Purification, Identification and Neuroprotective Effects of Proteins from Bombyx batryticatus in Glu-Stimulated PC12 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Total Proteins from BB
2.3. Purification of Neuroprotective Proteins from BBPs
2.4. Protein Patterns of PF-1 to PF-6 Analyzed by SDS-PAGE
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Determination of GABA, IL-1β, and TNF-α Contents in PC12 Cells
2.8. Laser Scanning Confocal Microscopy Analysis
2.9. MALDI-TOF/TOF-MS Analysis
2.10. Statistical Analysis
3. Results
3.1. BBPs Fractionated by Ion-Exchange Chromatography
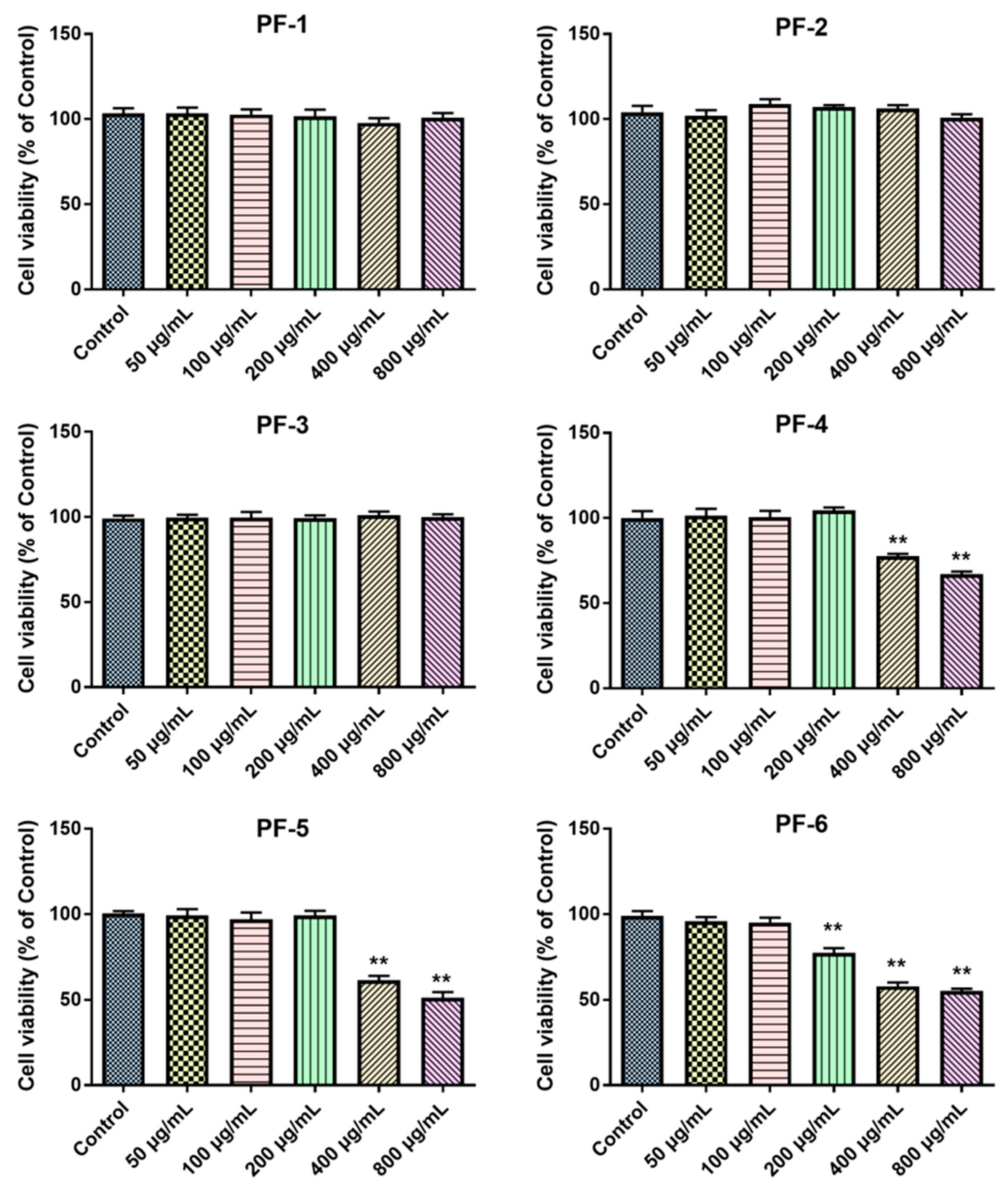
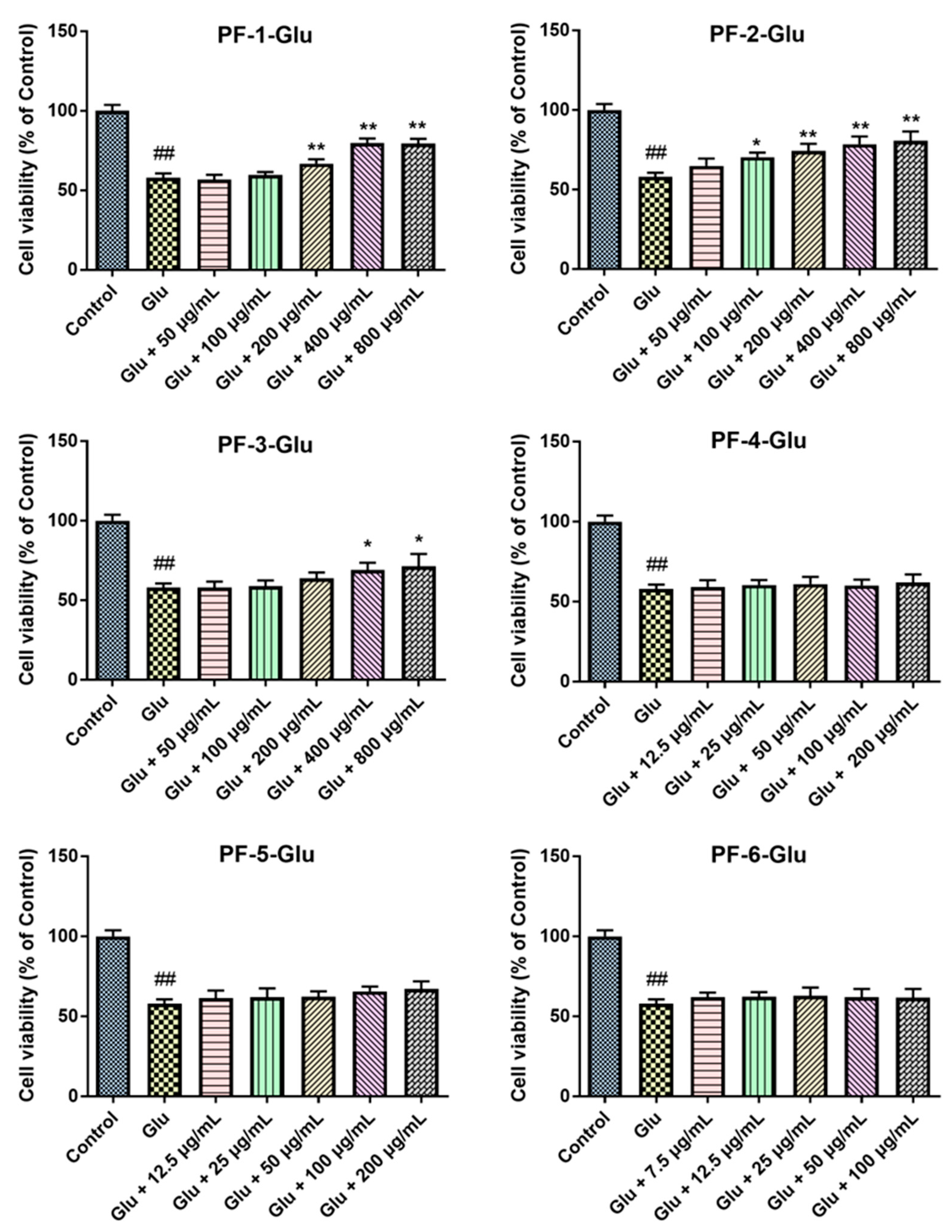
