Abstract
Commercial solid-phase microextraction fibers are available in a limited number of expensive coatings, which often contain environmentally harmful substances. Consequently, several different approaches have been used in the attempt to develop new sorbents that should possess intrinsic characteristics such as duration, selectivity, stability, and eco-friendliness. Herein we reported a straightforward, green, and easy coating method of silica fibers for solid-phase microextraction with polydopamine (PDA), an adhesive, biocompatible organic polymer that is easily produced by oxidative polymerization of dopamine in mild basic aqueous conditions. After FT-ATR and SEM characterization, the PDA fibers were tested via chromatographic analyses performed on UHPLC system using biphenyl and benzo(a)pyrene as model compounds, and their performances were compared with those of some commercial fibers. The new PDA fiber was finally used for the determination of selected PAHs in soot samples and the results compared with those obtained using the commercial PA fiber. Good reproducibility, extraction stability, and linearity were obtained using the PDA coating, which proved to be a very promising new material for SPME.
1. Introduction
The performances of analytical methods depend on the different steps of the process, such as sampling, sample preparation, instrumental analysis, and data processing [1]. Interference’s removal and analyte’s preconcentration are the main goals of sample preparation that is indeed a prerequisite for the attainment of reliable results and is often considered the bottleneck of analytical workflows. Chemical analysis could be greatly improved by technological advances of modern sample preparation techniques. Solid-phase extraction (SPE), with various solid-phase sorbents, has been widely used as sample preparation approach over the years, while other sorption-based techniques, such as solid-phase microextraction (SPME), stir-bar sorptive extraction (SBSE) and matrix solid-phase dispersion (MSPD) have been more recently introduced.
Solid-phase microextraction (SPME) is an evolving solventless technique for the extraction of organic compounds through the equilibration of the analytes between the sample matrix and a polymeric phase, generally coated onto a fiber. Its main advantages are rapidity, low cost, ability to perform in situ measurements, ease of use, and automation. SPME is proposed in many variants, in particular in-tube [2], hollow fiber [3], thin-film [4], coated-tip [5], and magnetic nanoparticles [6]. However, the fiber geometry is still the most widespread feature, even if the number of commercially available sorbents remains surprisingly limited. Thus, a new multidisciplinary research trend, involving materials science, nanotechnology, polymer synthesis, and analytical chemistry, has been recently introduced through the development and characterization of new SPME extraction sorbents [7,8,9,10,11,12], which should possess intrinsic characteristics such as long lifetime, selectivity, chemical, thermal, and mechanical stability, and good adsorptive properties. Consequently, many innovative sorbents have been developed, including carbon-based [13], metal organic frameworks [14,15], molecular imprinted polymers [15], ionic liquids [16,17,18], immunosorbents, sol-gel-based compounds [19,20], and nanomaterial-based [21], among others.
Unfortunately, many coatings are produced using environmentally harmful substances or precursors (organic alkoxysilanes, radical organic species, acrylic monomers). The production of fibers for SPME applications prior involves the synthesis of the bulk material (silica, stainless steel) and then the usual sol-gel coating processing for synthesizing the extractive layer. The bulk fused silica, for instance, should first undergo exposition of silanol groups onto the surface for further functionalization for creating the primary sites on sol solution and ensuring a dense sol–gel coating through chemical bonding, requiring alkali or acid solutions, organic and/or inorganic precursors, active organic solvents, a catalyst, and water [22].
Very recently, polydopamine (PDA) has been proposed as an interesting new material in sorption-based extraction methods for its extraordinary chemical and environmental stability, convenience for further modification [23] and eco-friendliness. In fact, polydopamine coating does not need pretreatments of the silica bulk fibers for material processing. PDA is a bioinspired polymer which, in vitro, mimics the protein sub-structures exploited by mussels for adhesion. It is produced via oxidative polymerization of dopamine (DA), a biological catecholamine neurotransmitter [24]. DA is a water-soluble monomer, easily polymerizable under alkaline conditions at room temperature, avoiding the use of toxic solvents and precursors, and directly exploiting the molecular oxygen as an abundant, cheap, and ecofriendly catalyst for polymerization [25]. The resulting polydopamine coating is soft, adhesive, biocompatible, and suitable for further chemical functionalization [26]. Quite recently, the presence of quinones and hydroxyindoles moieties into the polymer structures has been exploited for producing PDA coating surfaces capable of attracting and stably bonding aromatic pollutants via π-π stacking interactions. The PDA layers have been grown onto manifold surfaces, ranging from zeolites [27], ferromagnetic nanoparticles [28], nickel foam [29], and stainless-steel wires [30].
In this work, we prepared a novel SPME fiber with a silica bulk and a PDA coating, by a process of air-assisted self-polymerization of dopamine in aqueous medium. Surface properties of the coated fiber were characterized using scanning electron microscopy (SEM) and FT-ATR spectroscopy, while the extraction performances of the fiber were tested using polycyclic aromatic hydrocarbons (PAHs), namely Biphenyl and Benzo[a]Pyrene. PAH-enriched fibers were desorbed in a commercially available SPME interface for liquid chromatography (LC) and analyzed by LC with UV-diode array detection (UV-DAD). Since carbon compounds released into the atmosphere in the form of soot have a negative impact on the environment and human health, and simple, low-polluting, and fast methods of analysis for the determination of the main atmospheric pollutants are of great interest, the present method was eventually applied to the determination of the PAHs model, i.e., pyrene, chrysene, naphtalene, acenaphthene, biphenyl, fluorene, anthracene, benzo[a]pyrene, benzo[k]fluoranthene, and benzo[g,h,i]perylene, in soot samples.
2. Materials and Methods
2.1. Materials
Tris(hydroxymethyl)aminomethane hydrochloride (TRIS:HCl), potassium hydroxide, sodium chloride, dopamine hydrochloride (DA), ethanol, biphenyl, acenaphthene, anthracene, benzo(k)fluoranthene, benzo(ghi)perylene, benzo(a)pyrene, chrysene, naphthalene, and pyrene were purchased from Sigma-Aldrich. Stock solution (0.25 mg/mL) was prepared in ethanol and stored at 8 °C.
2.2. Polydopamine Fibers Coating
Silica fibers (Supelco, Bellefonte, PA, USA) were washed 2 times in stirred TRIS and water buffer (25 mM, pH 8.5). Then, the polydopamine coating (PDA) was carried out by adding dopamine hydrochloride (7.5, 15, 30 mg, for obtaining different morphologies and thickness of the polymer coatings) in the TRIS buffer solution (10 mL; pH 8.5, 25 mM), and gently immersing the silica tips into this stirred solution for 2 h at room temperature. Several washing steps with the TRIS buffer, bidistilled water, water/ethanol (50:50, v/v), ethanol, and ethanol/acetonitrile (50:50, v/v) water:acetonitrile:methanol (25:10:65, v/v/v), were performed after the coating treatment. The polymerization of dopamine around the tips was visible macroscopically following the browning of the silica bulks and confirmed by SEM and FTIR-ATR analysis.
2.3. Solid-Phase Microextraction
Home-made silica fibers coated with PDA and commercial silica fibers coated with polydimethylsiloxane (PDMS, 7 µm thickness), polydimethylsiloxane/divinylbenzene (PDMS/DVB, 60 µm), polyacrylate (PA, 85 µm), and polyethylene glycol (PEG, 60 µm) were conditioned for 30 min in the SPME-LC interface under a flow of 0.6 mL/min of mobile phase.
Analytes working solutions were prepared in 15 mL amber glass vials with screw cap and pierceable septum (PTFA) (Supelco) filled with a 10% NaCl solution. Extractions were carried out at room temperature for 30 min under magnetic stirring (800 rpm) using a cylindrical stirred bar (10 × 4 mm).
Desorption was carried out in the SPME-LC interface filled with 500 µL of mobile phase for 20 min in static mode, before switching the valve to the inject position. After 60 s, the valve was switched back to the load position, and the fiber was removed and cleaned with ethanol (5 min), mobile phase (10 min), and water (5 min). A second run was conducted to verify carryover.
All experiments were always conducted in triplicate.
