Google Scholar] [CrossRef]
- Aly, H.F.; Khalifa, S.M.; Navratil, J.D.; Saba, M.T. Extraction of some lanthanides and actinides by 15-crown-5 thenoyltrifluroacetone mixture in chloroform. Solvent Extr. Ion Exch. 1985, 3, 623–636. [Google Scholar] [CrossRef]
- Caceci, M.; Choppin, G.; Liu, Q. Calorimetric studies of the synergistic effect. The reaction of UO22+, Th4+ and Nd3+ TTA complexes with TBP and TOPO in benzene. Solvent Extr. Ion Exch. 1985, 3, 60–621. [Google Scholar] [CrossRef]
- Turanov, A.; Karandashev, V.; Baulin, V.; Baulin, D.; Tsivadze, A. Synergistic solvent extraction of lanthanides(III) with mixtures of 4-benzoyl-3-methyl-1-phenyl-5-pyrazolone and phosphoryl-containing podands. Acta Chim. Slov. 2020, 67, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y.; Kertes, A. Ion Exchange and Solvent Extraction of Metal Complexes; Wiley-Interscience: New York, NY, USA, 1969. [Google Scholar]
- Nash, K. A review of the basic chemistry and recent developments in trivalent f-elements separation. Solvent Extr. Ion Exch. 1993, 11, 729–768. [Google Scholar] [CrossRef]
- Taube, M.; Siekierski, S. General remarks on synergic effects in the extraction of uranium and plutonium compounds. Nucleonica 1961, 6, 489–501. [Google Scholar]
- Atanassova, M.; Kurteva, V. Synergism as a phenomenon in solvent extraction of 4f-elements with calixarenes. RSC Adv. 2016, 6, 11303–11324. [Google Scholar] [CrossRef]
- Peparrd, D.; Mason, G.; Sironen, R. Isolation of neptunium as a mono octyl phosphoric acid complex by liquid-liquid extraction. J. Inorg. Nucl. Chem. 1959, 10, 117–127. [Google Scholar] [CrossRef]
- Tagushi, S.; Goto, K. Pre-Concentration Techniques for Trace Elements; Alfassi, Z.B., Wai, C.M., Eds.; CRC Press: Boca Raton, FL, USA, 1989; p. 31. [Google Scholar]
- Hasegawa, Y.; Nakako, T.; Choppin, G. Synergistic extraction of europium(III) with benzoic acid and thenoyltrifluoroacetone. Solvent Extr. Ion Exch. 1983, 1, 745–753. [Google Scholar] [CrossRef]
- El-Reefy, J.A.; Dessouky, N.A.; Aly, H.F. Extraction of Am(III) and Eu(III) from nitrate medium by thenoyltrifluoroacetone mixed with some soft donor ligands. Solvent Extr. Ion Exch. 1993, 11, 19–32. [Google Scholar] [CrossRef]
- Ensor, D.D.; Shah, A.H. Extraction of trivalent lanthanides and actinides by a synergistic mixture of thenoyltrifluoroacetone and a linear polyether. Solvent Extr. Ion Exch. 1984, 2, 591–605. [Google Scholar] [CrossRef]
- Khopkar, P.; Mathur, J. Synergistic extraction of trivalent actinides by mixtures of thenoyltrifluoroacetone and neutral oxo donors. Sep. Sci. Technol. 1981, 16, 957–969. [Google Scholar] [CrossRef]
- Ramaknishna, V.; Patil, S.; Hara Prakas, B. Solvent extraction and spectrophotometric studies on synergistic extraction of plutonium(IV) by mixtures of thenoyltrifluoroacetone (HTTA) and tri-n-butylphosphate (TBP) in benzene. Sep. Sci. Technol. 1979, 14, 571–589. [Google Scholar] [CrossRef]
- Sekine, T.; Ono, M. Studies of the liquid-liquid partition systems. IV. The solvent extraction study of europium(III) adduct chelate complexes with six acetylacetone derivatives and tributylphosphate. Bull. Chem. Soc. Jpn. 1965, 38, 2087–2094. [Google Scholar] [CrossRef]
- Atanasova, M.; Kurteva, V. Synergism in the solvent extraction of europium(III) with thenoyltrifluoroacetone and CMPO in methylimidazolium ionic liquids. J. Solution Chem. 2019, 48, 15–30. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands. Part VII. Extration and separation of calcium and stroncium with TTA and TBP in carbone tetrachloride. Anal. Chim. Acta 1967, 37, 217–226. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands.-VI Extraction of In(III) and Eu(III) with mixtures of thenoyltrifluoroacetone and β-isopropyltropolone. J. Inorg. Nucl. Chem. 1967, 29, 1489–1498. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands-III. Adduct formation of Eu(III) and Th(IV) chelates with TTA and IPR. J. Inorg. Nucl. Chem. 1967, 29, 1457–1473. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands. V. Adduct formations of some tris-TTA complexes. J. Inorg. Nucl. Chem. 1967, 29, 1481–1487. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands. IV Extraction of Eu(III) with chelating acids and neutral adduct-forming ligands. J. Inorg. Nucl. Chem. 1967, 29, 1475–1480. [Google Scholar] [CrossRef]
- Choppin, G.; Morgenstern, A. Thermodynamics of solvent extraction. Solvent Extr. Ion Exch. 2000, 18, 1029–1049. [Google Scholar] [CrossRef]
- Upor, E. Synergistic and antagonistic effects in solvent extraction processes. In Proceedings of the 3rd Symp. “Coordination Chemistry” Debrecen, Hungary, 1970; Akádémiai. Kiado: Budapest, Hungary, 1970; Volume 1, pp. 143–155. [Google Scholar]
