Mechanism on the Separation of Vanadium and Titanium from Vanadium Slag by Roasting with Ammonium Sulfate
Abstract
:1. Introduction
2. Thermodynamic Analysis
3. Experiment
3.1. Raw Materials
3.2. Characterization Methods
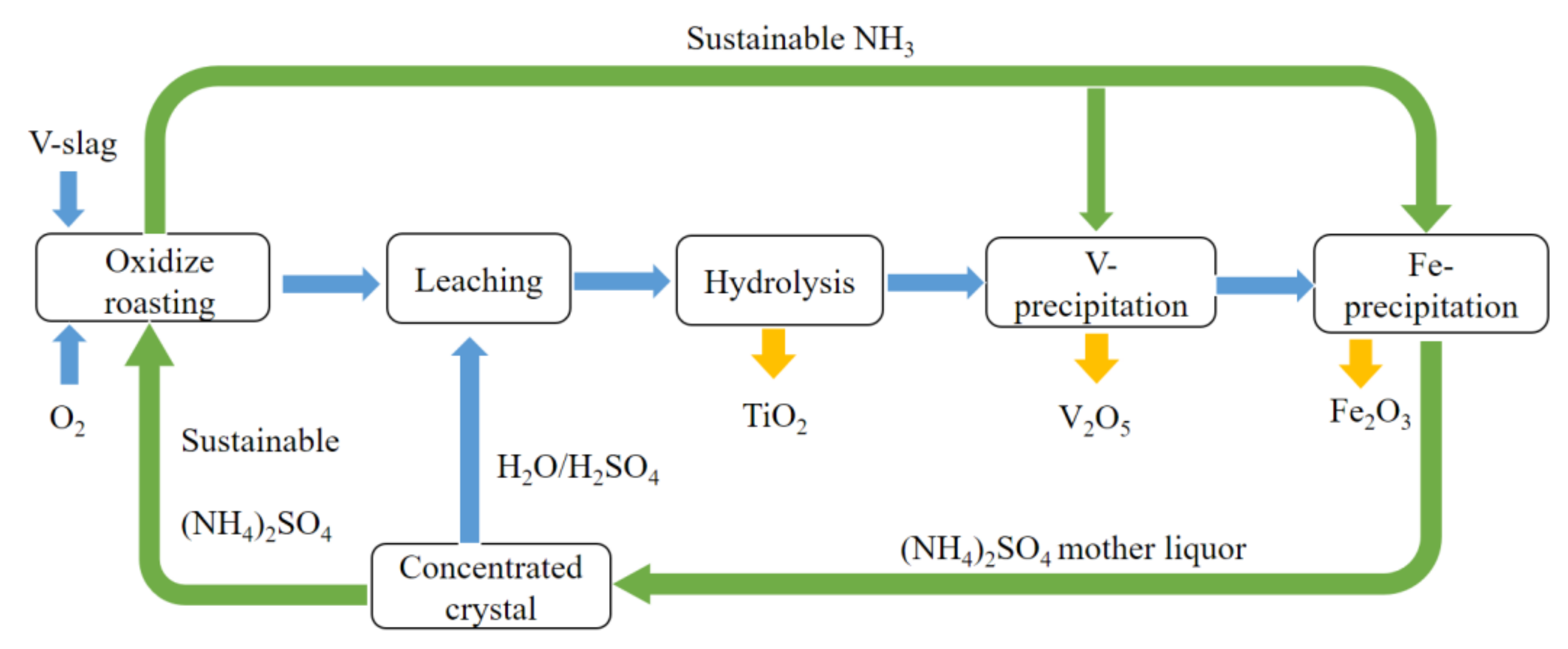
3.3. Experimental Procedure
4. Results and Discussions
4.1. Transformation of Functional Groups in the Spinel
4.2. Structures Evolution of Different Phases in the Vanadium Slag
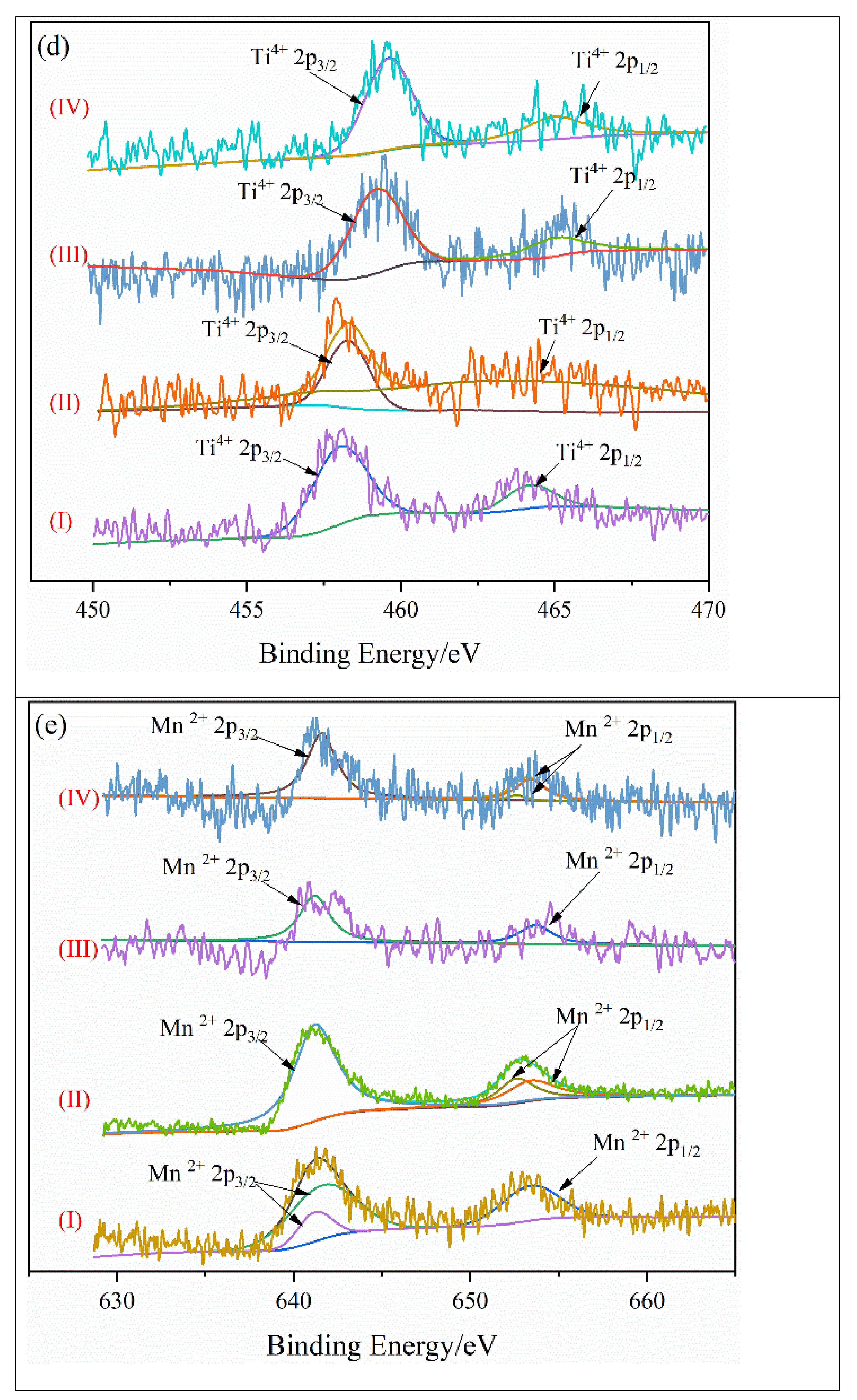
4.3. Valence State of Key Metal Elements
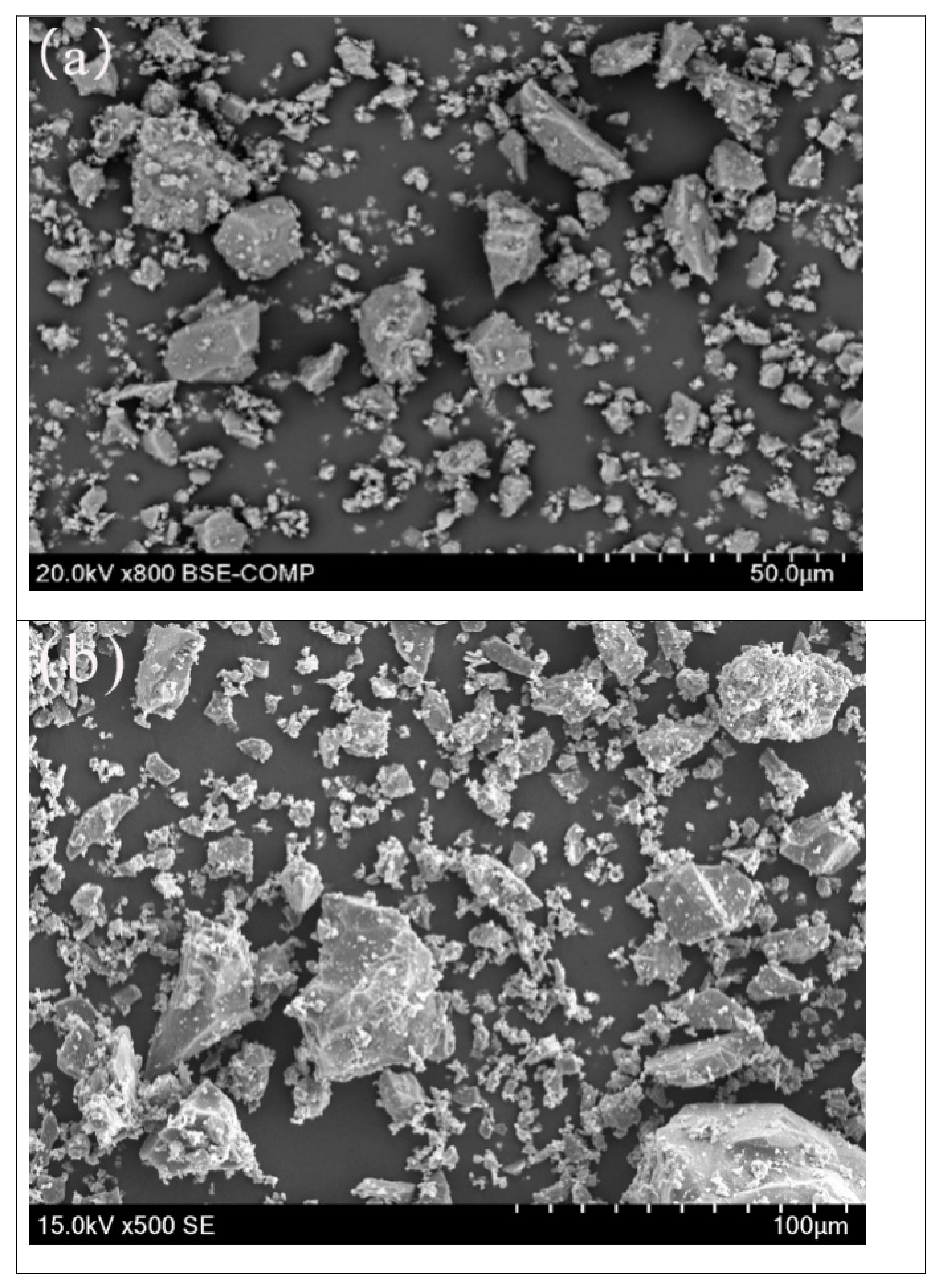
4.4. Morphology of the Roasted Slag
4.5. Effect of the ABS Amount on the Extraction Ratio of V and Ti from the Slag
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, C.; Yang, H.; Zhu, Q. Selective hydrolysis of trace TiCl4 from VOCl3 for preparation of high purity V2O5. Sep. Purif. 2017, 185, 196–201. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Lv, G.; Cao, X.; Zhu, H. Thermodynamic study on the V(V)-P(V)-H2O system in acidic leaching solution of vanadium-bearing converter slag. Sep. Purif. Technol. 2019, 218, 164–172. [Google Scholar] [CrossRef]
- Li, H.; Fang, H.; Wang, K.; Zhou, W.; Yang, Z.; Yan, X.; Ge, W.; Li, Q.; Xie, B. Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting-water leaching. Hydrometallurgy 2015, 156, 124–135. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Chou, K. Selective Chlorinated Extraction of Iron and Manganese from Vanadium Slag and Their Application to Hydrothermal Synthesis of MnFe2O4. ACS Sustain. Chem. Eng. 2017, 5, 10588–10596. [Google Scholar] [CrossRef]