2.4. Apparatus and Instrumental Conditions
Fourier Transformed Infrared-Attenuated Total Reflectance (FTIR-ATR) spectra were acquired with a Perkin Elmer Spectrum Two Spectrophotometer equipped with a 2 × 2 mm diamond crystal. Spectra were recorded in the range 4000–400 cm−1 with a 2 cm−1 resolution, using 0.25 cm−1 acquisition intervals and acquiring 32 scans for each sample. Scanning electron microscope (SEM), analyses were carried out by a VP Field emission SEM EDS Zeiss Sigma 300 equipped with an in lens backscattered and secondary electron detectors. An accelerating voltage of 7 kV and a 5 mm working distance were used. FE-SEM samples were placed onto stainless-steel sample holders with carbon tape. A graphite sputtering was performed on samples before analyses. UV–vis spectra were recorded on a Shimadzu UV—2401 PC spectrophotometer (acquisition parameters: slit width 1 nm; scan sampling 1 nm; single beam mode).
2.5. Chromatographic Conditions
Chromatographic analyses were performed using an UHPLC system (Shimadzu LC20ADXR, Milan, Italy) equipped with a binary pump, an SPME interface (Supelco, Bellefonte, PA, USA), and a UV-diode array detector (PDA-1, Shimadzu, Milan, Italy) with a flow cell (10 mm, 1/1600, steel, 2.4 mL, LWL). The chromatographic column was a Accucore XLC18 (150 × 4.6 mm, 4 µm) (Thermo Scientific, Milan, Italy) equipped with a Accucore XLC18 precolumn (10 × 4 mm, 4 µm) (Thermo Scientific). The mobile phase used was water:acetonitrile:methanol:2-propanol mixture (15:10:65:10, v/v/v/v). The flow rate was 1.2 mL/min. Spectra were acquired in the range of 220–450 nm, while meticulous composite chromatograms were obtained using the Max Plot software of the PDA-1 detector, which automatically selects and stores the maximum absorbances for each peak, thus providing the maximum signal for all compounds detected. The selected wavelengths were 226, 251, 306, 298, 295, 247, 267, 275, and 239 nm for acenaphthene, anthracene, benzo(k)fluoranthene, benzo(ghi)perylene, benzo(a)pyrene, biphenyl, chrysene, naphthalene, and pyrene, respectively.
2.6. Sample Preparation
Soot samples were obtained by scratching the walls of private fireplaces. Soot (34 mg) was directly weighed into a 15 mL amber vial and 1.5 mL of ethanol was added. After the insertion of a magnetic stir bar, the vials were sealed with a screw cap equipped with a pierceable PTFE septum, placed on a magnetic plate, and the solution was stirred for 30 min. Then, 13.5 mL of a 10% NaCl solution was added through the septum with a syringe and the sample finally subjected to DI-SPME.
For methods calibration, PAHs-free soot samples were prepared performing a series of repeated extractions. Briefly, 5 g of soot was extracted 5 folds with 50 mL of an acetonitrile: methanol:2-propanol (15:65:20, v/v/v) solution. After centrifugation (3.500 rpm, 5 min), the obtained PAHs free soot samples were dried, divided into aliquots, and added with variable amounts of PAHs to cover the concentration range 0.5–1000 µg/g for the calculation of calibration curves, coefficient of variations and recoveries. Three replicates for each concentration level were performed. The within-day (n = 6) and between-days (n = 6 over 6 days) coefficient of variation were at the concentration levels of 50, 100, and 200 µg/g. Recoveries were calculated at the same concentration levels as peak area ratios between analytes in spiked samples and analytes in standard solutions.
3. Results
3.1. Characterization of the PDA Coated Fiber
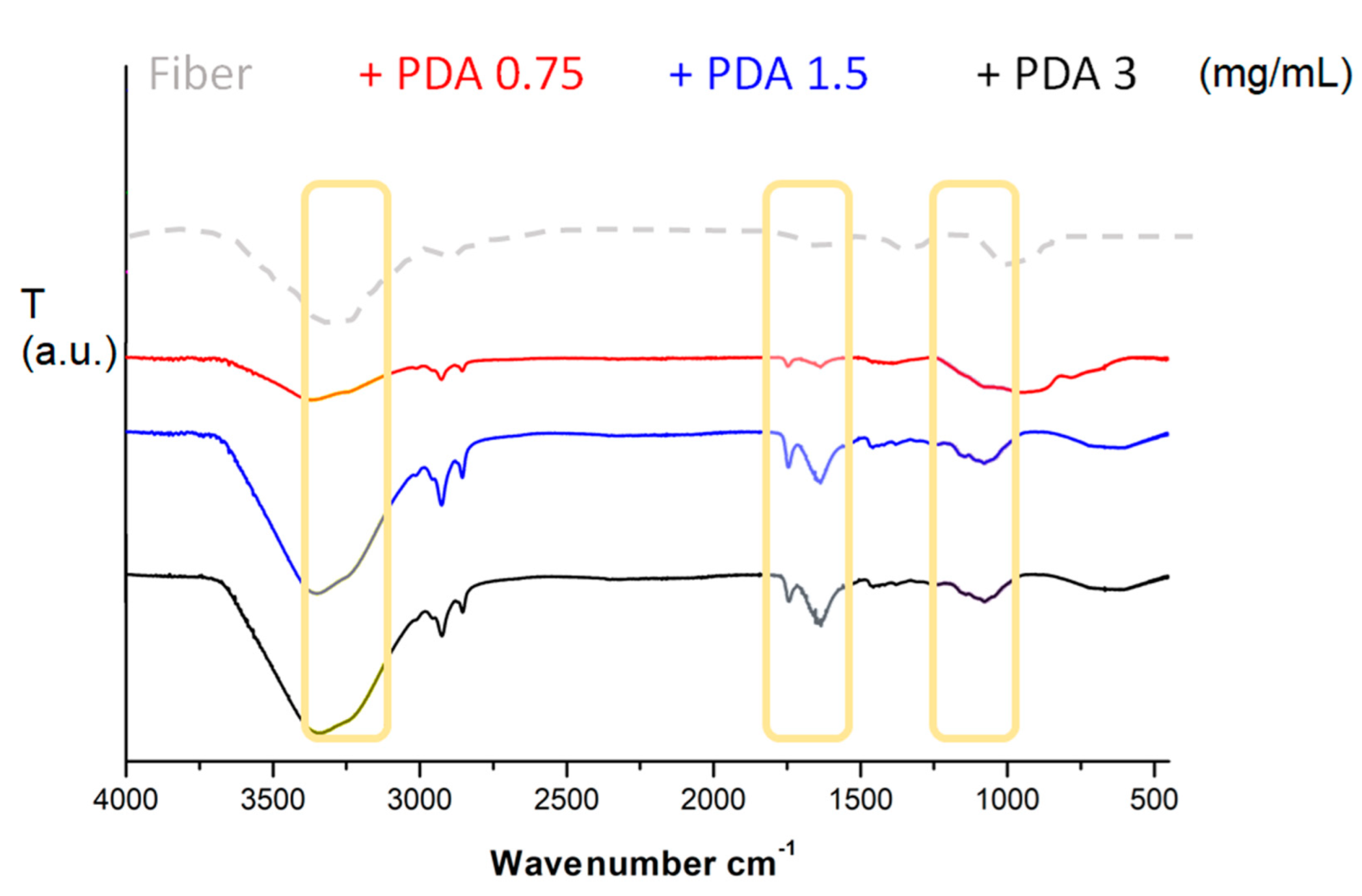
FT-ATR spectra (Figure 1) of bare and coated fibers contained Si-O stretching signal visible at 1150–1210 cm−1. Only coated fibers contained multiple and complex signals around 1435, 1625, and 1730 cm−1, corresponding to pyrrole intra-moiety, C=C quinone moiety, and C=O stretch, and related to polydopamine backbones. Moreover, signal of water around 3455 cm−1 exhibited a shoulder signal around 3260 cm−1, corresponding to internal amine-based moieties [31,32,33].
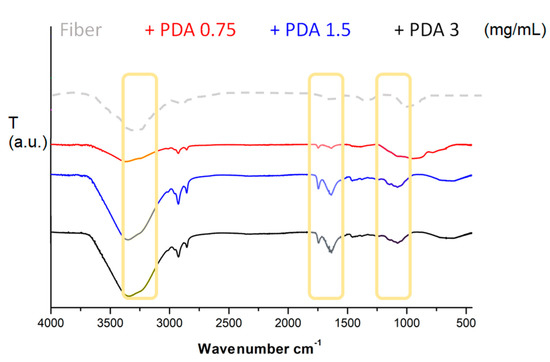
Figure 1.