- Zhao, Z.; Kubota, F.; Kamiya, N.; Goto, M. Selective extraction of scandium from transition metals by synergistic extraction with 2-thenoyltrifluroacetone and tri-n-octylphosphine oxide. Solv. Extr. Res. Dev. Jpn. 2016, 23, 137–143. [Google Scholar]
- Akiba, K.; Wada, M.; Kanno, T. Effect of diluent on synergic extraction of europium by thenoyltrifluoroacetone and tri-n-octyl phosphine oxide. J. Inorg. Nucl. Chem. 1981, 43, 1031–1034. [Google Scholar] [CrossRef]
- Irving, H.; Edgington, D.N. Synergic effects in the solvent extraction of the actinides—IV: Trivalent plutonium, americium and europium. J. Inorg. Nucl. Chem. 1961, 21, 169–180. [Google Scholar] [CrossRef]
- Murthy, K.S.R.; Krupadam, R.J.; Aujaneyuly, Y. Studies on the solvent extraction of trivalent lanthanides with hexafluoroacetylacetone (HFAA) and tri-n-octylphosphineoxide (TOPO). Proc. Indian Acad. Sci. Chem. Sci. 1998, 110, 83–92. [Google Scholar] [CrossRef]
- Favaro, D.; Atalla, L. Interaction effect between thenoyltrifluorocetone and tri-n-octylphosphine oxide in the synergistic extraction of trivalent lanthanides. Determination of the composition of the extracted species. J. Radioanal. Nucl. Chem. Art 1987, 111, 81–94. [Google Scholar] [CrossRef]
- Aly, H.F.; Khalifa, S.M.; Zakareia, N. Periodic variations of lanthanides synergistic extraction by thenoyltrifluoroacetone and triphenylphosphine oxide mixture. Solvent Extr. Ion Exch. 1984, 2, 887–898. [Google Scholar] [CrossRef]
- Sasayama, K.; Umetani, S.; Matsui, M. The substituent effect on the synergic extraction of europium and scandium with 1-phenyl-3-methyl-4-acylpyrazol-5-one and tri and tri-n-octylphosphine oxide. Anal. Chim. Acta 1983, 149, 253–258. [Google Scholar] [CrossRef]
- Akiba, K. Some regularities in the formation constants of TBP adducts of europium tris-TTA chelate. J. Inorg. Nucl. Chem. 1973, 35, 2525–2535. [Google Scholar] [CrossRef]
- Farbu, L.; Alstad, J.; Augustson, J.H. Synergistic solvent extraction of rare-earth metal ions with thenoyltrifluoroacetone admixed with tributylphosphate. J. Inorg. Nucl. Chem. 1974, 36, 2091–2095. [Google Scholar] [CrossRef]
- Sekine, T.; Koizumi, A.; Sakairi, M. Studies of scandium in various solutions. III. The solvent extraction and complex formation of scandium (III) with acetylacetone and thenoyltrifluoroacetone. Bull. Chem. Soc. Jpn. 1966, 39, 2681–2684. [Google Scholar] [CrossRef]
- Okamura, H.; Mizuno, M.; Hirayama, N.; Shimojo, K.; Naganawa, H.; Imura, H. Synergistic enhancement of the extraction and separation efficiencies of lanthanoid(III) ions by the formation of charged adducts in an ionic liquid. Ind. Eng. Chem. Res. 2020, 59, 329–340. [Google Scholar] [CrossRef]
- D’Olieslager, W.; Sannen, L. Extraction of Lanthanide ions with Benzoyltrifluroacetone-tri-n-Butylphosphate: Synergism and Antagonism. 967–972. ISEC’90, Solvent Extraction, Kyoto, Japan, 16–21 July 1990; Sekine, T., Ed.; Elsevier Science Publ. B.V.: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Shigematsu, T.; Tabushi, M.; Matsui, M.; Honjyo, T. The synergistic effect in solvent extraction-The correlation of the ionic radius of rare earth elements with the stability constants of rare earth benzoyltrifluoroacetonate adducts with n-hexyl alcohol, TBP and TOPO. Bull. Chem. Soc. Jpn. 1967, 40, 2807–2812. [Google Scholar] [CrossRef]
- Umetani, S.; Kawase, Y.; Le, Q.; Matsui, M. The synergistic extraction of lanthanides with trifluoroacetylcycloalkanones and trioctylphosphine oxide. Chem. Lett. 1997, 26, 771–772. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Nishiyama, Y.; Nagatani, H.; Okamura, H.; Imura, H. Synergistic extraction equilibrium of lanthanide(III) ions with benzoylacetone and a neutral ligand in an ionic liquid. Solvent Extr. Res. Dev. Jpn. 2018, 25, 79–89. [Google Scholar] [CrossRef]
- Kertes, A. The chemistry of solvent extraction. In Recent Advances in Liquid-Liquid Extraction, 1st ed.; Hadson, C., Ed.; Elsevier: Amsterdam, The Netherlands, 1971; Chapter 2; pp. 15–92. [Google Scholar]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Billard, I. Solvent extraction and separation of light lanthanoids with mixtures of two chelating extractants: Benzene vs. ionic liquid. Sep. Sci. Technol. 2016, 51, 290–299. [Google Scholar] [CrossRef]
- Hala, J. Some aspects of synergistic extraction with chelating extractants. J. Radioanal. Chem. 1979, 51, 15–25. [Google Scholar] [CrossRef]
- Woo, C.; Wagner, W.E.; Sands, D.F. Acetylacetone adducts of rare earth trithenoyltrifluoroacetonates in synergistic solvent extraction. J. Inorg. Nucl. Chem. 1972, 34, 307–312. [Google Scholar] [CrossRef]