- Zhang, G.; Luo, D.; Deng, C.; Lv, L.; Liang, B.; Li, C. Simultaneous extraction of vanadium and titanium from vanadium slag using ammonium sulfate roasting-leaching process. J. Alloys Compd. 2018, 742, 504–511. [Google Scholar] [CrossRef]
- Hoffmann, R.C.; Jeurgens, L.; Wildhack, S.; Bill, J.; Aldinger, F. Deposition of composite titania/vanadia thin films by chemical bath deposition. Chem. Mater. 2004, 16, 4199–4201. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Dreisinger, D.; Lv, C.; Lv, G.; Zhang, W. Recovery of vanadium from calcification roasted-acid leaching tailing by enhanced acid leaching. J Hazard. Mater. 2019, 369, 632–641. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Q.; Lv, X.; Bai, C. Extraction of vanadium from converter slag by two-step sulfuric acid leaching process. J. Clean. Prod. 2018, 170, 1089–1101. [Google Scholar] [CrossRef]
- Ji, Y.; Shen, S.; Liu, J.; Yan, S.; Zhang, Z. Green and Efficient Process for Extracting Chromium from Vanadium Slag by an Innovative Three-Phase Roasting Reaction. ACS Sustain. Chem. Eng. 2017, 5, 6008–6015. [Google Scholar] [CrossRef]
- Li, X.; Meng, F.; Dong, X.; Liang, D.; Gao, F.; Wei, Y. Effect of CaO addition on phase formation in the Fe-Fe2O3-V2O3 system. J. Alloys Compd. 2019, 772, 955–960. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Fan, G.; Li, C.; Deng, Z.; Li, X. Extraction of vanadium from black shale using pressure acid leaching. Hydrometallurgy 2009, 98, 308–313. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Ou, L.; Lu, Y. An environmentally-friendly technology of vanadium extraction from stone coal. Miner. Eng. 2007, 20, 1184–1186. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zhang, L.; Gu, S. Mechanism of vanadium slag roasting with calcium oxide. Int. J. Miner. Process. 2015, 138, 20–29. [Google Scholar] [CrossRef]
- Yin, S.; Li, S.; Zhang, B.; Peng, J.; Zhang, L. Mass transfer kinetics of lanthanum (III) extraction in the presence of two complexing agents by D2EHPA using a constant interfacial area cell with laminar flow. Chem. Eng. Res. Des. 2015, 104, 92–97. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Zhang, Y.; Lv, G.; Liu, Y.; Liu, Z. Pressure leaching of converter vanadium slag with waste titanium dioxide. Rare Met. 2016, 35, 576–580. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, S.; Wang, S.; Qin, Y.; Du, H.; Zhang, Y. Electrochemical decomposition of vanadium slag in concentrated NaOH solution. Hydrometallurgy 2015, 151, 51–55. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Q.; Lv, X.; Bai, C. Effect of Mechanical Activation Treatment on the Recovery of Vanadium from Converter Slag. Met. Mater. Trans. B 2017, 48, 2759–2767. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Chen, M.; Nueraihemaiti, A.; Du, J.; Fan, X.; Tao, C. Enhanced leaching of vanadium slag in acidic solution by electro-oxidation. Hydrometallurgy 2016, 159, 1–5. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Lu, G.; Zhang, Y.; Liu, Y.; Liu, Z. Extraction of vanadium from vanadium slag by high pressure oxidative acid leaching, International journal of minerals. Metall. Mater. 2015, 22, 21–26. [Google Scholar]
- Hu, J.; Liu, W.; Wang, L.; Liu, Q.; Chen, F.; Yue, H.; Liang, B.; Lu, L.; Wang, Y.; Zhang, G.; et al. Indirect mineral carbonation of blast furnace slag with (NH4)2SO4 as a recyclable extractant. J. Energy Chem. 2017, 26, 927–935. [Google Scholar] [CrossRef]
- Jiang, T.; Wen, J.; Zhou, M.; Xue, X. Phase evolutions, microstructure and reaction mechanism during calcification roasting of high chromium vanadium slag. J. Alloys Compd. 2018, 742, 402–412. [Google Scholar] [CrossRef]
- Shi, Y.; Shu, H.; Zhang, Y.; Fan, H.; Zhang, Y.; Yang, L. Formation and decomposition of NH4HSO4 during selective catalytic reduction of NO with NH3 over V2O5-WO3/TiO2 catalysts. Fuel Process. Technol. 2016, 150, 141–147. [Google Scholar] [CrossRef]
- Yang, T.; Xia, D.; Wang, Z.; Chen, Y. A novel anode material of Fe2VO4 for high power Lithium ion battery. Mater. Lett. 2009, 63, 5–7. [Google Scholar] [CrossRef]
- Brion, D. Etude par spectroscopie de photoelectrons de la degradation superficielle de FeS2, CuFeS2, ZnS et PbS a l’air et dans l’eau. Appl. Surf. Sci. 1980, 5, 133–152. [Google Scholar] [CrossRef]