FT-ATR output of bare silica fiber tip and PDA:coated silica fiber tip after the three different concentrations of dopamine monomer.
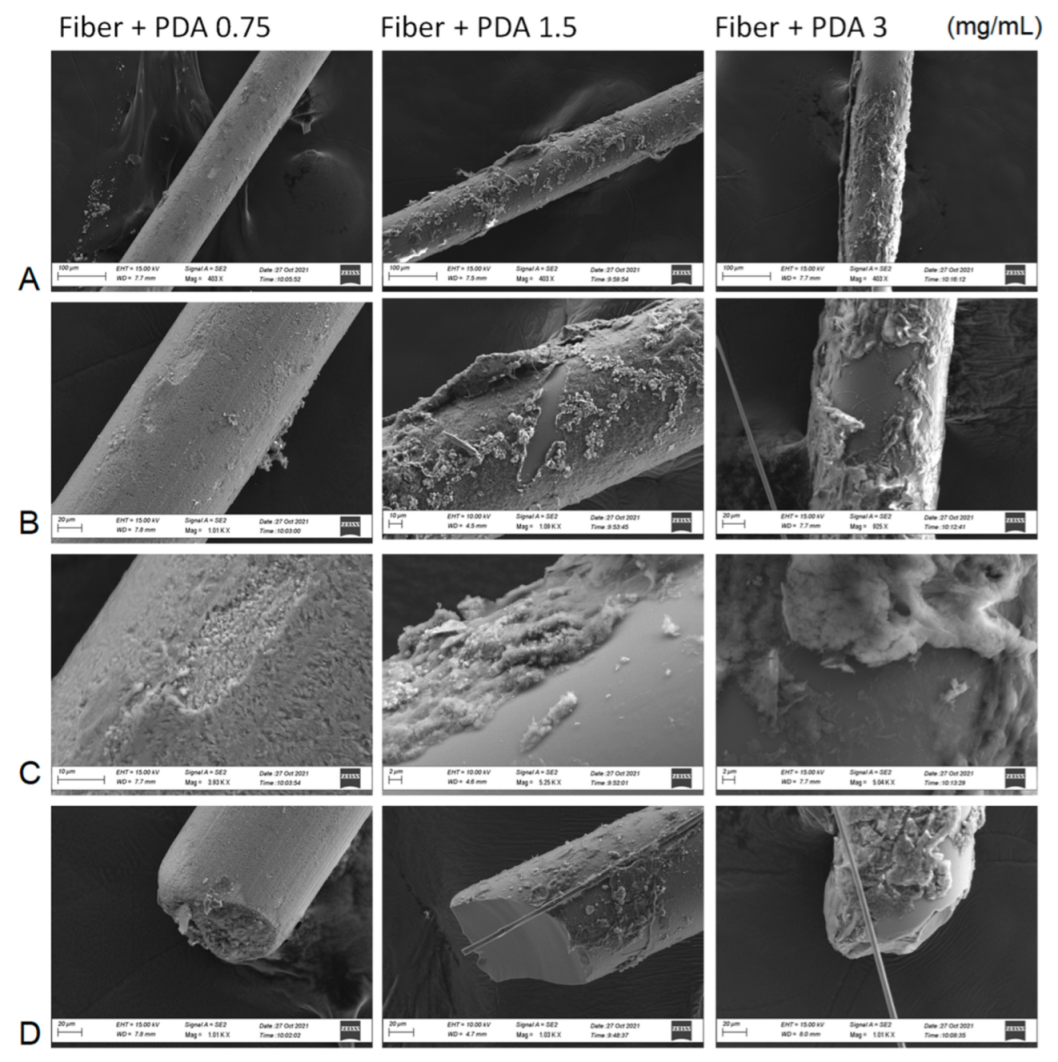
SEM characterization (Figure 2) showed differently coated bulks passing from 0.75 to 3 mg/mL of dopamine monomer used for polydopamine coatings. The scratched areas were analyzed to underline the contrasted images between the organic layers, becoming more heterogenous and robust by increasing the dopamine concentration, and the silica smooth bulk. Focus and tips pictures were exploited to calculate the average thickness of the PDA layers: 85 ± 110 nm for 0.75 mg/mL, 245 ± 125 nm for 1.5 mg/mL, and 510 nm ± 210 nm for 3 mg/mL. Fiber coating was also accomplished using higher dopamine concentrations, but the resulting polymers were easily released from the surface of the bulk material due to a strongly heterogenous polymer nucleation and were not used for the analysis.
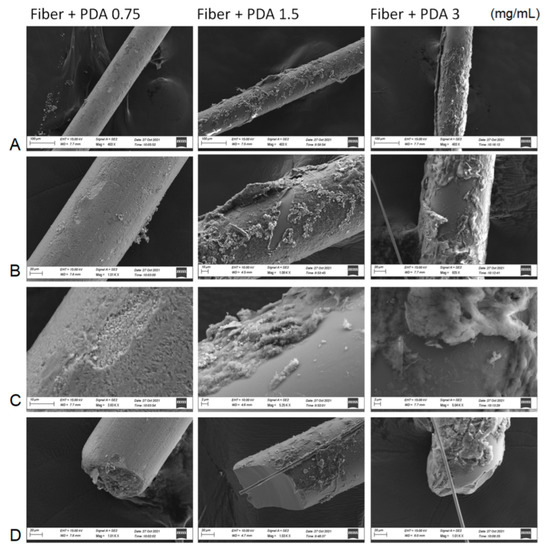
Figure 2.
SEM characterization of bare silica fiber and PDA:coated silica fiber after the three different concentrations of dopamine monomer. A set of three areas of the fibers ((A): bulk, (B): scretched area with (C): focus; (D): tip) has been investigated.
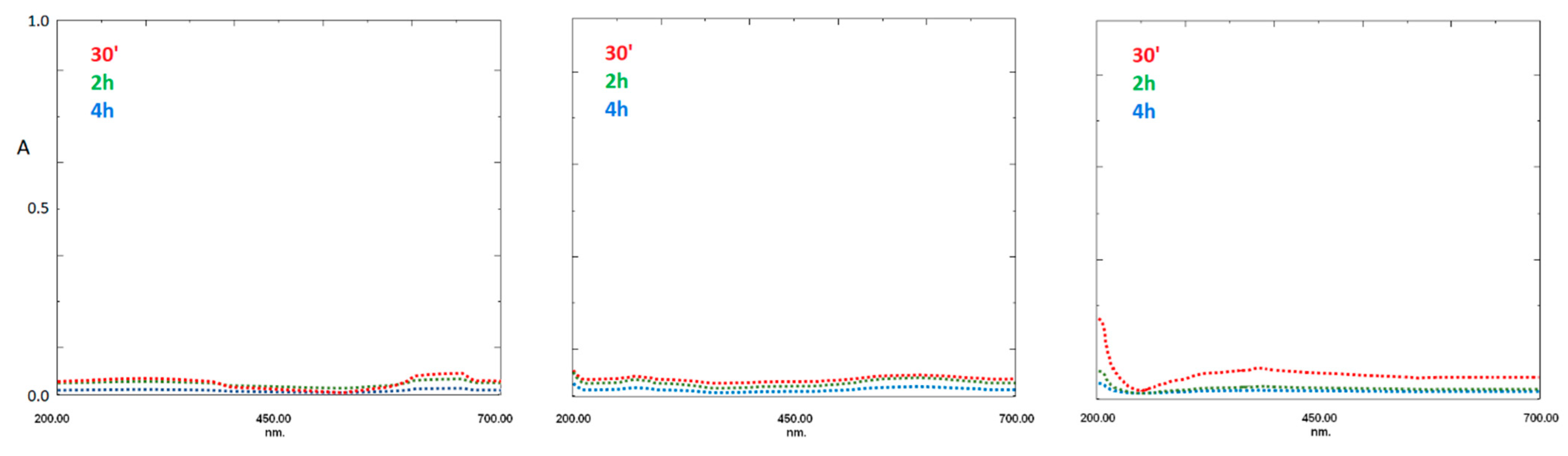
PDA-coated fibers having three different thicknesses were prepared using three dopamine concentrations, i.e., 0.75 mg/mL, 1.5 mg/mL, and 3 mg/mL, respectively. Before use, the effects of the solvent on PDA-SPME fibers were evaluated. For this purpose, the coating’s lifetime was investigated by immersing the fiber in the mix of water:acetonitrile:methanol (25:10:65, v/v/v), and measuring the UV signal of the eventually released dopamine, dopachrome, or dopamine-oligomers into aqueous solutions in a range of 200–700 nm. The absence of the typical peaks over 230 and around 340 nm (at 2 and 4 h) underlines the lack of leakage of monomers and monomer adducts from the fiber coatings (Figure 3).