- Atanasova, M.; Dukov, I. Crown ethers as synergistic agents in the solvent extraction of trivalent lanthanoids with thenoyltrifluoroacetone. Sep. Sci. Technol. 2005, 40, 1104–1113. [Google Scholar] [CrossRef]
- Kitatsuji, Y.; Meguro, Y.; Yoshida, Z.; Yamamoto, T.; Nishizawa, K. Synergistic ion-pair extraction of lanthanide(III) with thenoyltrifluoroacetone and crown ether into 1,2-dichloroethane. Solvent Extr. Ion Exch. 1995, 13, 289–300. [Google Scholar] [CrossRef]
- Mathur, J.; Choppin, G. The interaction of crown ethers with β−diketonate complexes of f-elements. Solvent Extr. Ion Exch. 1993, 111, 1–18. [Google Scholar] [CrossRef]
- Shyama, P. Sinha Structure and Bonding 30. Rare Earths; Springer: Berlin/Heidelberg, Germany, 1976; pp. 1–64. [Google Scholar]
- Zhang, A. Study of the antagonistic extraction of palladium (II) with 1-phenyl-3-methyl-4-propionylpyrazolone-5-one and an organic amine. Solvent Extr. Ion Exch. 2001, 19, 925–938. [Google Scholar]
- Zhang, A.; Wanyan, G.; Kumagai, M. Association behavior of 4-acylpyrazolone derivative and tertiary amine of high molecular weight in antagonistic synergistic extraction of palladium. J. Solut. Chem. 2004, 33, 1017–1028. [Google Scholar] [CrossRef]
- Pai, S.A.; Lohithakshan, K.V.; Mithapara, P.D.; Aggarwal, S.K. Solvent extraction of uranium (VI), plutonium (VI) and americium (III) with HTTA/HPMBP using mono-and bi-functional neutral donors: Synergism and thermodynamics. J. Radioanal. Nucl. Chem. 2000, 245, 623–628. [Google Scholar] [CrossRef]
- Santhi, P.B.; Reddy, M.L.P.; Ramamohan, T.R.; Damodaran, A.D. Radiochemical extraction of lanthanide thiocyanate complexes with bis-2-ethylhexyl sulphoxide. Radiochim. Acta 1994, 64, 121–125. [Google Scholar] [CrossRef]
- Sahu, S.K.; Chakravortty, V.; Reddy, M.L.P.; Ramamohan, T.R. Synergistic extraction of trivalent lanthanoids with 3-phenyl-4-benzoyl-5-isoxazolone and various sulphoxides. Radiochim. Acta 1999, 85, 107–111. [Google Scholar] [CrossRef]
- Bond, A.H.; Dietz, M.L.; Chiarizia, R. Incorporating size selectivity into synergistic solvent extraction: A review of crown ether-containing systems. Ind. Eng. Chem. Res. 2000, 39, 3442–3464. [Google Scholar] [CrossRef]
- Atanassova, M.; Dukov, I. A comparative study of the solvent extraction of the trivalent elements of the lanthanoid series with thenoyltrifluoroacetone and 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one using diphenylsulphoxide as synergistic agent. J. Solut. Chem. 2009, 38, 289–301. [Google Scholar] [CrossRef]
- Ramamohan, T.R.; Prasada Rao, T.; Iyer, C.S.P.; Damodaran, A.D.; Mathur, J.N.; Murali, M.S.; Iyer, R.H. Mixed ligand chelate extraction of trivalente lanthanides and actinides with 3-phenyl-4-benzoyl-5-isoxazolone and neutral oxo-donors. Radiochim. Acta 1995, 69, 55–60. [Google Scholar]
- Nakamura, S.; Suzuki, N. Adduct formation constant in the synergic extraction of several rare earth elements with acetylacetone and 1,10-phenanthroline in nonpolar organic solvents. J. Radional. Nucl. Chem. Artic. 1986, 99, 145–152. [Google Scholar] [CrossRef]
- Nakamura, S.; Suzuki, N. Effect of an acidic chelating agent in the synergic extraction systems of rare earths(III)-β-diketones-2,2’-bipyridine. Inorg. Chim. Acta 1986, 114, 101–107. [Google Scholar] [CrossRef]
- Nakamura, S.; Suzuki, N. A new trend in the synergistic extraction of rare-earth(III) complexes with various β-diketones and 1,10-phenanthroline. Polyhedron 1986, 5, 1805–1813. [Google Scholar] [CrossRef]
- Matsubayshi, I.; Hasegawa, Y. Thermodynamic consideration for solvent effects in the synergistic extraction of europium(III) with pivaloyltrifluoroacetone and 1,10-phenanthrolone. Anal. Sci. 2001, 17, 221–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kassierer, E.F.; Kertes, A.S. Synergic extraction of some lanthanides and transition metals by two bidentate chelating agents. J. Inorg. Nucl. Chem. 1972, 34, 3221–3231. [Google Scholar] [CrossRef]
- Kassierer, E.F.; Kertes, A.S. Thermodynamics of solvent extraction processes. Synergic adducts of copper and zinc diketonates with bidentate nitrogen bases. J. Inorg. Nucl. Chem. 1972, 34, 3209–3214. [Google Scholar] [CrossRef]
- Bhatti, M.S.; Desreux, J.F.; Duyckaerts, G. Behavior of bidentate amines in the synergic extraction of lanthanides by thenoyltrifluoroacetone. J. Inorg. Nucl. Chem. 1980, 42, 767–770. [Google Scholar] [CrossRef]
- Xia, Y.-X.; Chen, J.-F.; Choppin, G. Solubility, dissociation and complexation with Nd(III) and Th(IV) of oxine, thenoyltrifluoroacetone and 1,10-phanantroline in 5.0 m NaCl. Talanta 1996, 43, 2073–2081. [Google Scholar] [CrossRef]