- Langevoort, J.C.; Sutherland, I.; Hanekamp, L.J.; Gellings, P.J. On the Oxide Formation on Stainiess-Steels Aisi-304 and Incoloy-800H Investigated with XPS. Appl. Surf. Sci. 1987, 28, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P. X-RAY Photoelectron-Spectroscopy Studies of Solvated Metal Atom Dispersed Catalysis-Monometallic Iron and Bimetallic Iron Cobalt Particles on Alumina. Chem. Mater. 1990, 2, 186–191. [Google Scholar] [CrossRef]
- Nag, N.K.; Massoth, F.E. ESCA and Gravimetric Reduction Studies on V/Al2O3 and V/SiO2 Catalysts. Cheminform 1990, 21, 127–132. [Google Scholar] [CrossRef]
- Horvath, B.; Strutz, J.; Geyerlippmann, J.; Horvath, E.G. Prearation, Properties, and ESCA Characterization of Vanadium Surface-Compounds on Silica-Gel. 1. Z. Anorg. Allg. Chem. 1981, 483, 181–192. [Google Scholar] [CrossRef]
- Sanjines, R.; Tang, H.; Berger, H.; Gozzo, F.; Margaritondo, G.; Levy, F. Electronic structure of anatase TiO2 oxide. J. Appl. Phys. 1994, 75, 2945–2951. [Google Scholar] [CrossRef]
- Castillo, R.; Koch, B.; Ruiz, P.; Delmon, B. Influence of the amount of titania on the texture and structure of titania supported on silica. J. Catal. 1996, 161, 524–529. [Google Scholar] [CrossRef]
- Oku, M.; Hirokawa, K.; Ikeda, S. X-ray photoelectron spectroscopy of manganese—oxygen systems. J. Electron Spectrosc. 1975, 7, 465–473. [Google Scholar] [CrossRef]
- Allen, G.C.; Harris, S.J.; Jutson, J.A.; Dyke, J.M. A study of a number of mixed transition metal oxide spinels using X-ray photoelectron spectroscopy. Appl. Surf. Sci. 1989, 37, 111–134. [Google Scholar] [CrossRef]