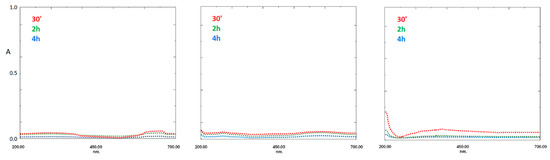
Figure 3.
Stability test of bare silica fiber tip and PDA:coated silica fiber tip after the three different concentrations of dopamine monomer, tested using reference washing solutions at three different times ((left), 0.75 mg/mL, (center) 1.5 mg/mL, (right) 3 mg/mL).
3.2. Optimization of the SPME Procedure
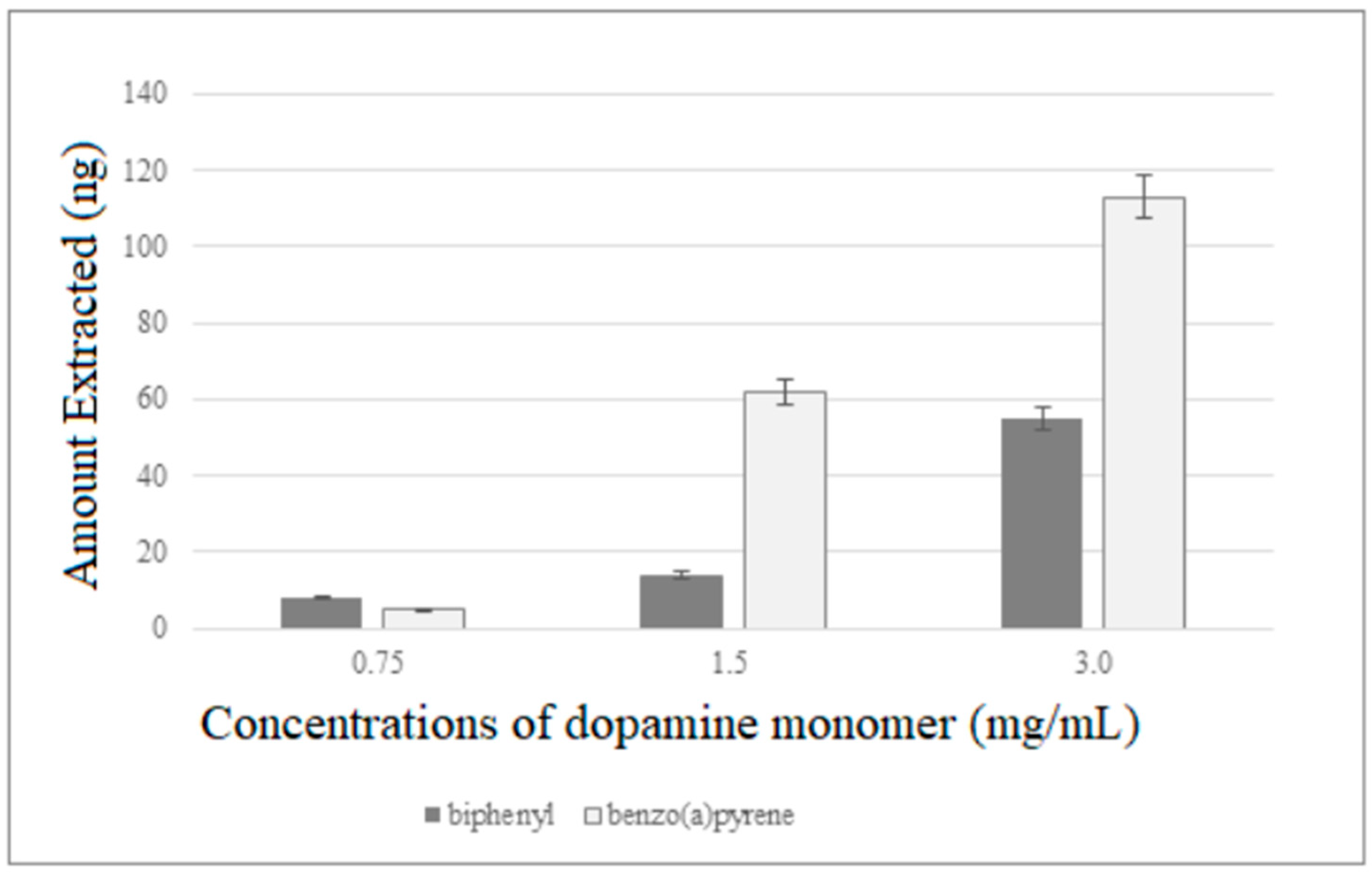
Biphenyl and benzo(a)pyrene were used as model compounds to test the performances of the new fiber. Initially, PDA fibers of different thickness were tested conducting extractions on working solution (1 µg/mL) of the two analytes for 30 min at room temperature and under constant stirring (800 rpm). The fibers were then transferred into the SPME/LC interface to be desorbed in dynamic mode by switching the valve from the INJECT to LOAD position after 10 min. As inferable by Figure 4, the extraction efficiencies of the coatings were found to increase with the dopamine concentration used for their preparation, and the thickest PDA fiber was then selected for subsequent experiments.
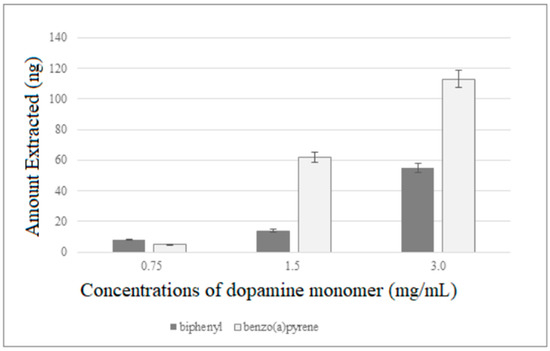
Figure 4.
Extraction efficiencies of PDA fibers of different thickness.