- Nakamura, S.; Imura, H.; Suzuki, N. Synergic extraction of thulium(III) with thenoyltrifluoroacetone and neutral unidentate and bidentate heterocyclic amines. Inorg. Chim. Acta 1985, 110, 101–105. [Google Scholar] [CrossRef]
- Nakamura, S.; Suzuki, N. Synergic extraction of lanthanide(III) ions with 2-thenoyltrifluoroacetone in the presence of 2,2’-bipyridine or pyridine. Polyhedron 1988, 7, 155–159. [Google Scholar] [CrossRef]
- Ishimori, K.; Imura, H.; Ohashi, K. Effect of 1,10-phenantroline on the extraction and separation of lithium(I), sodium(I) and potassium with thenoyltrifluoroacetone. Anal. Chim. Acta 2002, 454, 241–247. [Google Scholar] [CrossRef]
- Nakamura, S.; Takai, S.; Akiba, K. Synergic extraction of lanthanoids by mixtures of LIX 54 (High molecular weight β-diketone) and bidentate neutral amines. Anal. Sci. 2002, 18, 319–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roy, A.; Nag, K. Investigation on the lanthanoid chelates of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone. An examination of the phenomenon of hypersensitivity. Bull. Chem. Soc. Jpn. 1978, 51, 1525–1529. [Google Scholar] [CrossRef]
- Kikuta, Y.; Watarai, H.; Suzuki, N. Synergic effect of some heterocyclic bidentate amines on the extraction of zinc with 2-thenoyltrifluoroacetone. Correlation between formation constants of amine-complex in organic and aqueous phases. Polyhedron 1982, 4, 387–392. [Google Scholar] [CrossRef]
- Satake, S.; Tsukahara, S.; Suzuki, N. Synergistic extraction equilibrium of lanthanoids(III) with 2-thenoyltrifluoroacetone and nitrogen-containing bidentate ligands, ethylenediamine derivatives. Solvent Extr. Ion Exch. 1999, 17, 259–275. [Google Scholar] [CrossRef]
- Atanassova, M. Adduct formation constants in the synergistic solvent extraction of several lanthanoids with two chelating extractants: β-Diketone and bidentate nitrogen bases. J. Chem. Eng. Data 2010, 55, 5791–5796. [Google Scholar]
- Atanassova, M.; Lachkova, V.; Vassilev, N.; Varbanov, S.; Dukov, I. Complexation of trivalent lanthanoid ions with 4-benzoyl-3-phenyl-5-isoxazolone and p-tert-butylcalix[4]arene fitted with phosphinoyl pendant arms in solution during synergistic solvent extraction and structural study of solid complexes by IR and NMR. Polyhedron 2010, 29, 655–663. [Google Scholar] [CrossRef]
- Kertes, A.; Kassierer, E. Thermochemistry of lanthanide complexes in the thenoyltrifluoroacetone-bipyridyl system. Inorg. Chem. 1972, 11, 2108–2111. [Google Scholar] [CrossRef]
- Saved, S.A.; Sami, T.M.; Abd El Tawab, A.A. Synergistic extraction of some rare earths(III) from nitrate media by thenoyltrifluoroacetone and tri-n-octylamine. Sep. Sci. Technol. 1996, 31, 2579–2587. [Google Scholar] [CrossRef]
- Dukov, I.; Genov, L. On the mechanism of the synergistic extraction of lanthanides with thenoyltrifluoroacetone and Aliquat-336S. Acta Chim. Acad. Sci. Hung. 1980, 104, 329–335. [Google Scholar]
- Dukov, I.; Atanassova, M. Effect of diluents on the synergistic solvent extraction of some lanthanides with thenoyltrifluoroacetone and quaternary ammonium salt. Hydrometallurgy 2003, 68, 89–96. [Google Scholar] [CrossRef]
- Atanassova, M.; Dukov, I. Synergistic solvent extraction and separation of trivalent lanthanide metals with mixtures of 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one and Aliquat 336. Sep. Purif. Technol. 2004, 40, 171–176. [Google Scholar] [CrossRef]
- Brunette, J.P.; Lakkis, Z.; Lakkis, M.; Leroy, M.J.F. Effect of inorganic aqueous anions on the synergic extraction of cadmium and zinc with 1-phenyl—3-methyl-4-benzoyl-pyrazol-5-one (HPMBP) and lipophilic ammonium salts. Polyhedron 1985, 4, 577–582. [Google Scholar] [CrossRef]
- Sekine, T.; Murai, R.; Konno, H. Solvent extraction equilibria of tris(benzoyl-trifluroacetonato)complex of cobalt(II) and nickel(II) as ion-pairs with tetrabutylammonium. Solvent Extr. Ion Exch. 1984, 2, 213–225. [Google Scholar] [CrossRef]
- Peppard, D.F.; Mason, G.W.; Lewey, S. A tetrad effect in the liquid-liquid extraction ordering of lanthanides. J. Inorg. Nucl. Chem. 1969, 31, 2271–2272. [Google Scholar] [CrossRef]
- Dukov, I.; Jordanov, V.; Atanassova, M. Stoichiometry of the praseodymium complex extracted by a mixture of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone and Aliquat 336. Solv. Extr. Ion Exch. 2001, 19, 619–628. [Google Scholar] [CrossRef]
- Dukov, I.; Atanassova, M. Synergistic solvent extraction and separation of lanthanides using mixtures of 1-phenyl-3-methyl-4-benzoyl-pyrazol-5-one and Aliquat 336: Influence of the ammonium salt anion. Sep. Sci. Technol. 2004, 39, 227–239. [Google Scholar] [CrossRef]