- Mills, P.; Sullivan, J.L. A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy. J. Phys. D Appl. Phys. 2000, 16, 723. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Cook, J.M. Interactions of SO2 with sodium deposited on silica. J. Colloid Interface Sci. 1985, 108, 414–422. [Google Scholar] [CrossRef]
- Seyama, H.; Soma, M. Fe 2p spectra of silicate minerals. J. Electron Spectrosc. 1987, 42, 97–101. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, G.; Wang, K.; Luo, D.; Tang, S.; Yue, H. Two-stage cyclic ammonium sulfate roasting and leaching of extracting vanadium and titanium from vanadium slag. Chin. J. Chem. Eng. 2022, 47, 39–47. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, G.; Luo, D.; Li, C.; Yue, H.; Liang, B. Separation of titanium from vanadium and iron in leach solutions of vanadium slag by solvent extraction with trioctyl tertiary amine (N235). Hydrometallurgy 2019, 188, 216–221. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, G.; Xiong, Y.; Luo, D.; Li, C.; Tang, S.; Yue, H.; Liang, B. Recovery of vanadium from leach solutions of vanadium slag using solvent extraction with N235. Hydrometallurgy 2020, 192, 105259. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Duan, H. A New Process of Extracting Titanium from Vanadium–Titanium Magnetite. Crystals 2021, 11, 327. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, T.; Liao, W.; Ma, X. An energy-efficient process of leaching vanadium from roasted tablet of ammonium sulfate, vanadium slag and silica. J. Environ. Chem. Eng. 2021, 9, 105332. [Google Scholar] [CrossRef]