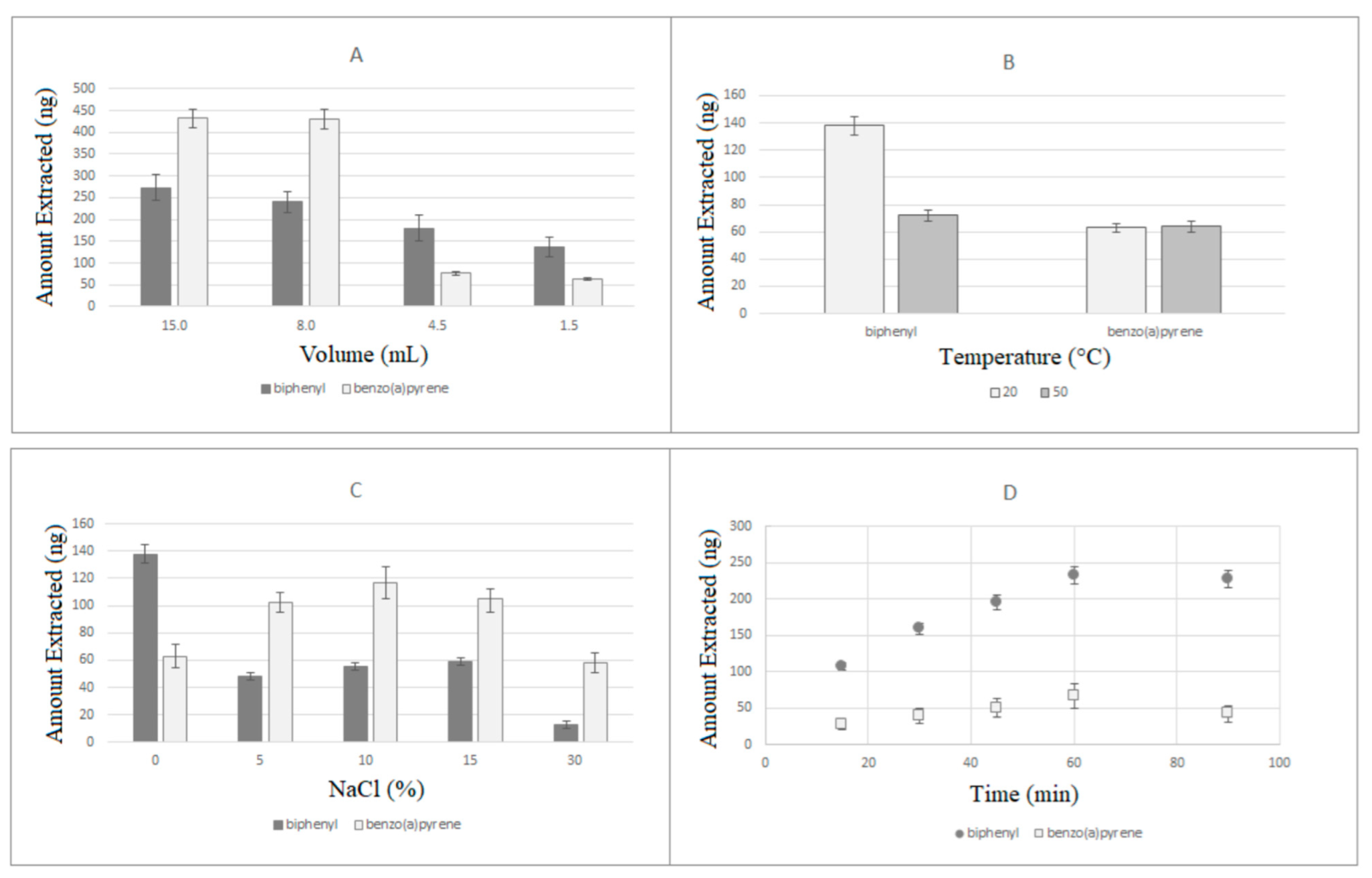
Then, the SPME-LC-UV-DAD analysis of standard solutions (1.0 µg/mL) of the analytes was performed with the selected PDA fiber in different conditions to optimize the extraction parameters, namely sample volume, temperature, ionic strength, and extraction time, before validating the analytical method. The relevant results are reported in Figure 5. Extraction performed using different volumes (ranging from 1.5 and 15 mL) of standard solutions showed a direct proportionality between sample volume and extraction efficiency (see Figure 5A), clearly indicating 15 mL as the optimal volume. Testing the temperature conditions, as showed in Figure 5B, two different extraction temperatures (20 and 50 °C) were explored in this case, and the best results were observed at 20 °C, which was selected as the working temperature for the following experiments. Regarding the salinity of the used buffer, salt addition has often shown variable effects on SPME, usually increasing the extraction efficiency due to the salting-out phenomenon, sometimes showing the opposite behavior. In this case, the presence of sodium chloride (0%, 5%, 10%, 15%, and 30% (w/v)) showed variable effects on the extraction of the two analytes (Figure 5C), leading to a general decrease at 30%, an always negative effect for biphenyl, and positive effects for benzo(a)pyrene between 5% and 15%. The addition of 10% NaCl was then individuated as a good compromise for an efficient simultaneous extraction of the analytes under study. Extraction time profiles were then established by plotting the area counts versus the extraction time, and the relevant extraction kinetics are reported in Figure 5D. Biphenyl kinetics concretely followed huge time-dependent slope variations with respect to benzo(a)pyrene moiety. However, equilibrium conditions were reached after 60 min for both the analytes and since SPME quantitation is feasible even before adsorption equilibrium is reached, an extraction time of 30 min was eventually chosen for further experiments to obtain a considerable gain on the analysis time.
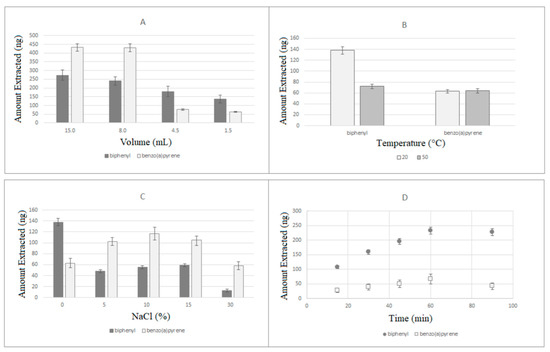
Figure 5.
Extraction efficiencies of PDA fibers obtained with varying sample volume (A), temperature (B), ionic strength (C), and extraction time (D).
Two desorption steps are distinguishable using the on-line SPME/LC commercial interface: static, in which the fiber is soaked in mobile phase in the interface for a certain time before switching the valve in the inject position, and dynamic, when the fiber coating is exposed to the moving stream of mobile phase. These phases must be carefully blended to balance chromatographic efficiency and satisfactory desorption yields. The best conditions were obtained by combining 20 min of static and 6 s of dynamic desorption. To minimize the carry-over effects, the fibers were soaked in ethanol for 5 min and rinsed with water after each extraction. It is worth noting that the fiber allowed up to 100 consecutive extractions before the polymer was damaged.
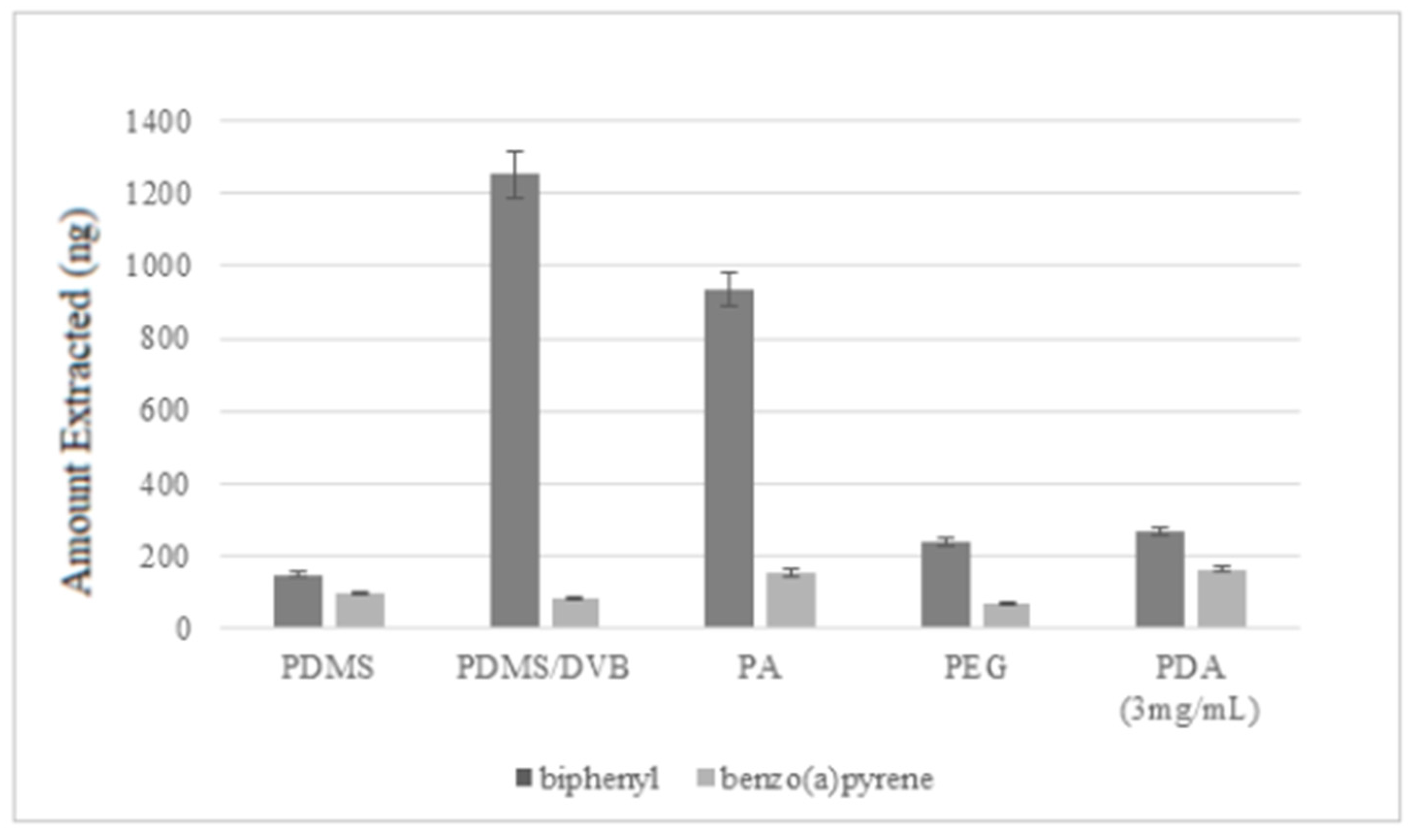
Furthermore, the performances of the home-made PDA SPME fiber were compared with those of four commercial SPME fibers, namely polydimethylsiloxane (PDMS), polydimethylsiloxane/divinylbenzene (PDMS/DVB), polyacrylate (PA), and polyethylene glycol (PEG), and the obtained results were reported in Figure 6 and discussed in the Discussion section. Extractions with all the fibers were performed using the conditions optimized for the PDA fiber, since they also produced good extractions yields for commercial fibers in a reasonable amount of time.