- Noro, J.; Sekine, T. Effect of several quarternary ammoniums on the solvent extraction of europium (III) 2-thenoyltrifluoroacetonate anionic complex into chloroform. Bull. Chem. Soc. Jpn. 1993, 66, 804–809. [Google Scholar] [CrossRef]
- Mikkola, J.-P.; Virtanen, P.; Sjöholm, R. Aliquat 336-a versatile and affordable cation source for an entirely new family of hydrophobic ionic liquids. Green Chem. 2006, 8, 250–255. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanides and actinides in ionic liquids. In Comprehensive Inorganic Chemistry II; Reedijk, J., Peoppelmeir, K., Eds.; Elsevier: Oxford, UK, 2013; Volume 12, pp. 641–673. [Google Scholar]
- Billard, I. In Ionic liquids: New hopes for efficient lanthanide/actinide extraction and separation. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.C.G., Pecharsky, V., Eds.; Elsevier Science Publ. B. V.: Amsterdam, The Netherlands, 2013; Volume 43, Chapter 256; pp. 213–273. [Google Scholar]
- Kolaric, Z. Ionic liquids: How far do they extend the potential of solvent extraction of f-elements? Solvent. Extr. Ion Exch. 2013, 31, 24–60. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Q.; Sun, T.; Shen, Y. Extraction of Th(IV) from aqueous solution by room temperature ionic liquids and coupled with supercritical carbon dioxide. Sep. Purif. Technol. 2013, 119, 66–71. [Google Scholar] [CrossRef]
- Hirayama, N.; Okamura, H.; Kidani, K.; Imura, H. Ionic liquid synergistic cation-exchange system for the selective extraction of lanthanum(III) using 2-thenoyltrifluoroacetone and 18-crown-6. Anal. Sci. 2008, 24, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Borkowski, M.; Laszak, I.; Beitz, J.; Rickert, P.; Dietz, M. Anion effects in the extraction of lanthanide 2-thenoyltrifluoroacetone complexes into an ionic liquid. Sep. Sci. Technol. 2012, 47, 233–243. [Google Scholar] [CrossRef]
- Ranjbar, L.; Yamini, Y.; Saleh, A.; Seidi, J.; Faraji, M. Ionic liquid based dispersive liquid-liquid microextraction combined with ICP-OES for the determination of trace quantities of cobalt, copper, manganese, nickel and zinc in environmental water samples. Microchim. Acta 2012, 177, 119–127. [Google Scholar] [CrossRef]
- Pandey, S.; Baker, G.; Sze, L.; Pandey, S.; Kamath, G.; Zhao, H.; Baker, S. Ionic liquids containing fluorinated β-diketonate anions: Synthesis, characterization and potential applications. New J. Chem. 2013, 37, 909–919. [Google Scholar] [CrossRef]
- Kidani, K.; Imura, H. Solvent effect of ionic liquids on the distribution constants of 2-thenoyltrifluoroacetone and its nickel(II) and copper(II) chelates and the evaluation of the solvent properties based on the regular solution theory. Talanta 2010, 83, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Rout, A.; Venkatesan, K.; Srinivasan, T.; Vasudeva Rao, P. Ionic liquid extractants in molecular diluents: Extraction behavior of plutonium(IV) in 1,3-diketone ionic liquids. Solvent Extr. Ion Exch. 2011, 29, 602–618. [Google Scholar] [CrossRef]
- Okamura, H.; Sakae, H.; Kidani, K.; Hirayama, N.; Aoyagi, N.; Saito, T.; Shimojo, K.; Naganawa, H.; Imura, H. Laser-induced fluorescence and infrared spectroscopic studies on the specific solvation of tris(1-(2-thienyl)-4,4,4-trifluoro-1,3-butanedionato)europium(III) in an ionic liquid. Polyhedron 2012, 31, 748–753. [Google Scholar] [CrossRef]
- Jensen, M.; Neuefeind, J.; Beitz, J.; Skanthakumar, S.; Soderholm, L. Mechanism of metal ion transfer into room-temperature ionic liquids: The role of anion exchange. J. Am. Chem. Soc. 2003, 125, 15466–15473. [Google Scholar] [CrossRef]
- Petrova, M. The Crucial Performance of Mutual Solubility among Water and Ionic Liquids in the Time of Liquid-Liquid Extraction of Metallic Species; Academic Solutions: Sofia, Bulgaria, 2020; pp. 1–159. ISBN 978-954-2940-23-4. [Google Scholar]
- Atanassova, M.; Mazan, V.; Billard, I. Modulating the solubilities of ionic liquid components in aqueous-ionic liquid biphasic systems: A Q-NMR investigation. Chem. Phys. Chem. 2015, 16, 1703–1711. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V. Peculiar synergistic extraction behavior of Eu(III) in ionic liquids: Benzoylacetone and CMPO fusion. Sep. Purif. Technol. 2017, 183, 226–236. [Google Scholar] [CrossRef]
- Mazan, V.; Billard, I.; Papaiconomou, N. Experimental connections between aqueous aqueous and aqueous ionic biphasic systems. RSC Adv. 2014, 4, 13371–13384. [Google Scholar] [CrossRef]
- Depuydt, D.; Dehaen, W.; Binnemans, K. Solvent extraction of scandium(III) by an aqueous biphasic system with a nonfluorinated functionalized ionic liquid. Ind. Eng. Chem. Res. 2015, 54, 8988–8996. [Google Scholar] [CrossRef]