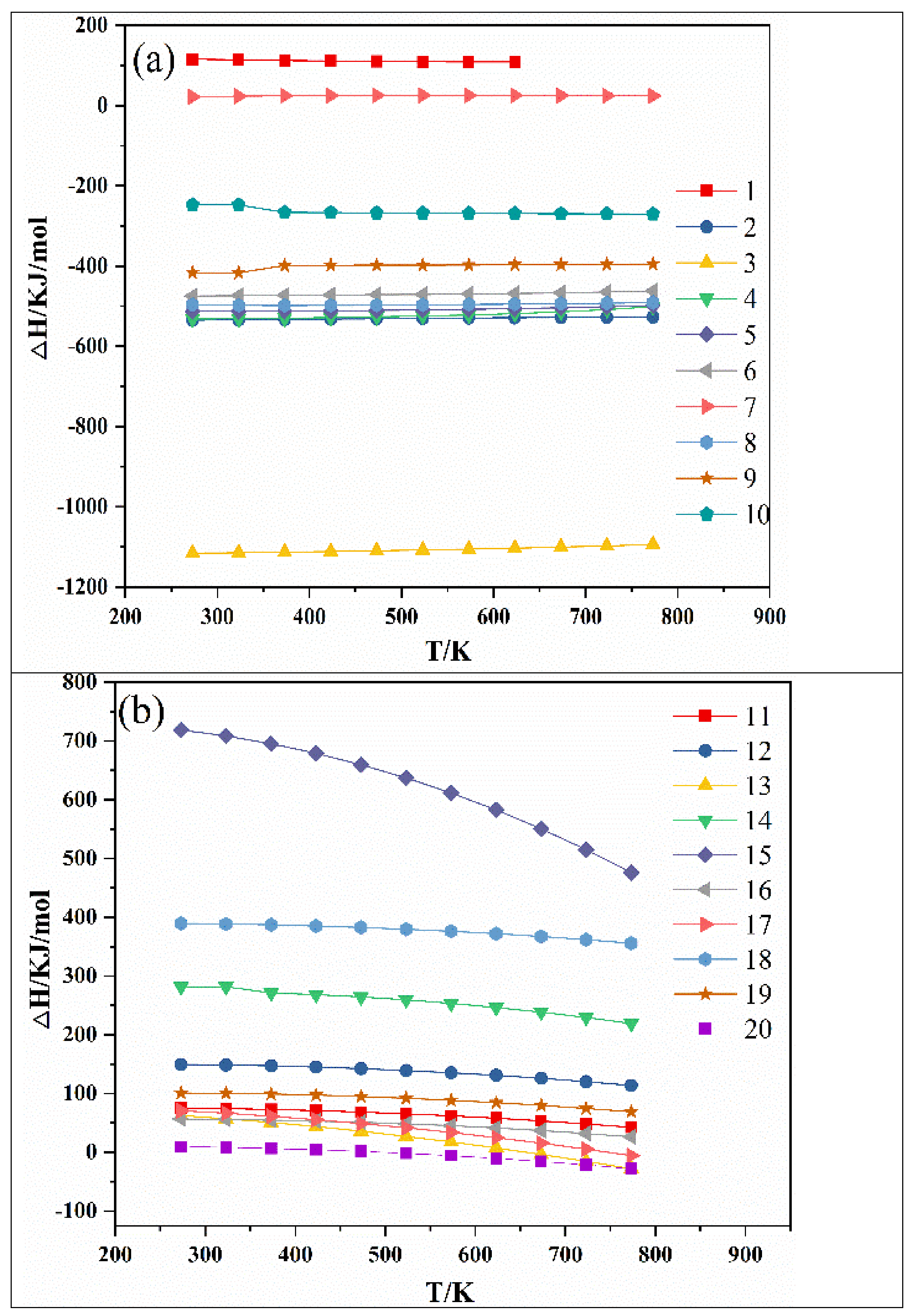

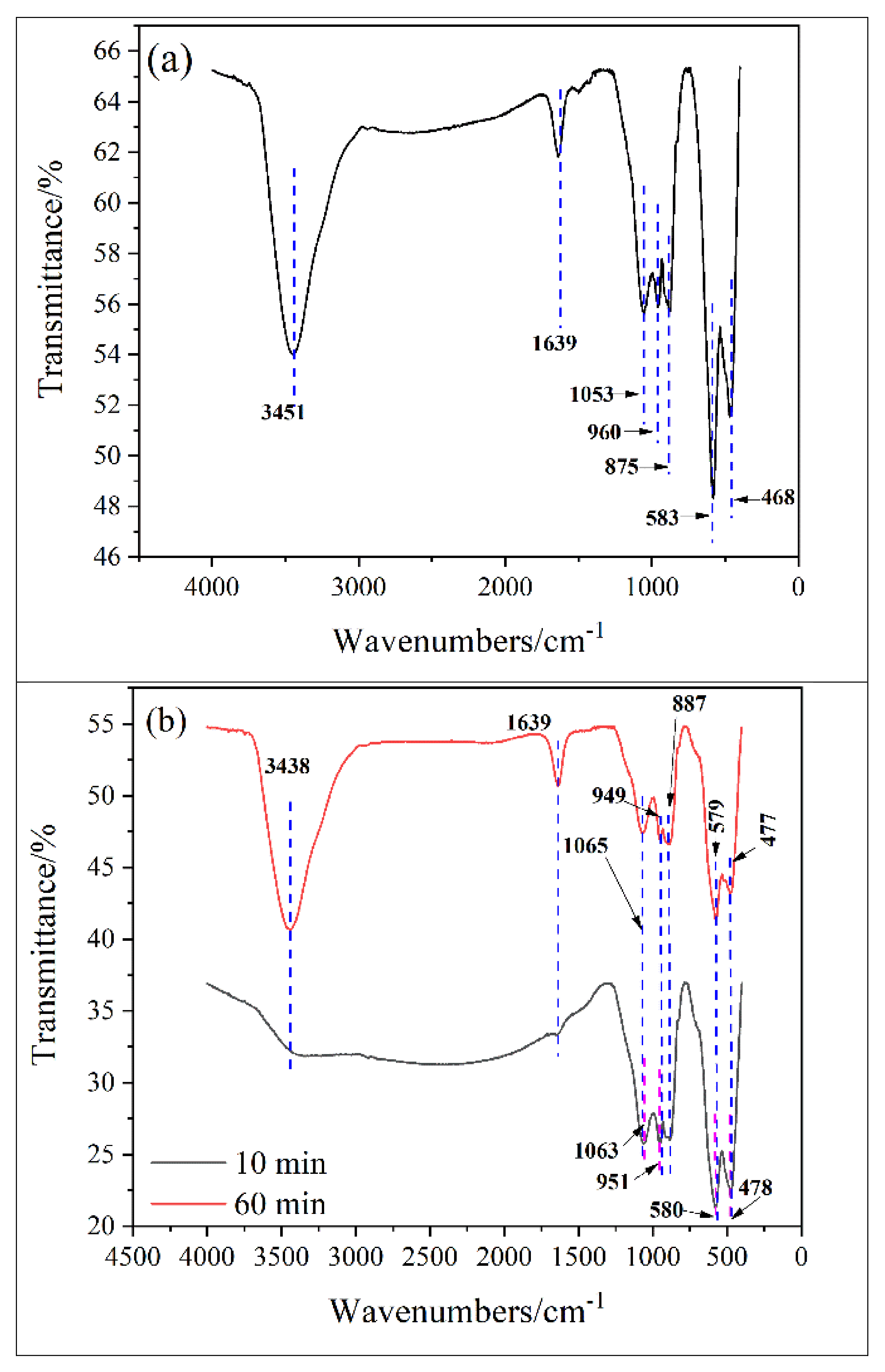
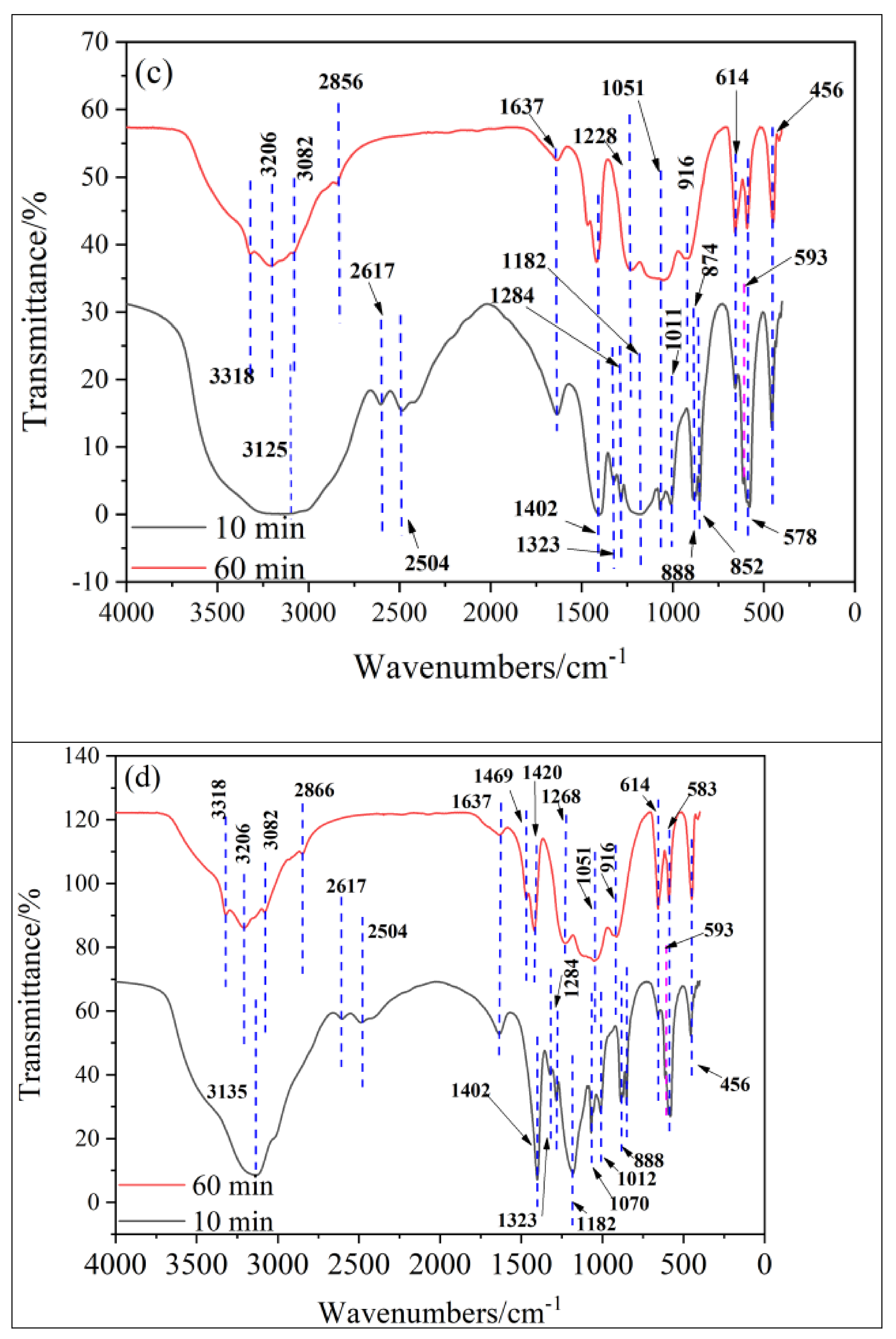



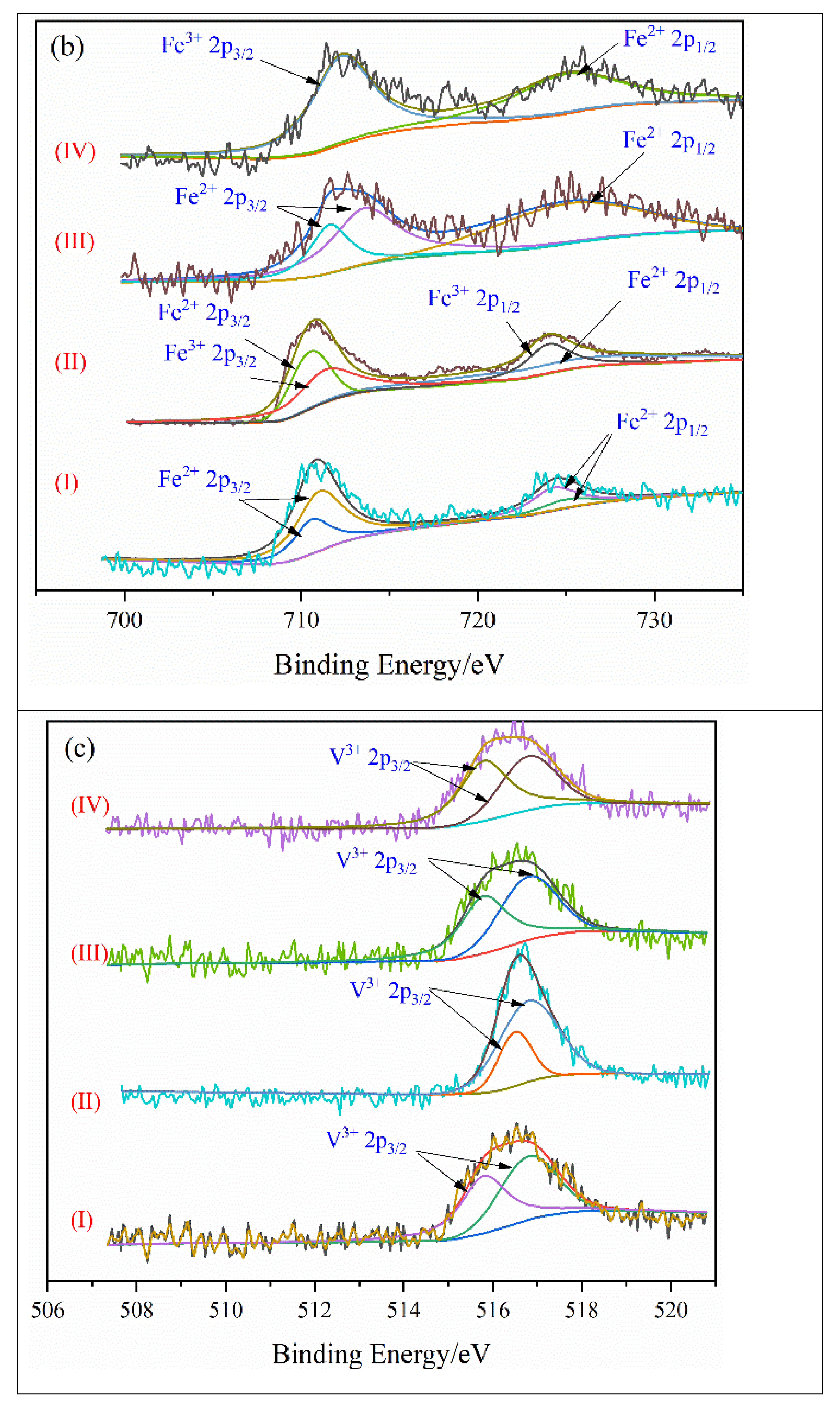


| NO. | Reaction Equation | NO. | Reaction Equation |
|---|---|---|---|
| 1 | (NH4)2SO4 = NH4HSO4 + NH3(g) | 11 | NH4HSO4 + FeO = FeSO4 + NH3(g) + H2O(g) |
| 2 | 2Fe + O2(g) = 2FeO | 12 | NH4HSO4 + 1/3Fe2O3 = 1/3Fe2(SO4)3 + NH3(g) + H2O(g) |
| 3 | 3/2Fe + O2(g) = 1/2Fe3O4 | 13 | NH4HSO4 + 1/3V2O3 = 1/3V2(SO4)3 + NH3(g) + H2O(g) |
| 4 | 3Fe2SiO4 + O2(g) = 2Fe3O4 + 3SiO2 | 14 | NH4HSO4 + 1/2V2O4 = VOSO4 + NH3(g) + H2O(g) |
| 5 | 2Fe2SiO4 + O2(g) = 2Fe2O3 + 3SiO2 | 15 | NH4HSO4 + MnO = MnSO4 + NH3(g) + H2O(g) |
| 6 | 4CaFe(SiO3)2 + O2(g) = 2Fe2O3 + 4CaSiO3 + 4SiO2 | 16 | NH4HSO4 + 1/3Fe2TiO4 = 1/3TiOSO4 + 2/3FeSO4 + NH3(g) + H2O(g) |
| 7 | CaMgSiO6 = MgSiO3 + CaSiO3 | 17 | NH4HSO4 + 1/4FeV2O4 = 1/4V2(SO4)3 + 1/4FeSO4 + NH3(g) + H2O(g) |
| 8 | 4FeV2O4 + O2(g) = 2Fe2O3 + 4V2O3 | 18 | NH4HSO4 + 1/2Fe2SiO4 = FeSO4 + NH3(g) + 1/2SiO2(g) + H2O(g) |
| 9 | 2V2O3 + O2(g) = 2V2O4 | 19 | NH4HSO4 + CaFe(SiO3)2 = FeSO4 + NH3(g) +CaSiO3 + H2O(g) + SiO2 |
| 10 | 2V2O4 + O2(g) = 2V2O5 | 20 | NH4HSO4 + 2FeSO4 + 1/2O2(g) = Fe2(SO4)3 + NH3(g) + H2O(g) |
| Composition | Fe2O3 | SiO2 | V2O5 | TiO2 | MnO | CaO | MgO | Cr2O3 | Al2O3 |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 38.50 | 27.75 | 18.88 | 12.75 | 10.40 | 2.27 | 2.33 | 1.90 | 2.19 |