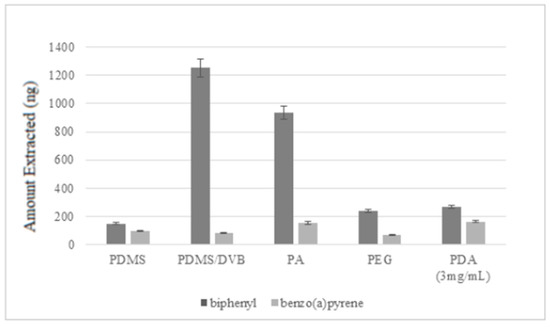
Figure 6.
Comparison of the extraction efficiencies of the PDA fiber with those of four commercial SPME fibers, i.e., polydimethylsiloxane (PDMS), polydimethylsiloxane/divinylbenzene (PDMS/DVB), polyacrylate (PA), and polyethylene glycol (PEG).
Table 1 reports the validation parameters obtained with the PDA fiber; compared with those obtained with the PA, the commercial fiber showed the best extraction performances for the target analytes.

Table 1.
Comparison of the analytical performances obtained with PDA and PA fibers.
3.3. Soot Samples Analysis
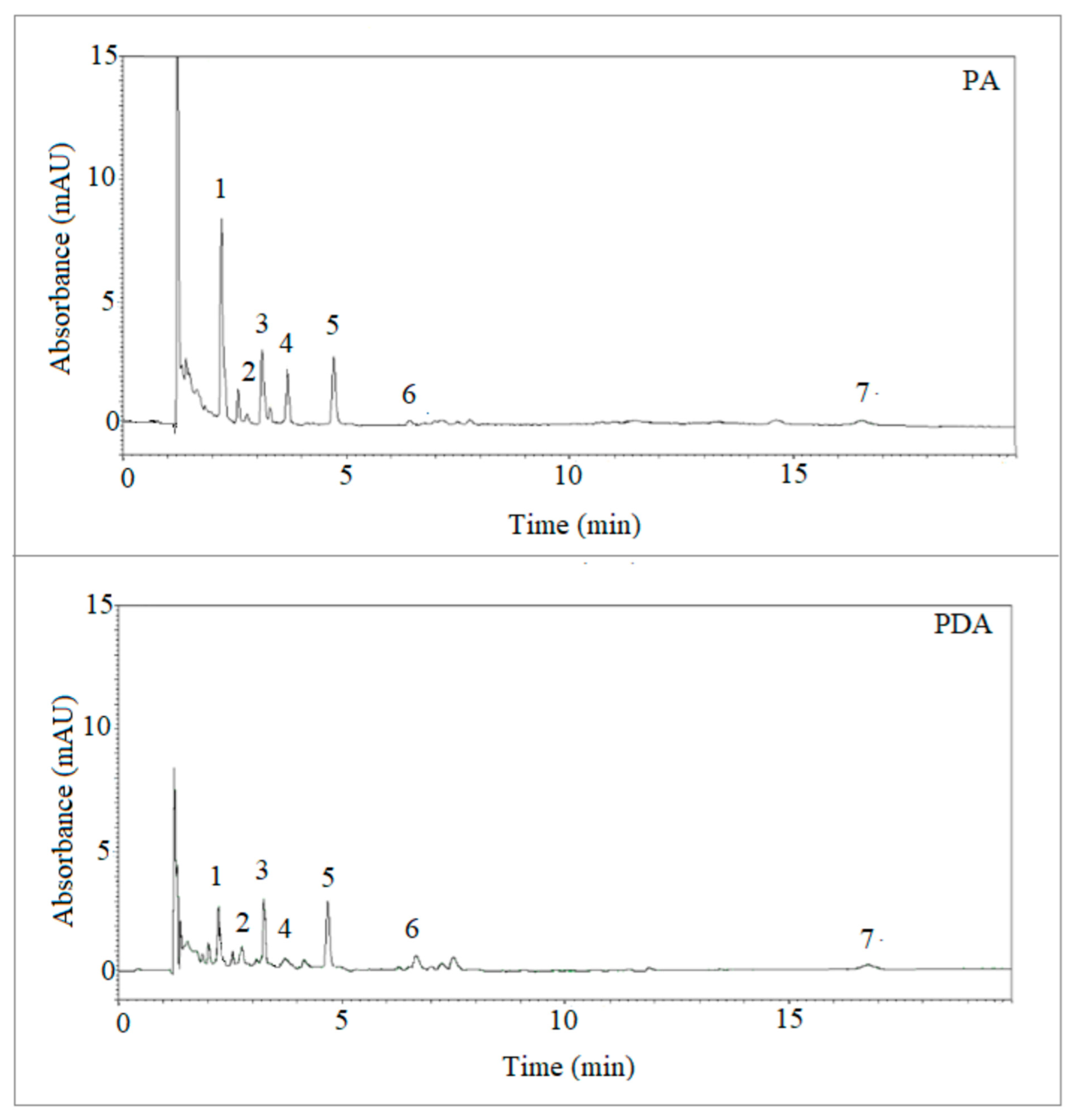
The analysis of selected PAHs was then successfully performed in soot samples with the PDA fiber using the optimized conditions, and the results compared with those obtained using the commercial PA fiber. Table 2 reports the validation parameters obtained with the two fibers using PAHs-free soot samples prepared as described in the experimental section. Figure 7 reports the LC-UV-DAD “max plot” chromatograms, generated by plotting the maximum spectral absorbance measured at each time point, of the same soot sample extracted with the PA and PDA fibers, respectively. As observable, the detection of various target analytes from soot samples was possible using both the extraction devices, which showed slightly different extraction yields. Then, the quantification of PAHs found in soot using the PDA fiber was accomplished and the relevant results reported in Table 3, demonstrating the potential of the fiber developed in the present work.

Table 2.
Validation parameters obtained with PA and PDA fibers, respectively, for the analysis of PAHs in soot samples.
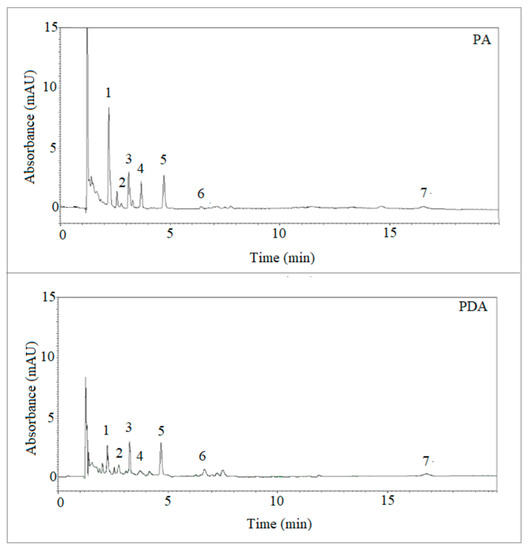
Figure 7.
LC-UV-DAD chromatograms (max plots) of the same soot samples extracted with the PA and PDA fibers, respectively. The numbers refer to the analytes detected at ʎ max for each peak and are: (1) naphthalene (275 nm); (2) biphenyl (247 nm); (3) acenaphthene (226 nm); (4) anthracene (251 nm); (5) pyrene (239 nm); (6) chrysene (267 nm); (7) benzo(ghi)perylene (298 nm).

Table 3.
Estimated concentration of PAHs found in soot using the PDA fiber (n = 3).