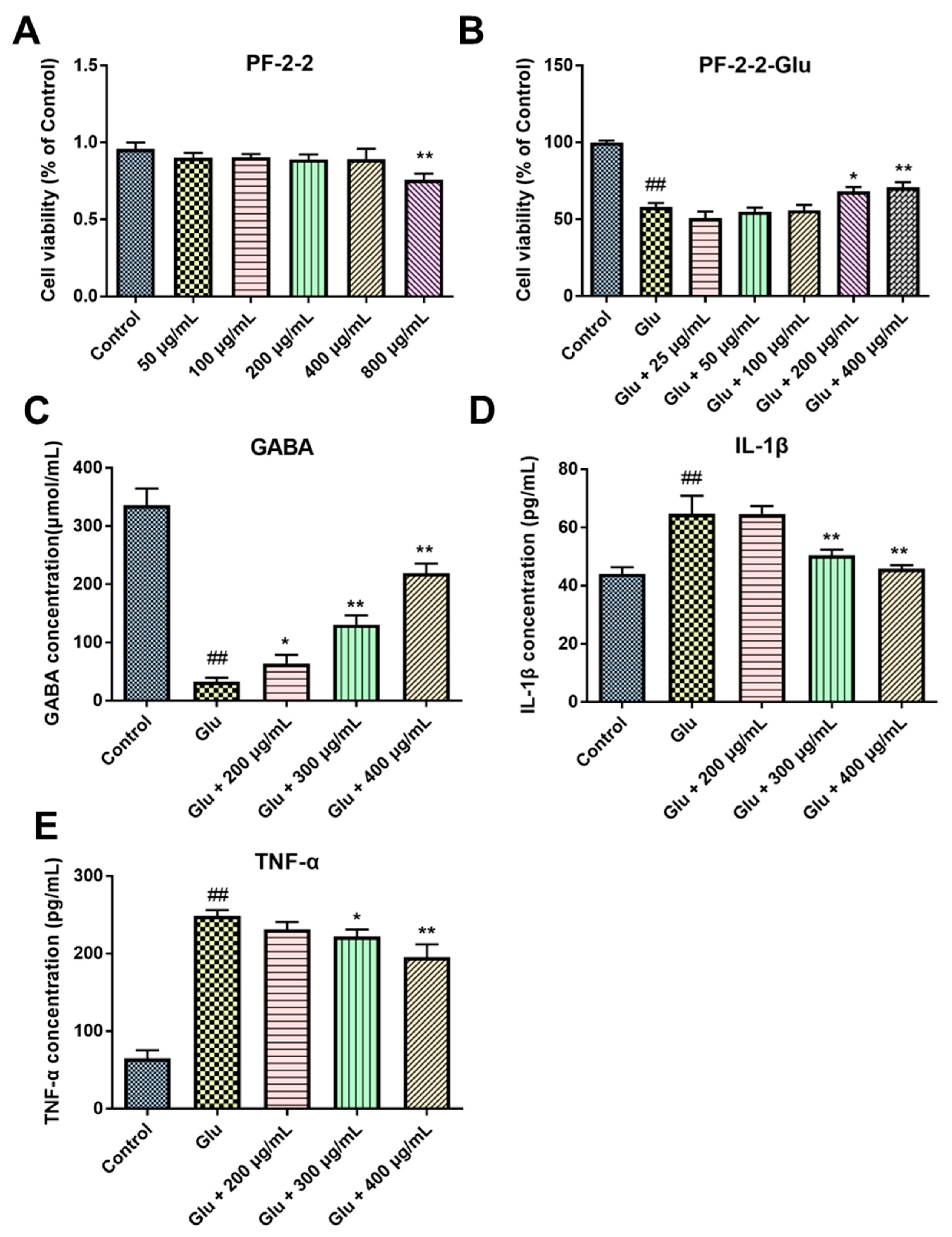
3.2. Effects of PF-1 to PF-6 on the Cell Viability of Normal and Glu-Injured PC12 Cells
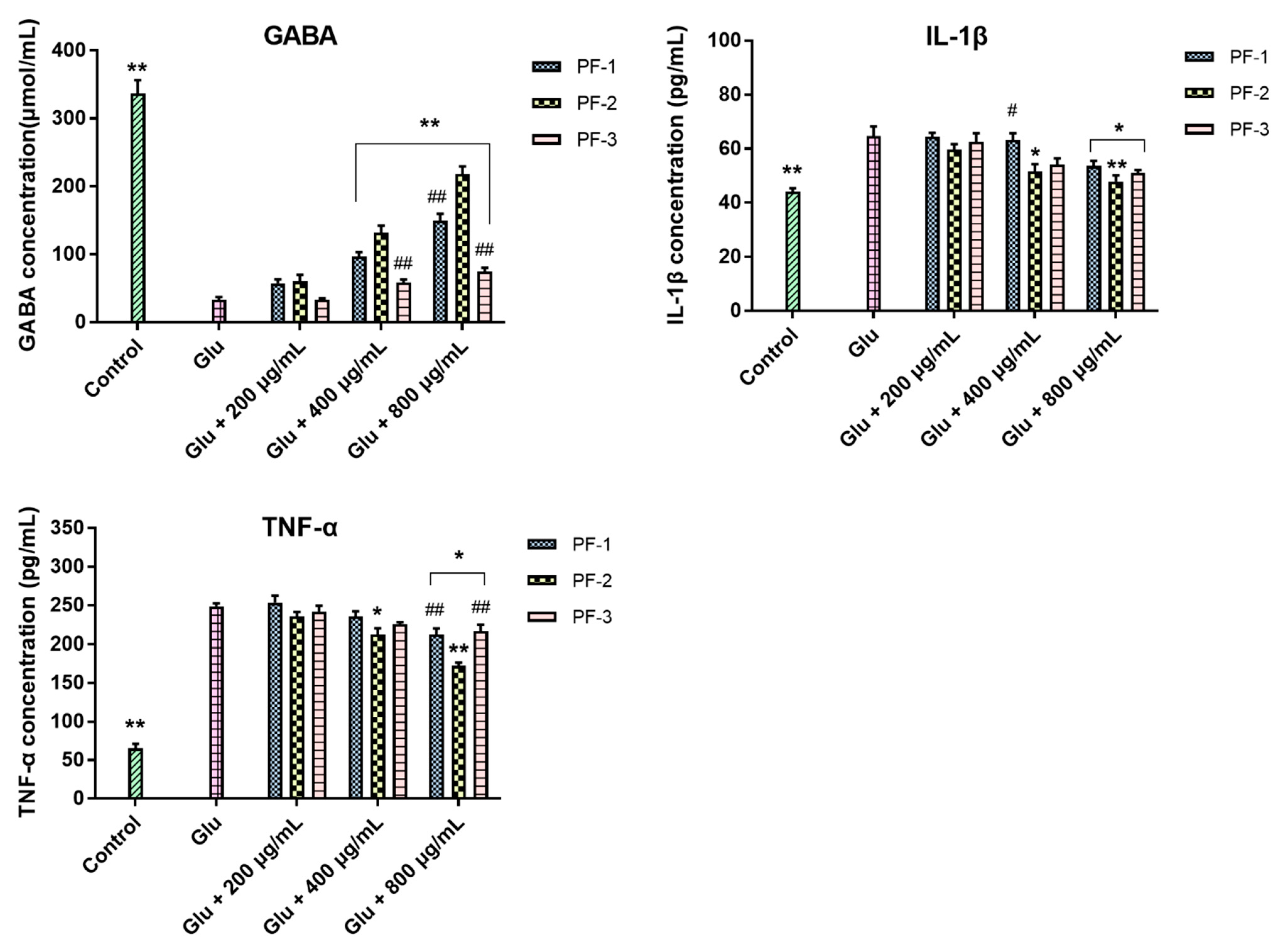
3.3. Effects of PF-1, PF-2 and PF-3 on the Levels of GABA, IL-1β, and TNF-α in Glu-Injured PC12 Cells
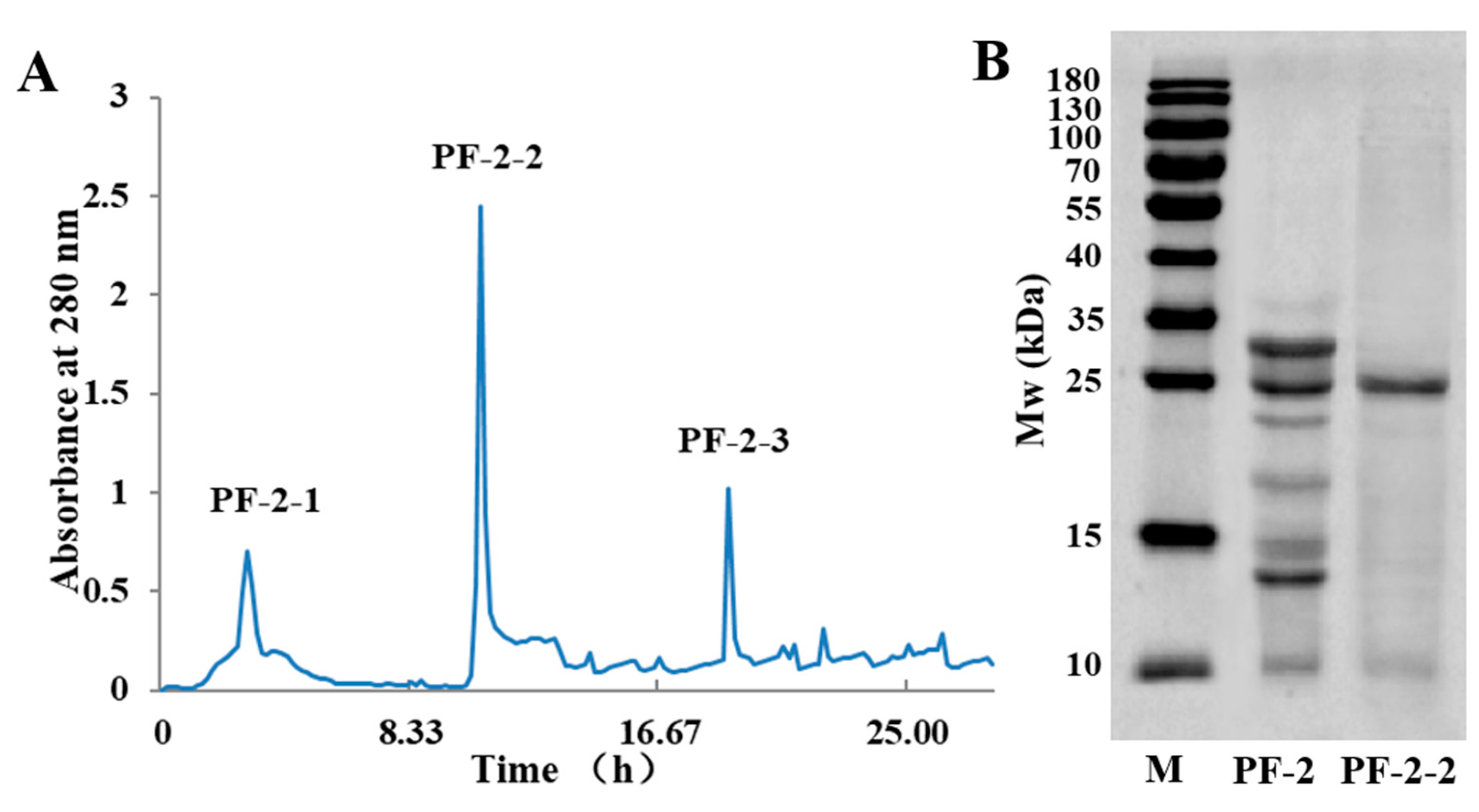
3.4. Size-Exclusion Chromatography of PF-2-2
3.5. The Neuroprotective Effects of PF-2-2 in Glu-Stimulated PC12 Cells
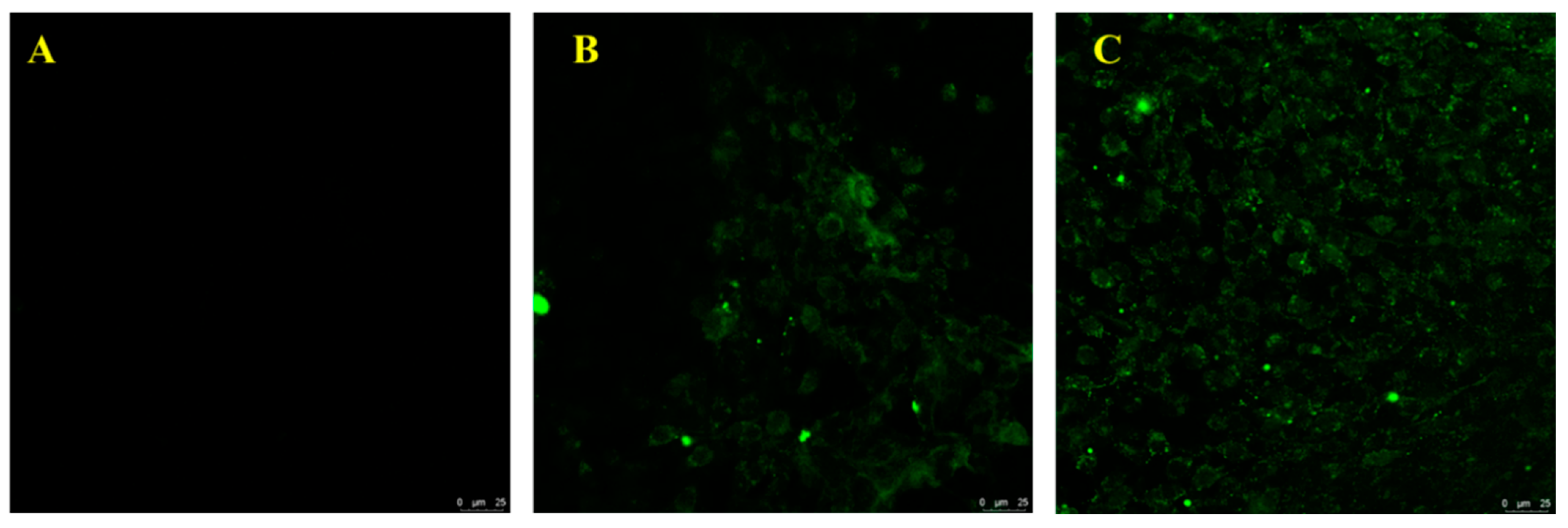
3.6. Interaction Evaluation between PF-2-2 and PC12 Cells
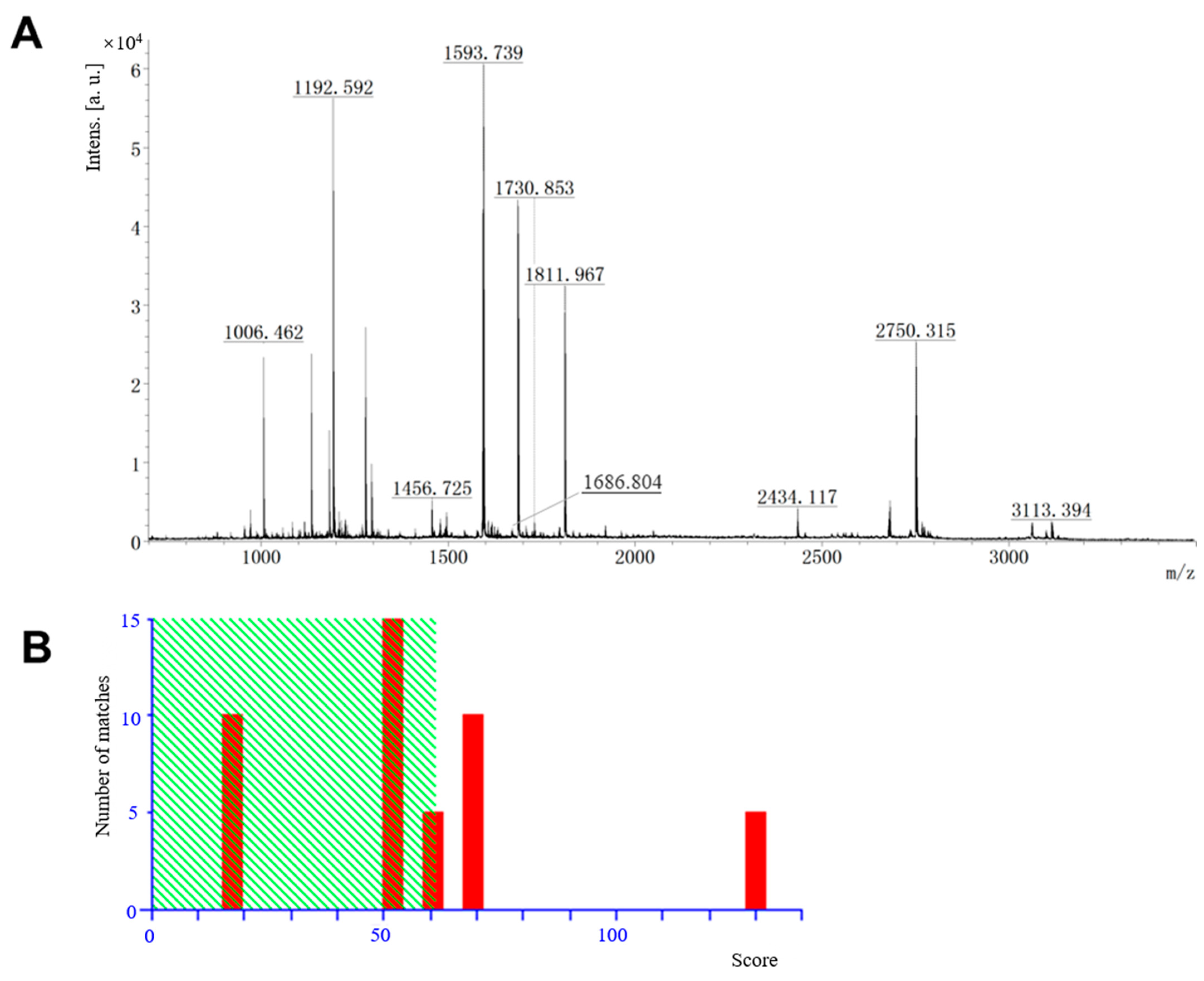