- Atanassova, M.; Billard, I. Determination of pKaIL values of three chelating extractants in ILs. Consequences on extraction process of 4f-elements. J. Solut. Chem. 2015, 44, 606–620. [Google Scholar] [CrossRef]
- Bouby, M.; Billard, I.; Duplâtre, G.; Simonin, J.P.; Bernard, O.; Brunette, J.P.; Goetz-Grandmont, G. Determination of the thermodynamic acidity constant of 3-phenyl-4-benzoylisoxazol-5-one (HPBI) using the binding mean spherical approximation model. Phys. Chem. Chem. Phys. 1999, 1, 3765–3769. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Billard, I. Comparing extraction, synergism and separation of lanthanoids using acidic and neutral compounds in chloroform and one ionic liquid: Is the latter always “better”? RSC Adv. 2014, 4, 38820–38829. [Google Scholar] [CrossRef]
- Kurteva, V.; Atanassova, M.; Billard, I. NMR study on the possible interaction between imidazolium based ionic liquids and extractants widely applied in solvent extraction and separation of f-ions. J. Solut. Chem. 2015, 44, 2416–2430. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Billard, I. Coordination chemistry of europium(III) ion towards acylpyrazolone ligands. Anal. Sci. 2015, 31, 917–922. [Google Scholar] [CrossRef]
- Hirayama, N. Chelate extraction of metals into ionic liquids. Solvent Extr. Res. Dev. Jpn. 2011, 18, 1–14. [Google Scholar] [CrossRef]
- Hirayama, N.; Deguchi, M.; Kawasumi, H.; Honjo, T. Use of 1-alkyl-3-methylimidazolium hexafluorophosphate room temperature ionic liquids as chelate extraction solvent with 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione. Talanta 2005, 65, 255–260. [Google Scholar] [CrossRef]
- Kumar, P.; Vincent, T.; Khanna, A. Extraction of UO22+ into ionic liquids using TTA and TBP as extractants. Sep. Sci. Technol. 2015, 50, 2668–2675. [Google Scholar] [CrossRef]
- Dietz, M.; Jensen, M.; Beitz, J.; Dzielawa, J. Room Temperature Ionic Liquids as Diluents for the Liquid-Liquid Extraction of Metal Ions: Promise and limitations. Hydrometallurgy 2003-Fifth International Conference in Honor of Prof. I. Ritchie; TMS (The Minerals, Metals&Materials Socieaty): Warrendale, PA, USA, 2003; Volume 1, pp. 929–939. [Google Scholar]
- Dietz, M.; Dzielawa, J.; Jensen, M.; Beitz, J.; Borkowski, M. The Road to Partition。 The Road to Partition. Mechanism of Metal Ion Transfer into Ionic Liquids and Their Implications for the Application of Ionic Liquids as Extraction Solvents. ACS Symposium Series, Ionic Liquids IIIB: Fundamentals, Progress, Challenges and Opportunities. 2005, Volume 902, Chapter 1. pp. 2–18. Available online: https://global.oup.com/academic/product/ionic-liquids-iiib-fundamentals-progress-challenges-and-opportunities-9780841238947?cc=us&lang=en&# (accessed on 28 April 2022).
- Okamura, H.; Hirayama, N. Recent progress in ionic liquid extraction for the separation of rare earth elements. Anal. Sci. 2021, 37, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.; Marcias-Ruvalcaba, N.; Aguilar-Martinez, M.; Kobrak, M. Metal extraction to ionic liquids: The relationship between structure, mechanism and application. Int. Revs. Phys. Chem. 2015, 34, 591–622. [Google Scholar] [CrossRef]
- Okamura, H.; Aoyagi, N.; Shimojo, K.; Naganawa, H.; Imura, H. Role of Tf2N- anions in the ionic liquid-water distribution of europium(III) chelates. RSC Adv. 2017, 7, 7610–7618. [Google Scholar] [CrossRef]
- Sieffert, N.; Wipff, G. Alcali cation extraction by calix[4]crown-6 to room-temperature ionic liquids. The effect of solvent anion and humidity investigation by molecular dynamics simulations. J. Phys. Chem. A 2006, 110, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Kidani, K.; Hirayama, N.; Imura, H. Extraction behavior of divalent metal cations in ionic liquid chelate extraction system using 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides and thenoyltrifluoroacetone. Anal. Sci. 2008, 24, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, N.; Deguchi, M.; Honjo, T. The behavior of divalent transition metal cations on extraction into 1-bytul-3-methylimidazolium hexafluorophosphate room temperature ionic liquid with β-diketones having a trifluoromethyl group. Solvent Extr. Res. Dev. Jpn. 2006, 13, 83–88. [Google Scholar]
- Matsumiya, M.; Yamada, T.; Muramaki, S.; Kohno, Y.; Tsunashima, K. Evaluation of the extraction properties and stability of extracted rare earth complexes in ionic liquid extraction system using β-diketone. Solvent Extr. Ion Exch. 2016, 34, 454–468. [Google Scholar] [CrossRef]
- Stepinski, D.; Jensen, M.; Dzielawa, J.; Dietz, M. Synergistic effects in the facilitated transfer of metal ions into room-temperature ionic liquids. Green Chem. 2005, 7, 151–158. [Google Scholar] [CrossRef]