4. Discussion
In this paper, a novel SPME fiber bearing a silica core and a cheap, fully organic polymer as coating active layers was proposed. Silica fiber is a robust, smooth, functionalizable matrix which can undergo further functionalization processes assisted by different manufacture techniques. Here, the active layer was polydopamine (PDA), a mussel inspired soft polymer produced by cheap, simple air-assisted self-polymerization of dopamine in aqueous medium at ecological and mild conditions. Scanning electron microscopy (SEM) and FT-ATR spectroscopy were used here for the surface characterization of the systems, demonstrating the different morphologies exhibited passing from low to high dopamine concentration in the reaction vessels, and the presence of PDA functional groups on silica surfaces, respectively (Figure 1 and Figure 2). Thanks to steady-state UV-visible assays, an absence of the leakage of monomer or oligomer species from PDA coatings was demonstrated using different solvent mixtures (Figure 3). Then, these new SPME hybrid fibers were tested for the extraction of polycyclic aromatic hydrocarbons (PAHs) in combination with LC-UV-DAD using a commercial SPME-LC interface. First, the extraction aptitude of the fibers was tested on biphenyl and benzo(a)pyrene species, in relation with dopamine concentration used for producing the fibers, the thickness, and consistency of the active PDA layers (Figure 4), underlining that the extraction efficiencies increased along the dopamine concentration used for their preparation, and consequently the thickest PDA fiber was selected for all the experiments (dopamine 3 mg/mL). PDA fibers using the chosen dopamine concentration were tested using different optimization conditions, such as sample volume, temperature, ionic strength, and extraction time, before validating the analytical method. As previously mentioned, commenting on Figure 5, the volume obviously positively affected the extraction yields (Figure 5A), while the temperature, which influences both kinetics and thermodynamics of the extraction process, had a negative effect. In fact, a temperature increase (from 20 to 50 °C) produced a decrease in the extraction yields (Figure 5B), and 20 °C was consequently selected as the working thermal condition for the following experiments. It is likely that, in this case, even if higher temperatures reduce the time needed to reach equilibrium by enhancing the diffusion of the analyte towards the organic phase, they could have altered the distribution coefficients of the analytes between the aqueous phase and the fiber. The influence of salt addition has been also investigated, discovering a different behavior of the fibers for biphenyl and benzo(a)pyrene, respectively. The presence of salts led to a general decrease on the extraction efficiency of biphenyl, and a positive effect in the case of benzo(a)pyrene (Figure 5C). Extraction time profiles were visualized in Figure 5D as graphs obtained by plotting the area counts versus the extraction time, describing specific kinetics with huge slope variabilities vs time for biphenyl with respect to benzo(a)pyrene. SPME is a non-exhaustive process in which analytes are partitioned between the sample and the polymer coating. The equilibrium time refers to the time after which the amount of analyte extracted remains constant and corresponds to the amount extracted after an infinite time, within the limits of the experimental error. Apparently, equilibrium conditions for these molecules were reached after 60 min, and 30 min was chosen for further experiments.
After the optimization of the analytical parameters, a complete investigation on the present PDA-based extractive systems in comparison with four commercial SPME fibers (polydimethylsiloxane (PDMS), polydimethylsiloxane/divinylbenzene (PDMS/DVB), polyacrylate (PA), and polyethylene glycol (PEG)) was performed. The PDA coating allowed better extraction efficiencies than commercial fibers for benzo(a)pyrene, while the most efficient extraction of biphenyl was shown by the PA and PDMS/DVB fibers, probably due to the higher thickness of their coatings. Finally, the PDA-based fiber was successfully used for the extraction of selected PAHs from soot samples exploiting the conditions optimized for biphenyl and benzo(a)pyrene. Even if great advantages in terms of extraction performances in comparison with commercial polymers were not obtained, the new fibers proposed here are clearly innovative in manufacture. Coatings of commercial silica fibers for SPME often involve pre-treating processes. For instance, the bulk fused silica should first undergo exposition of silanol groups (via acid oxidative treatments) onto the surface for further functionalization, or the creation of the primary sites using sol gel methods or organic activators [34], in addition to the use of some active organic solvents [35]. On the contrary, the polydopamine coating does not need particular pretreatments of the silica bulk fibers for material processing. Besides, active extractive layers in commercial fibers are based on polydimethylsiloxane (PDMS), polyacrylate (PA), polyethyleneglycol (PEG), and divinylbenzene (DVB) coatings and so on, which are produced via sol-gel, electrospun, and deposition methods [36]. The most used sol-gel methods for producing these industrial extractive layers often involve organic:inorganic precursors, aqueous and organic solvents, a catalyst, and water [22], and in the sol solutions toxic alkoxysilanes are used as sol-gel precursors. Hydroxy-PDMS, terminated-PDMS, and hexamethyldisilazane-(HMDS) derivatives are used for producing PDMS-coated fibers, together with toxic solvents like methylene chloride and 1-butanol [35], and this functional coating is performed only after hydrothermal and alkaline treatment of the bulk fibers. MTMOS, TEOS, and vinyltetraethoxysilane (VTEOS) are used as UV-cross linkable precursors (together with some glycol-based species and other typical alkoxysilanes used for silica and biosilica functionalization) [37] for producing acrylate co-polymers for SPME coatings [38], again under co-solvent (aqueous:organic) conditions. In the case of PDA, the use of toxic solvents, precursors, and catalysts is completely avoided, and the processing is performed in a totally waterish buffer, at room temperature, exploiting the molecular oxygen as an abundant, cheap, and ecofriendly catalyst for polymerization.
5. Conclusions
SPME is a widely used sample pretreatment approach that suffers of limitations, such as high cost and use of chemical precursors for producing the coating layers, which are often environmentally harmful. Polydopamine, the coating material proposed herein, represents an ideal candidate to be used as sorption material in extraction techniques, possessing a great number of advantages, such as ease and rapidity of synthesis, chemical and environmental stability, cost-effectiveness, and eco-friendliness. After FT-ATR and SEM characterization, two model compounds, namely biphenyl and benzo(a)pyrene, were used to test the performances of the new PDA fibers and for comparison with some commercial fibers. The analysis of selected PAHs was then performed in soot samples using the PDA fiber, and the results compared with those obtained using the commercial PA fiber. The extraction yields shown by PDA throughout the work were comparable to those observed for commercial polymers, but the approach presented here is innovative in manufacture, being easily polymerizable under alkaline conditions at room temperature, and exploiting the molecular oxygen as abundant, cheap, and ecofriendly catalysts. Besides, the PDA coating was reusable for up to 100 consecutive extractions before the polymer was damaged. Even if the process of polymerization of the monomers around the silica bulks is still under study of the process, since the oxygen-related polymerization needs further optimization before a hypothetical industrial integration for massive production, the PDA coating proved to be a very promising sorbent for SPME.
Author Contributions
Conceptualization, C.Z. and G.M.F.; methodology, A.A., D.V. and S.R.C.; validation, A.A. and D.V.; formal analysis, A.A. and D.V.; resources, A.A., C.Z. and G.M.F.; data curation, A.A. and D.V.; writing—original draft preparation, A.A., D.V. and C.Z.; writing—review and editing, A.A., D.V. and C.Z.; supervision C.Z.; project administration, A.A., D.V. and C.Z.; funding acquisition, A.A., C.Z. and G.M.F. All authors have read and agreed to the published version of the manuscript.