3.7. Protein Identification by MALDI-TOF/TOF-MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.C.; Tewari, B.P.; Chaunsali, L.; Sontheimer, H. Neuron-glia interactions in the pathophysiology of epilepsy. Nat. Rev. Neurosci. 2019, 20, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, E.; Paudel, Y.N.; Polat, A.K.; Dundar, H.E.; Angelopoulou, E. Enlightening the neuroprotective effect of quercetin in epilepsy: From mechanism to therapeutic opportunities. Epilepsy Behav. 2021, 115, 107701. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Shaikh, M.F.; Shah, S.; Kumari, Y.; Othman, I. Role of inflammation in epilepsy and neurobehavioral comorbidities: Implication for therapy. Eur. J. Pharmacol. 2018, 837, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Nandini, H.S.; Paudel, Y.N.; Krishna, K.L. Envisioning the neuroprotective effect of Metformin in experimental epilepsy: A portrait of molecular crosstalk. Life Sci. 2019, 233, 116686. [Google Scholar]
- Yang, N.; Guan, Q.W.; Chen, F.H.; Xia, Q.X.; Yin, X.X.; Zhou, H.H.; Mao, X.Y. Antioxidants Targeting Mitochondrial Oxidative Stress: Promising Neuroprotectants for Epilepsy. Oxid. Med. Cell Longev. 2020, 2020, 6687185. [Google Scholar] [CrossRef]
- Sanz, P.; Garcia-Gimeno, M.A. Reactive Glia Inflammatory Signaling Pathways and Epilepsy. Int. J. Mol. Sci. 2020, 21, 4096. [Google Scholar] [CrossRef]