| Exp. No. | Amount of Slag | Flow Rate of Atmosphere |
|---|---|---|
| 1 | 3 g vanadium slag | 250 mL/min air |
| 2 | 1 g vanadium slag + 3 g ABS | 250 mL/min N2 gas |
| 3 | 1 g vanadium slag + 3 g ABS | 250 mL/min air |
| Materials | Roasting Atmosphere | Main Phase | Main Valance Sate |
|---|---|---|---|
| raw slag | without roasting | FeV2O4 | Fe2+, V3+, Ti4+, Mn2+ |
| Fe2TiO4 | |||
| MnV2O4 | |||
| raw slag | air | Fe2+VnFe2−nO4 (0 < n < 2) | Fe2+, Fe3+, V3+, Ti4+, Mn2+ |
| Fe2TiO4 | |||
| MnV2O4 | |||
| raw slag + ABS | N2 | (NH4)2Fe(SO4)2 | Fe2+, V3+, Ti4+, Mn2+ |
| NH4V(SO4)2·2H2O/NH4V(SO4)2 | |||
| TiOSO4 | |||
| MnSO4 | |||
| raw slag + ABS | air | NH4Fe(SO4)2 | Fe2+, Fe3+, V3+, Ti4+, Mn2+ |
| NH4V(SO4)2·2H2O/NH4V(SO4)2 | |||
| MnSO4 | |||
| TiOSO4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, K.; Luo, M. Mechanism on the Separation of Vanadium and Titanium from Vanadium Slag by Roasting with Ammonium Sulfate. Separations 2022, 9, 196. https://doi.org/10.3390/separations9080196
Zhang G, Wang K, Luo M. Mechanism on the Separation of Vanadium and Titanium from Vanadium Slag by Roasting with Ammonium Sulfate. Separations. 2022; 9(8):196. https://doi.org/10.3390/separations9080196
Chicago/Turabian StyleZhang, Guoquan, Kun Wang, and Mingzhi Luo. 2022. "Mechanism on the Separation of Vanadium and Titanium from Vanadium Slag by Roasting with Ammonium Sulfate" Separations 9, no. 8: 196. https://doi.org/10.3390/separations9080196