Funding
Project n° 87429C9C—Alghe vive per la bonifica dell’ambiente marino (AlgAmbiente) and Horizon Europe Seeds: A Holistic approach for the assessment of envirOnment and human health risks due to Pollution in a transitional watEr system (HOPE, Scientific Responsible Roberto Carlucci); project cup n° H99J21016870006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
D.V. acknowledges the financial support from Fondo Sociale Europeo “Research for Innovation (REFIN)”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zambonin, C.; Aresta, A. Recent Applications of Solid Phase Microextraction Coupled to Liquid Chromatography. Separations 2021, 8, 34. [Google Scholar] [CrossRef]
- Moliner-Martinez, Y.; Herráez-Hernández, R.; Verdú-Andrés, J.; Molins-Legua, C.; Campíns-Falcó, P. Recent advances of in-tube solid-phase microextraction. Trends Anal. Chem. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.B.; Li, K.Y.; Cao, Y.Q.; Wu, S.; Wu, L. Advances in different configurations of solid-phase microextraction and their applications in food and environmental analysis. Trends Anal. Chem. 2015, 72, 141–152. [Google Scholar] [CrossRef]
- Seidi, S.; Tajik, M.; Baharfar, M.; Rezazadeh, M. Micro solid-phase extraction (pipette tip and spin column) and thin film solid-phase microextraction: Miniaturized concepts for chromatographic analysis. Trends Anal. Chem. 2019, 118, 810–827. [Google Scholar] [CrossRef]
- Bagheri, H.; Piri-Moghadam, H. Recent advances in capillary microextraction. Trends Anal. Chem. 2015, 73, 64–80. [Google Scholar] [CrossRef]
- Ozturk, E.E.; Bozyigit, G.D.; Buyukpınar, C.; Bakırdere, S. Magnetic Nanoparticles Based Solid Phase Extraction Methods for the Determination of Trace Elements. Crit. Rev. Anal. Chem. 2022, 52, 231–249. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, J.; Tian, J.; Zhu, F.; Zeng, F.; Su, C.; Ouyang, G. New materials in solid-phase microextraction. Trends Anal. Chem. 2013, 47, 68–83. [Google Scholar] [CrossRef]
- Yin, L.; Xu, J.; Huang, Z.; Chen, G.; Zheng, J.; Ouyang, G. Solid-Phase Microextraction Fibers Based on Novel Materials: Preparation and Application. Prog. Chem. 2017, 29, 1127–1141. [Google Scholar]
- Piri-Moghadam, H.; Alam, M.N.; Pawliszyn, J. Review of geometries and coating materials in solid phase microextraction: Opportunities, limitations, and future perspectives. Anal. Chim. Acta 2017, 984, 42–65. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, B.; Zohrabi, P.; Shamsipur, M. Recent developments and applications of different sorbents for SPE and SPME from biological samples. Talanta 2018, 187, 337–347. [Google Scholar] [CrossRef]
- Lashgari, M.; Yamini, Y. An overview of the most common lab-made coating materials in solid phase microextraction. Talanta 2019, 191, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Khaled Nazal, M.; Rutkowska, M.; Szczepa´nska, N.; Namie´snik, J.; Płotka-Wasylka, J. Solid Phase Microextraction: Apparatus, Sorbent Materials, and Application. Crit. Rev. Anal. Chem. 2019, 49, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Park, S.J.; Meng, L.Y.; Jin, X. Applications of carbon-based materials in solid phase micro-extraction: A review. Carbon Lett. 2017, 24, 10–17. [Google Scholar]
- Rocío-Bautista, P.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Are metal-organic frameworks able to provide a new generation of solid-phase microextraction coatings? A review. Anal. Chim. Acta 2016, 939, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Row, K.H. Recent Applications of Molecularly Imprinted Polymers (MIPs) on Micro-extraction Techniques. Sep. Purif. Rev. 2018, 47, 1–18. [Google Scholar] [CrossRef]
- Yu, H.; Ho, T.D.; Anderson, J.L. Ionic liquid and polymeric ionic liquid coatings in solid-phase microextraction. Trends Anal. Chem. 2013, 45, 219–232. [Google Scholar] [CrossRef]
- Patinha, D.J.S.; Silvestre, A.J.D.; Marrucho, I.M. Poly (ionic liquids) in solid phase microextraction: Recent advances and perspectives. Prog. Polym. Sci. 2019, 98, 101148. [Google Scholar] [CrossRef]
- Yavir, K.; Konieczna, K.; Marcinkowski, L.; Kloskowski, A. Ionic liquids in the microextraction techniques: The influence of ILs structure and properties. Trends Anal. Chem. 2020, 45, 219–232. [Google Scholar] [CrossRef]
- McLean, M.; Malik, A. Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 311–329. [Google Scholar]
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in sol-gel microextraction phases for solvent-free sample preparation in analytical chemistry. Trends Anal. Chem. 2013, 45, 197–218. [Google Scholar] [CrossRef]
- Murtada, K. Trends in nanomaterial-based solid-phase microextraction with a focus on environmental applications—A review. Trends Envrion. Anal. 2020, 25, 00077. [Google Scholar] [CrossRef]
- Amiri, A. Solid-phase microextraction-based sol–gel technique. Trends Anal. Chem. 2016, 75, 57–74. [Google Scholar] [CrossRef]
- Che, D.; Cheng, J.; Ji, Z.; Zhang, S.; Li, G.; You, J. Recent advances and applications of polydopamine-derived adsorbents for sample pretreatment. Trends Anal. Chem. 2017, 97, 1–14. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.A.; Park, E.; Lee, H. Polydopamine and Its Derivative Surface Chemistry in Material Science: A Focused Review for Studies at KAIST. Adv. Mater. 2020, 32, 1907505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Xu, L.; Feng, L.; Ji, Y.; Tao, L.; Wei, Y. Biocompatible polydopamine fluorescent organic nanoparticles: Facile preparation and cell imaging. Nanoscale 2012, 4, 5581–5584. [Google Scholar] [CrossRef] [PubMed]
- Zhanga, S.; Yao, W.; Ying, J.; Zhao, H. Polydopamine-reinforced magnetization of zeolitic imidazolate framework ZIF-7 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from the air-water environment. J. Chromatogr. A 2016, 1452, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wanga, Y.; Wanga, S.; Niu, H.; Ma, Y.; Zeng, T.; Cai, Y.; Meng, Z. Preparation of polydopamine coated Fe3O4 nanoparticles and their application for enrichment of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2013, 1283, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yan, Z.; Yang, M.; Huang, X.; Min, W.; Wang, L.; Cai, Q. Polydopamine decorated 3D nickel foam for extraction of sixteen polycyclic aromatic hydrocarbons. J. Chromatogr. A 2016, 1478, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Fenga, J.; Suna, M.; Li, J.; Xua, L.; Liu, X.; Jiang, S. Polydopamine supported preparation method for solid-phase microextraction coatings on stainless steel wire. J. Chromatogr. A 2011, 1218, 3601–3607. [Google Scholar] [CrossRef]
- Vona, D.; Cicco, S.R.; Ragni, R.; Vincente-Garcia, C.; Leone, G.; Giangregorio, M.M.; Palumbo, F.; Altamura, E.; Farinola, G.M. Polydopamine coating of living diatom microalgae. Photochem. Photobiol. Sci. 2022, 21, 945–958. [Google Scholar] [CrossRef]
- Grieco, C.; Kohl, F.R.; Hanes, A.T.; Kohler, B. Probing the heterogeneous structure of eumelanin using ultrafast vibrational fingerprinting. Nat. Commun. 2020, 11, 4569. [Google Scholar] [CrossRef]
- Vona, D.; Cicco, S.R.; Ragni, R.; Leone, G.; Lo Presti, M.; Farinola, G.M. Biosilica/polydopamine/silver nanoparticles composites: New hybrid multifunctional heterostructures obtained by chemical modification of Thalassiosira weissflogii silica shells. MRS Commun. 2018, 8, 911–917. [Google Scholar] [CrossRef]
- Yun, L. High extraction efficiency solid-phase microextraction fibers coated with open crown ether stationary phase using sol–gel technique. Anal. Chim. Acta 2003, 486, 63–72. [Google Scholar] [CrossRef]
- Kim, T.Y.; Alhooshani, K.; Kabir, A.; Fries, D.P.; Malik, A. High pH-resistant, surface-bonded sol–gel titania hybrid organic–inorganic coating for effective on-line hyphenation of capillary microextraction (in-tube solid-phase microextraction) with high-performance liquid chromatography. J. Chromatogr. A 2004, 1047, 165–174. [Google Scholar] [CrossRef]
- Kumar, A.; Malik, A.K.; Tewary, D.K.; Singh, B. A review on development of solid phase microextraction fibers by sol–gel methods and their applications. Anal. Chim. Acta 2008, 610, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Presti, M.L.; Ragni, R.; Vona, D.; Leone, G.; Cicco, S.; Farinola, G.M. In vivo doped biosilica from living Thalassiosira weissflogii diatoms with a triethoxysilyl functionalized red emitting fluorophore. MRS Adv. 2018, 3, 1509–1517. [Google Scholar] [CrossRef]
- Stanisław, P.; Nawała, J.; Czupryński, K. Preparation and application of sol–gel acrylate and methacrylate solid-phase microextraction fibres for gas chromatographic analysis of organoarsenic compounds. Anal. Chim. Acta 2014, 837, 52–63. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).