- Perucca, P.; Perucca, E. Identifying mutations in epilepsy genes: Impact on treatment selection. Epilepsy Res. 2019, 152, 18–30. [Google Scholar] [CrossRef]
- Steriade, C.; French, J.; Devinsky, O. Epilepsy: Key experimental therapeutics in early clinical development. Expert Opin. Investig. Drugs 2020, 29, 373–383. [Google Scholar] [CrossRef]
- Beghi, E.; Beghi, M. Epilepsy, antiepileptic drugs and dementia. Curr. Opin. Neurol. 2020, 33, 191–197. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, Q.; He, M.; Ye, X.; Zhang, X. Extensive characterization and differential analysis of endogenous peptides from Bombyx batryticatus using mass spectrometric approach. J. Pharm. Biomed. Anal. 2019, 163, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Antihypertensive peptides of animal origin: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, S.; Fan, F.; Tu, M.; Xu, Z.; Du, M. Identification and molecular mechanism of antithrombotic peptides from oyster proteins released in simulated gastro-intestinal digestion. Food Funct. 2019, 10, 5426–5435. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Tian, C.; Jiang, J.; Zhang, G.L.; Hao, H.; Hou, H.M. Antibacterial Activity and Potential Application in Food Packaging of Peptides Derived from Turbot Viscera Hydrolysate. J. Agric. Food Chem. 2020, 68, 9968–9977. [Google Scholar] [CrossRef]