- Mathur, J.; Pai, S.; Khopkar, P.; Sumramanian, M. Thermodynamics of synergistic extraction of europium(III) by mixtures of thenoyltrifluoroacetone and some neutral oxo-donors. J. Inorg. Nucl. Chem. 1977, 39, 653–657. [Google Scholar] [CrossRef]
- Petrova, M.; Kurteva, V.; Lubenov, L. Synergistic effect in the solvent extraction and separation of lanthanoids by 4-(4-fluorobenoyl)-3-methyl-1-phenyl-pyrazol-5-one in the presence of monofunctional neutral organophosphorus extractants. Ind. Eng. Chem. Res. 2011, 50, 12170–12176. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Varbanov, S.; Billard, I. Are fancy acidic or neutral ligands really needed for synergism in ionic liquids? A comparative study of lanthanoid extraction in CHCl3 and an ionic liquid. New J. Chem. 2015, 39, 7932–7941. [Google Scholar] [CrossRef]
- Zhao, Z.; Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Synergistic extraction of rare earth metals and separation of scandium using 2-thenoyltrifluoroacetone and tri-n-octylphosphine oxide in an ionic liquid system. J. Chem. Eng. Jpn. 2014, 47, 656–662. [Google Scholar] [CrossRef]
- Okamura, H.; Hirayama, N.; Morita, K.; Shimojo, K.; Naganawa, H.; Imura, H. Synergistic effect of 18-crown-6 derivatives on chelate extraction of lanthanoids (III) into an ionic liquid with 2-thenoyltrifluoroacetone. Anal. Sci. 2010, 26, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chen, J.; Wang, W.; Cui, H.M.; Liu, W.G.; Liu, Y. Extraction mechanism of rare earths from chloride acidic solution with ammonium-bifunctionalized ionic liquid extractants. Sci. China Chem. 2016, 59, 532–537. [Google Scholar] [CrossRef]
- Kassierer, E.; Kertes, A. Displacement of water in synergic extraction systems. J. Inorg. Nucl. Chem. 1972, 34, 778–780. [Google Scholar] [CrossRef]
- Healy, T. Synergism in the solvent extraction of di-, tri-and tetravalent metal ions—II: Synergic effects in so-called inert diluents. J. Inorg. Nucl. Chem. 1961, 19, 328–339. [Google Scholar] [CrossRef]
- Choppin, G. Studies of the synergistic effect. Sep. Sci. Technol. 1981, 16, 1113–1126. [Google Scholar] [CrossRef]
- Healy, T.; Peppard, D.; Mason, G. Synergism in the solvent extraction of di, tri and tetravalent metal ions—III: Antisynergism with thenoyltrifluoracetone. J. Inorg. Nucl. Chem. 1962, 24, 1429–1448. [Google Scholar] [CrossRef]
- Atanassova, M. Investigation of synergism and selectivity using mixture of two neutral extractants in IL media for lanthanoids extraction. Sep. Purif. Technol. 2016, 169, 253–261. [Google Scholar] [CrossRef]
- Atanassova, M. Solvent extraction chemistry in ionic liquids: An overview of f-ions. J. Mol. Liq. 2021, 343, 117530. [Google Scholar] [CrossRef]

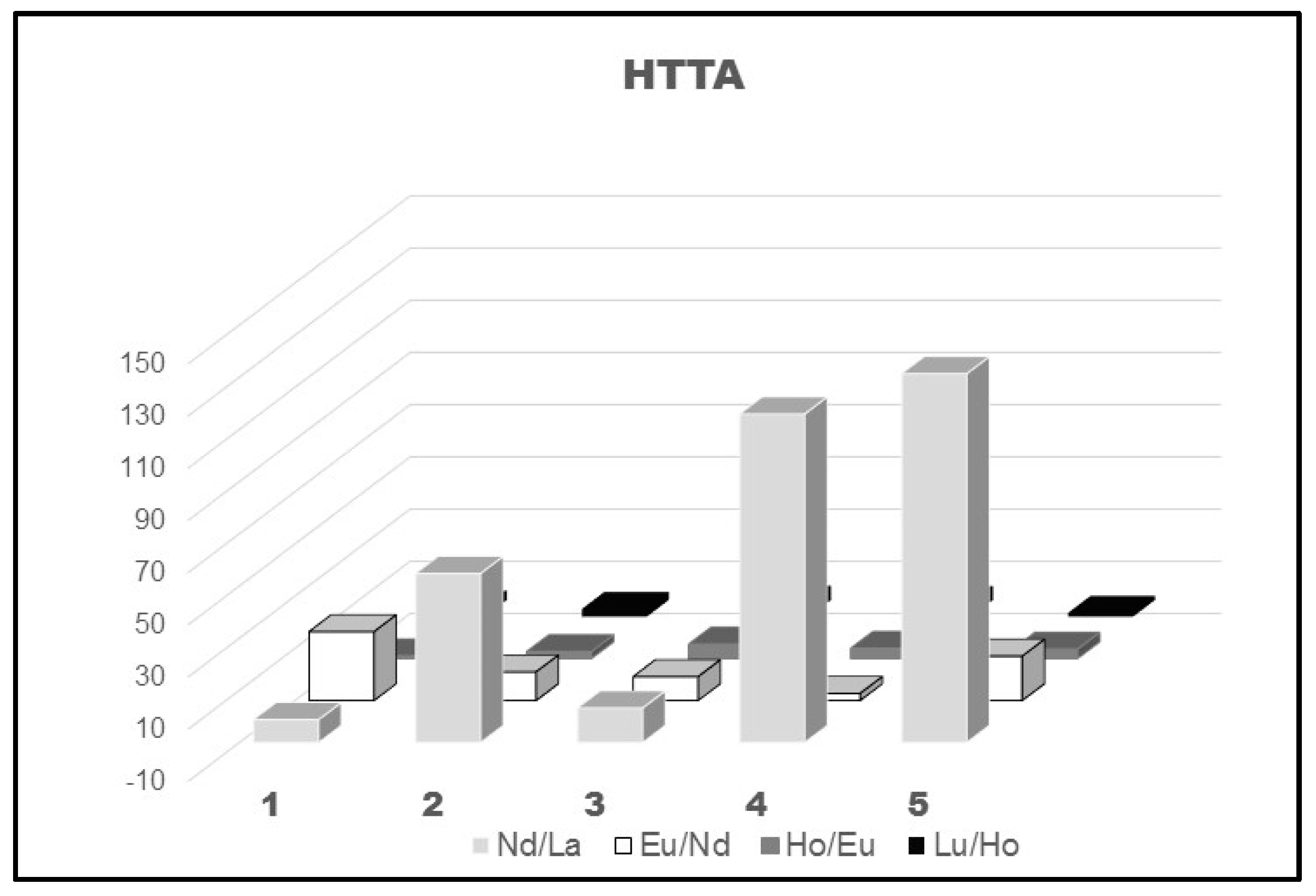
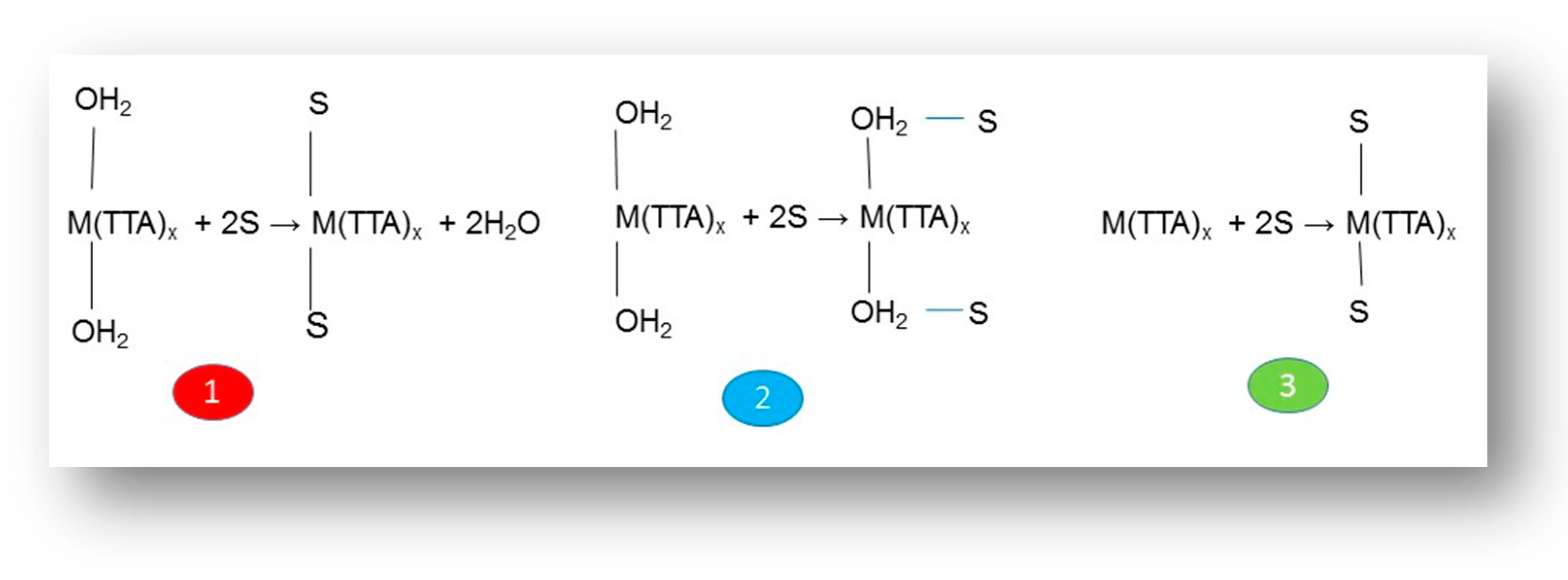

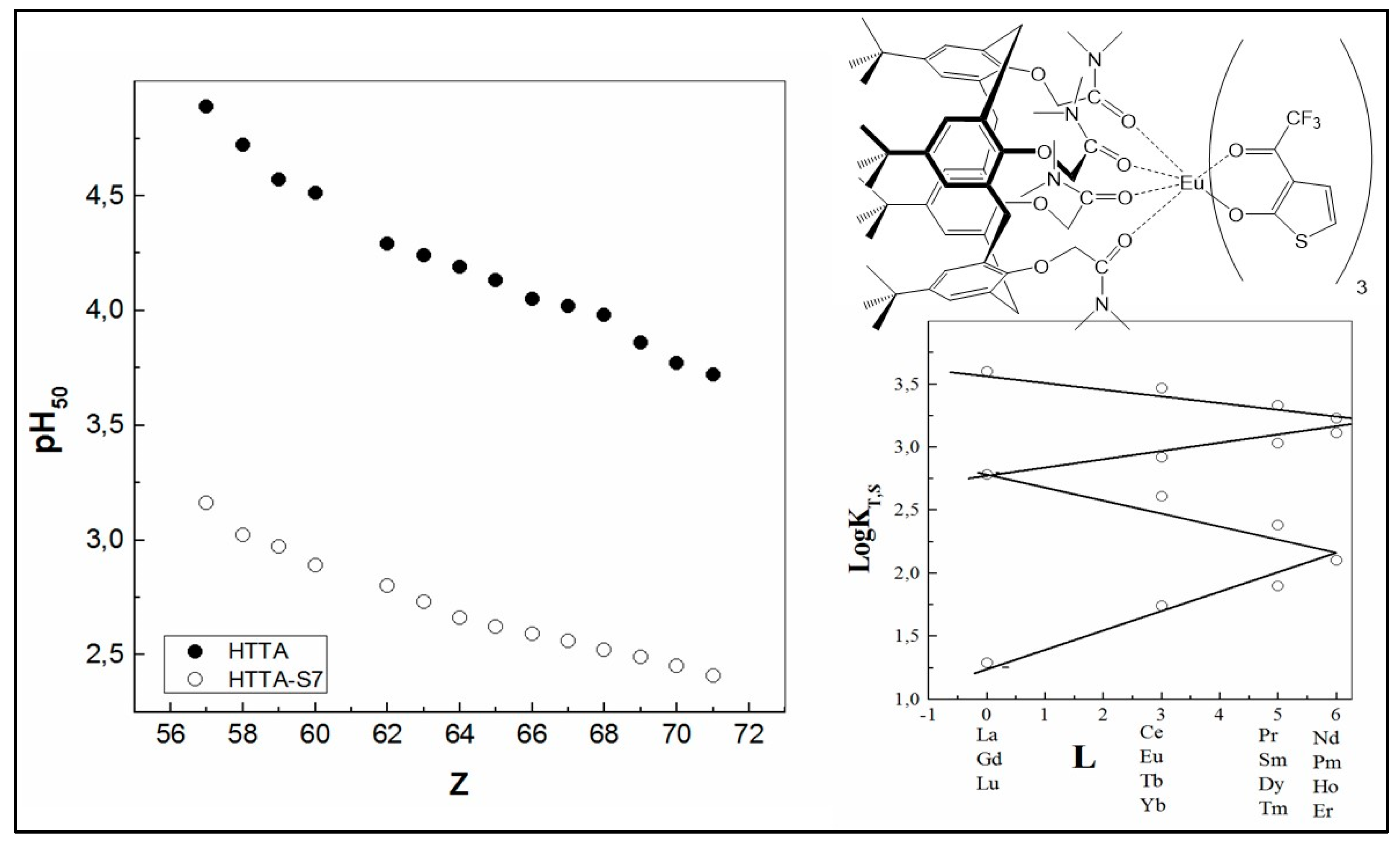
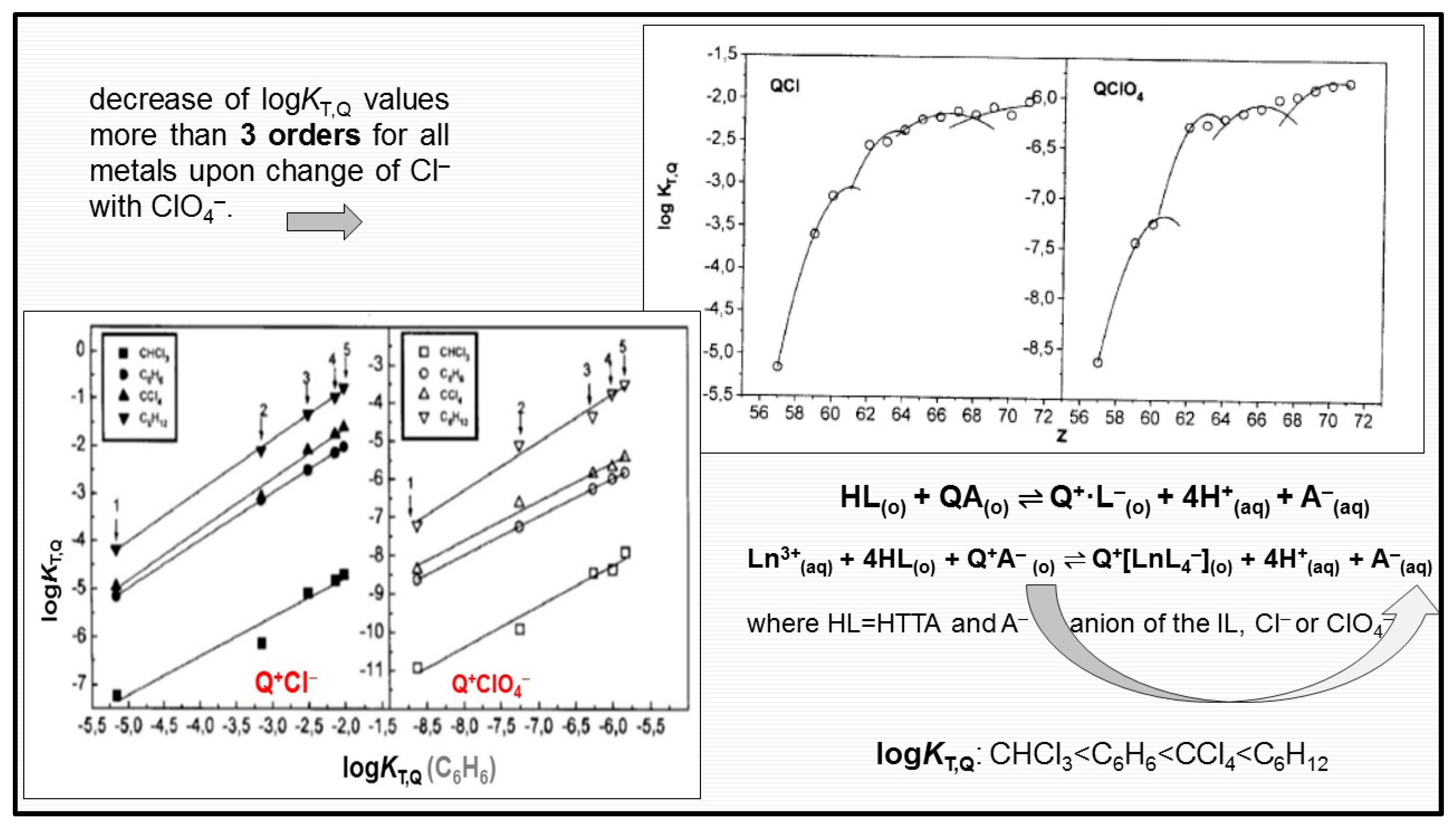

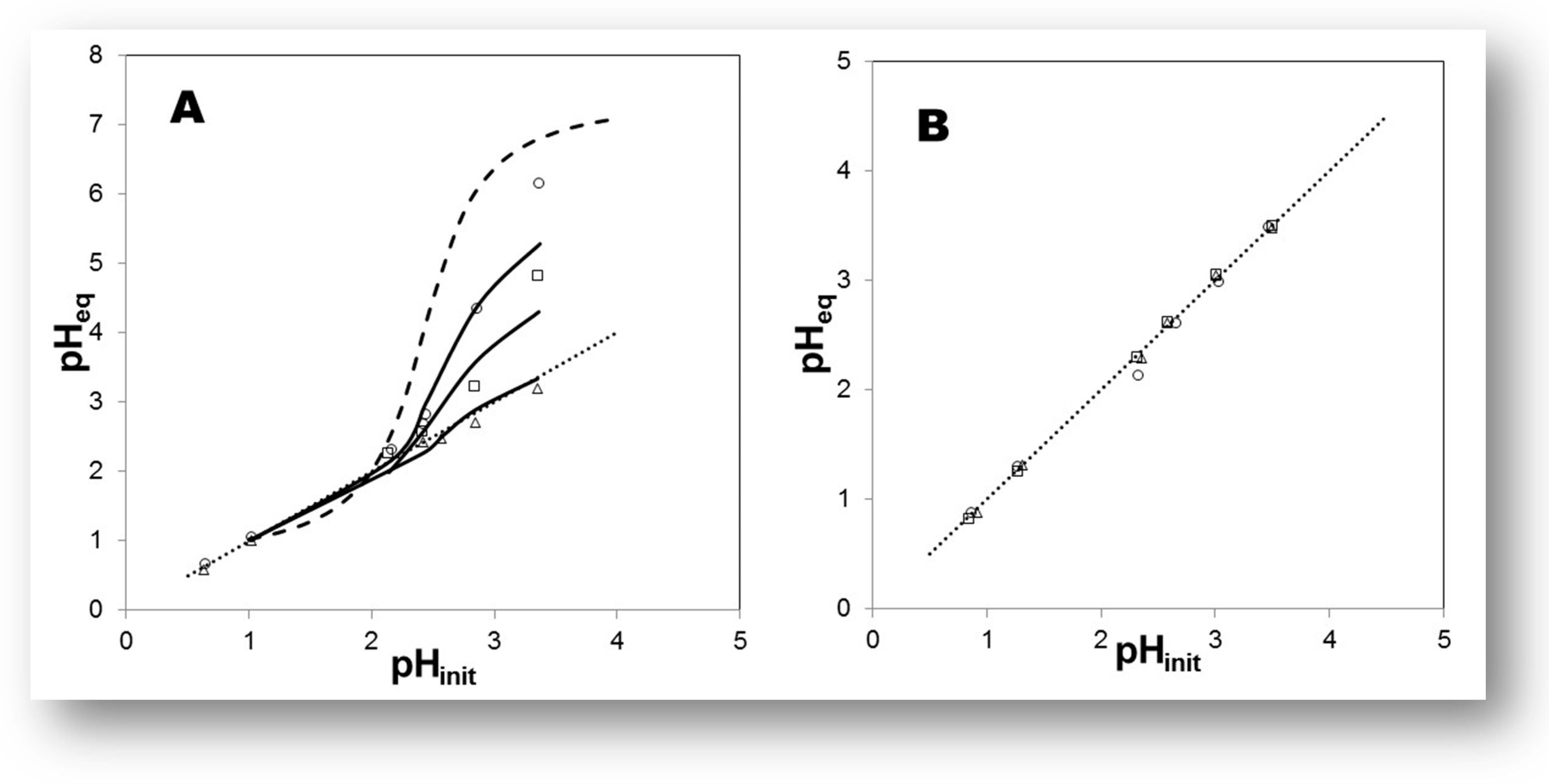
| Extractants | Diluent | SFEu/Am |
|---|---|---|
| dibutylphosphoric acid | CHCl3 | 22.9 |
| dioctylphosphoric acid | CHCl3 | 14.1 |
| 1-phenyl-3-methyl-4-acetylpyrazolone-5 | CHCl3 | 3.47 |
| thenoyltrifluoroacetone | CHCl3 | 3.02 |
| neocupferron | CHCl3 | 1.74 |
| N-benzoylphenylhydroxylamine | CHCl3 | 1.66 |
| N-2,4-dichlorobenzoylphenylhydroxylamine | CHCl3 | 1.32 |
| β-isopropyltropolon | CHCl3 | 0.98 |
| 1-hydroxy-2-napthoic acid | hexone | 1.07 |
| 2-hydroxy-1-napthoic acid | hexone | 1.02 |
| 3-hydroxy-2-naphtoic acid | hexone | 0.95 |
| 5,7-dichloroxine | CHCl3 | 0.10 |
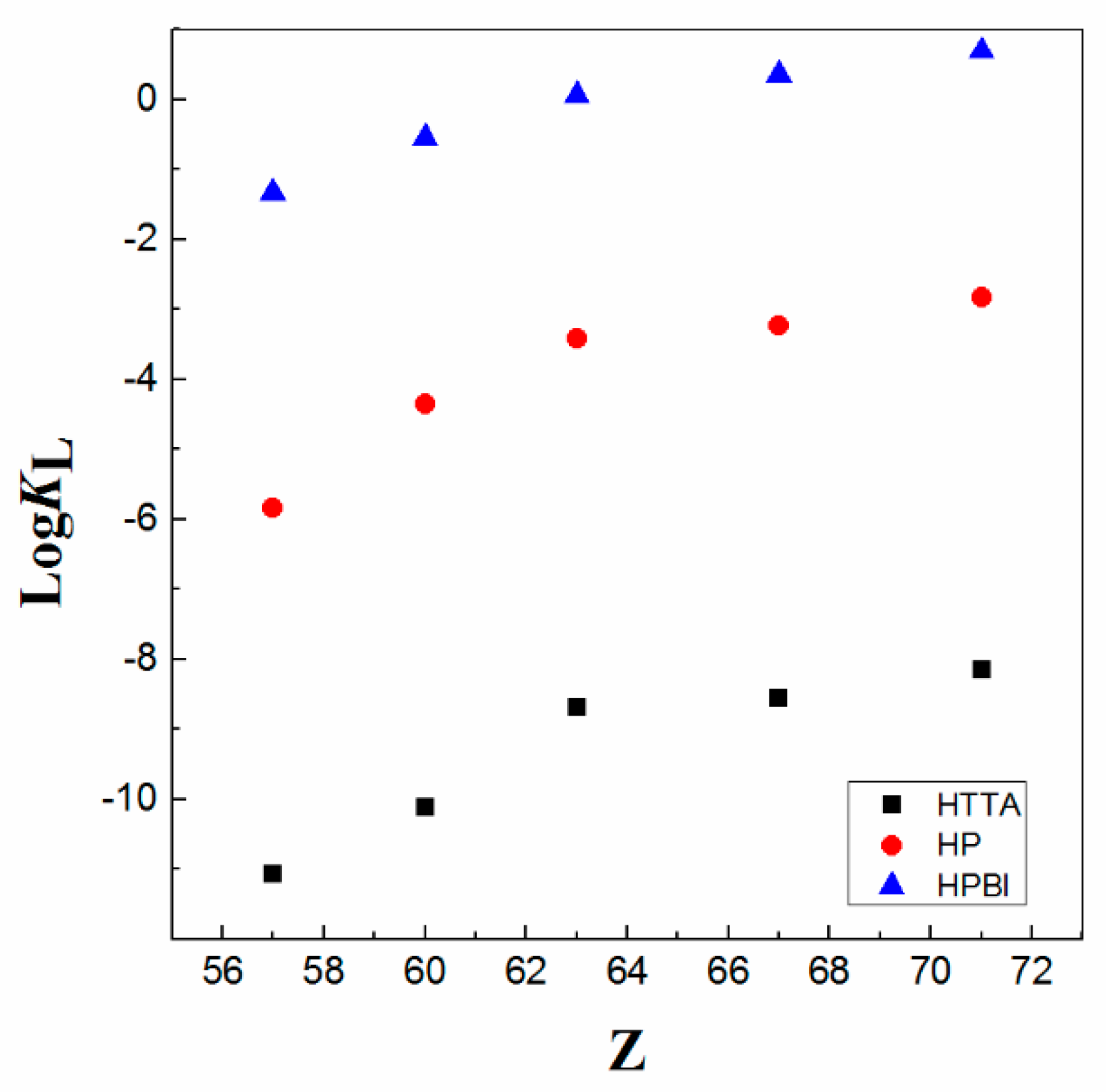
| Ln3+ | HBA | HTTA | HPPMBP | HPMMBP | HP | HPMFBP | HPMTFBP | HPBI |
|---|---|---|---|---|---|---|---|---|
| La | −21.81 | −11.06 | −6.21 | −6.12 | −5.84 | −5.16 | −3.24 | −1.33 |
| Nd | −10.12 | −5.84 | −4.17 | −4.35 | −3.91 | −2.74 | −0.54 | |
| Eu | −8.68 | −5.57 | −3.89 | −3.42 | −3.37 | −2.47 | 0.06 | |
| Ho | −8.56 | −4.97 | −3.37 | −3.24 | −2.84 | −1.88 | 0.36 | |
| Lu | −8.15 | −4.58 | −3.12 | −2.83 | −2.62 | −1.62 | 0.70 | |
| U6+ | −2.44 | 0.63 | 1.40 | |||||
| Th4+ | 2.25 | 6.96 | 8.26 | |||||
| Np4+ | 5.68 | 10.11 | ||||||
| Pu4+ | 7.31 | 13.75 | 10.76 | |||||
| pKa | 8.96 | 6.23 | 4.23 | 4.02 | 3.92 | 3.52 | 4.40 | 1.23 |
| Lns Pair | SF | |||||
|---|---|---|---|---|---|---|
| HTTA * | HFPA *** | HP ** | HPMMBP | HPMFBP | HPBI **** | |
| Ce/La | 12.02 | 6.45 | 8.13 | 16.22 | 5.75 | 2.18 |
| Pr/Ce | 3.80 | 2.63 | 1.86 | 6.30 | 2.04 | 1.44 |
| Nd/Pr | 2.0 | 2.57 | 3.80 | 2.29 | 8.12 | 2.08 |
| Sm/Nd | 10 | 2.51 | 2.39 | 2.34 | 1.90 | 2.75 |
| Eu/Sm | 1.09 | 1.99 | 1.34 | 1.66 | 1.28 | 2.82 |
| Gd/Eu | 1.47 | 2.04 | 0.89 | 2.08 | 2.08 | 2.82 |
| Extractant | Separation | Factors | |
|---|---|---|---|
| Lu/La | Eu/La | Lu/Eu | |
| HPBI | 6.0 × 102 | 51.28 | 11.74 |
| HP | 1.2 × 103 | 1.8 × 102 | 6.30 |
| HTTA | 5.4 × 103 | 7.0 × 102 | 8.04 |
| Cation | Diluents | Equilibrium | Refs |
|---|---|---|---|
| Eu | cyclohexane | Eu (TTA)3/Eu (TTA)3·2TBP | [89] |
| 152,154 Eu | n-hexane, n-heptane, cyclohexane, methylenechloride, chloroform, benzene, carbon tetrachloride, bromoform, toluene, isopropylbenzene, chlorobenzene, o-dichlorobenzene | Eu (TTA)3·TBP/ Eu (TTA)3·2TBP | [93] |
| All REs | CCl4 | Ln (TTA)3 | [94] |
| Sc | CHCl3 | Sc(TTA)3 | [95] |
| Sc | CHCl3 | Sc(acac)3 | [95] |
| Sc, La, Eu, Lu | CCl4 | Ln (TTA)3/ Ln (TTA)3·TBP/ Ln (TTA)3·2TBP | [82] |
| Eu | CHCl3, CCl4 | Eu (TTA)3·TBP/ Eu (TTA)3·2TBP/ Eu (TTA)3·TOPO/ Eu (TTA)3·2TOPO | [83] |
| Tm, Eu | C6H6, cyclohexane | Tm(TTA)3/Tm(TTA)3·TBP/ Tm(TTA)3·TOPO/ Tm(TTA)3·2TBP/ Tm(TTA)3·2TOPO | [58] |
| 152,154 Eu | pentane, hexane, heptane, cyclohexane, isopropylbenzene, carbon tetrachloride, toluene, benzene, chloroform, chlorobenzene, methylene chloride, o-dichlorobenzene, bromoform | Eu (TTA)3·TOPO/ Eu (TTA)3·2TOPO | [87] |
| 169Yb, 140La | cyclohexane | Ln (TTA)3·TOPO/ Ln (TTA)3·2TOPO | [90] |
| All Ln3+ | benzene | Ln (TTA)3/ Ln (TTA)3·2TPPO | [91] |
| La, Nd, Eu, Dy, Lu | [C1C4im+][Tf2N−] | Ln (TTA)3 | [95] |
| La, Nd, Eu, Dy, Lu | [C1C4im+][Tf2N−] | Ln (TTA)4− | [96] |
| La,Nd, Eu, Dy, Lu | [C1C4im+][Tf2N−] | Ln (TTA)3·2TOPO/ Ln (TTA)2·2TOPO (Ln = Nd)/ Ln (TTA)2·3TOPO (Ln = La,Nd, Eu, Dy,Lu)/ Lu (TTA)·3TOPO | [96] |
| Cation | Diluents | Equilibrium | Refs. |
|---|---|---|---|
| Eu | CHCl3 | EuL3/ EuL3·TBP (L: acetylacetone, benzoylacetone, trifluoroacetylacetone, benzoyltrifluoroacetone, froyltrifluoroacetone, thenoyltrifluoroacetone) | [77] |
| La, Eu, Tb, Lu | C6H6 | LnL3/LnL3·TBP/LnL3·2TBP/LnL3·TOPO/LnL3·2TOPO L: benzoyltrifluoroacetone | [98] |
| La, Ce, Pr, Nd, Sm, Eu, Gd | C6H6 | LnL3/LnL3·2TOPO/LnL3·2TBPO/LnL3·TPPO L:4,4,4-trifluoro-1-(biphenyl-4-yl)butane-1,3-dione | [42] |
| La, Pr, Eu, Ho, Yb | CHCl3 | LnL3/ ML3·TOPOL:2-trifluoroacetyl-cyclopentanone, 2-trifluoroacetyl-cyclohaxanone, 2-trifluoroacetyl-cycloheptanone | [99] |
| La, Nd, Eu, Ho, Lu | CHCl3 | LnL3/LnL3∙2S1; L: HTTA; S: S1 | [13] |
| All Ln3+ | CCl4 | LnL3/LnL3·2S; L: HTTA; S: S7 | [61] |
| All Ln3+ | CCl4 | Ln (TTA)3·S; S: S8 | [59] |
| La, Nd, Eu, Dy, Lu | [C1C4im+][Tf2N−] | Ln (ba)2+; L: benzoylacetone | [100] |
| La, Nd, Eu, Dy, Lu | LnL3; L: benzoylacetone | ||
| La, Nd, Eu, Dy | [C1C4im+][Tf2N−] | LnL2(TOPO)2+ L: benzoylacetone | [100] |
| La, Nd, Eu, Dy, Lu | LnL(TOPO)4+;L: benzoylacetone |
| Ln3+ | logKT | logKT,S | logβT,S | pH50 | ||||
|---|---|---|---|---|---|---|---|---|
| HTTA–bipy | HTTA–phen | HTTA–bipy | HTTA–phen | HTTA | HTTA–bipy | HTTA–phen | ||
| La | −11.06 | −5.85 | −3.95 | 5.21 | 7.11 | 5.08 | 4.13 | 3.48 |
| Nd | −10.12 | −3.71 | −1.78 | 6.41 | 8.34 | 4.77 | 3.40 | 2.76 |
| Eu | −8.68 | −2.23 | −0.43 | 6.45 | 8.25 | 4.29 | 2.92 | 2.31 |
| Ho | −8.56 | −1.75 | 0.08 | 6.81 | 8.64 | 4.25 | 2.74 | 2.13 |
| Lu | −8.15 | −0.96 | 0.64 | 7.19 | 8.79 | 4.11 | 2.52 | 1.95 |
| Ln3+ | SC | SF | ||||
|---|---|---|---|---|---|---|
| HTTA–bipy | HTTA–phen | HTTA | HTTA–bipy | HTTA–phen | ||
| La | 2.91 | 4.82 | Nd/La | 8.70 | 138.09 | 147.9 |
| Nd | 4.11 | 6.04 | Eu/Nd | 27.54 | 30.19 | 22.38 |
| Eu | 4.15 | 5.95 | Ho/Eu | 1.31 | 3.02 | 3.23 |
| Ho | 4.51 | 6.34 | Lu/Ho | 3.54 | 6.16 | 3.63 |
| Lu | 4.89 | 6.49 |
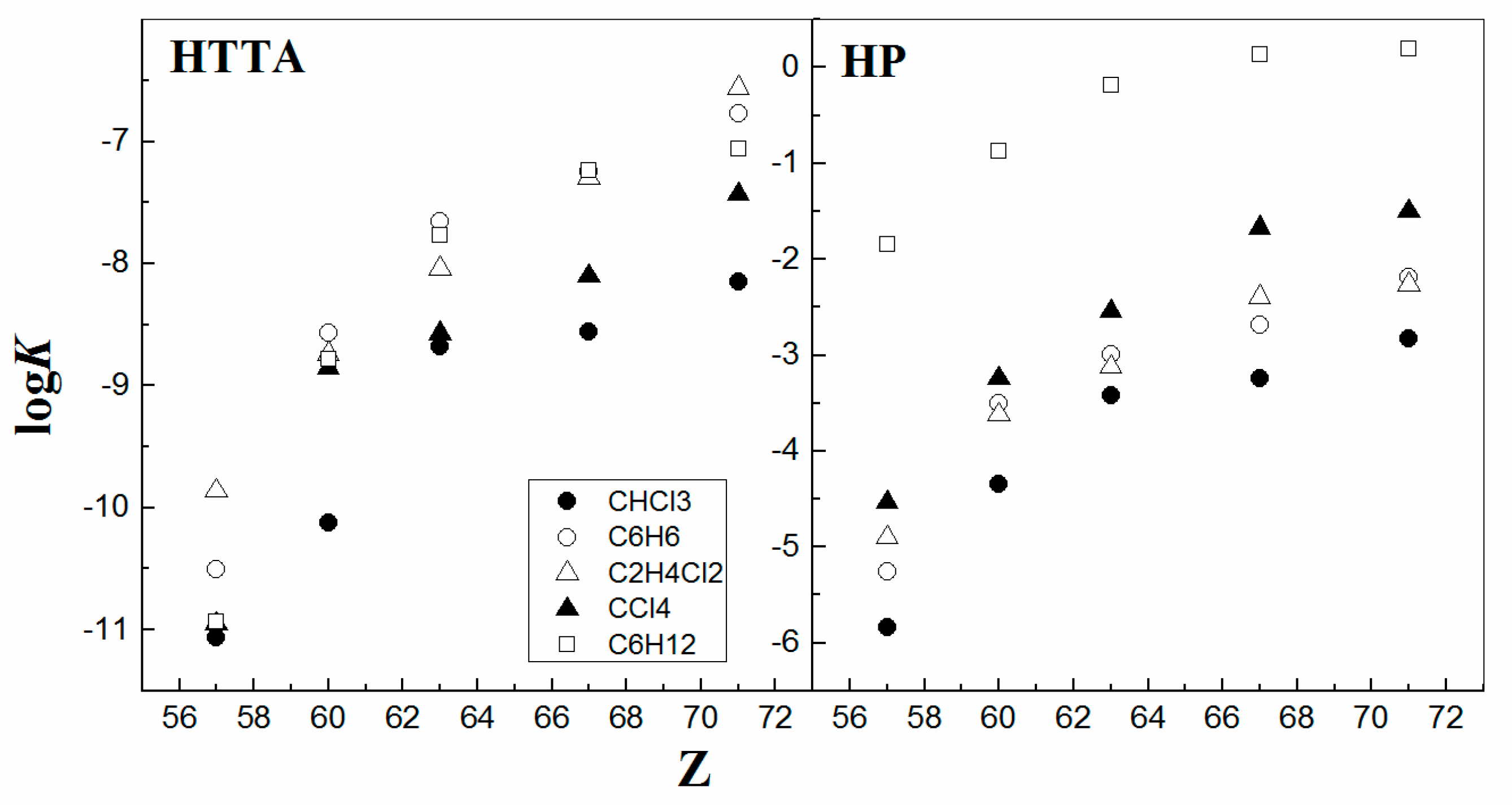
| Diluent | C6H12 | CCl4 | CHCl2CCl3 | C6H6 | CHCl3 | CHCl2CHCl2 |
|---|---|---|---|---|---|---|
| log[H2O]o, mol∙dm−3 | −2.8 | −2.1 | −1.55 | −1.46 | −1.14 | −1.02 |
| logK ≤ 10% | −7.6 | −8.2 | −8.5 | −7.4 | −8.6 | −8.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanassova, M. Thenoyltrifluoroacetone: Preferable Molecule for Solvent Extraction of Metals—Ancient Twists to New Approaches. Separations 2022, 9, 154. https://doi.org/10.3390/separations9060154
Atanassova M. Thenoyltrifluoroacetone: Preferable Molecule for Solvent Extraction of Metals—Ancient Twists to New Approaches. Separations. 2022; 9(6):154. https://doi.org/10.3390/separations9060154
Chicago/Turabian StyleAtanassova, Maria. 2022. "Thenoyltrifluoroacetone: Preferable Molecule for Solvent Extraction of Metals—Ancient Twists to New Approaches" Separations 9, no. 6: 154. https://doi.org/10.3390/separations9060154
APA StyleAtanassova, M. (2022). Thenoyltrifluoroacetone: Preferable Molecule for Solvent Extraction of Metals—Ancient Twists to New Approaches. Separations, 9(6), 154. https://doi.org/10.3390/separations9060154