- Kachel, H.S.; Buckingham, S.D.; Sattelle, D.B. Insect toxins—Selective pharmacological tools and drug/chemical leads. Curr. Opin. Insect Sci. 2018, 30, 93–98. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Y.; Li, Y.; Fu, H.; Hu, J.; Zhou, Y.; Xu, Y.; Xia, G.; Sun, X.; Yang, H.; et al. Identification of signature proteins of processed Bombyx batryticatus by comparative proteomic analysis. Int. J. Biol. Macromol. 2020, 153, 289–296. [Google Scholar] [CrossRef]
- Song, H.Y.; Han, J.M.; Byun, E.H.; Kim, W.S.; Seo, H.S.; Byun, E.B. Bombyx batryticatus Protein-Rich Extract Induces Maturation of Dendritic Cells and Th1 Polarization: A Potential Immunological Adjuvant for Cancer Vaccine. Molecules 2021, 26, 476. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Sheikho, A.; Ma, H.; Li, T.C.; Zhao, Y.Q.; Zhang, Y.L.; Wang, D. Molecular mechanisms of Bombyx batryticatus ethanol extract inducing gastric cancer SGC-7901 cells apoptosis. Cytotechnology 2017, 69, 875–883. [Google Scholar] [CrossRef]
- He, L.Y.; Hu, M.B.; Li, R.L.; Zhao, R.; Fan, L.H.; Wang, L.; Peng, W.; Liu, Y.J.; Wu, C.J. The Effect of Protein-Rich Extract from Bombyx batryticatus against Glutamate-Damaged PC12 Cells Via Regulating gamma-Aminobutyric Acid Signaling Pathway. Molecules 2020, 25, 553. [Google Scholar] [CrossRef]
- Hu, M.; Liu, Y.; He, L.; Yuan, X.; Peng, W.; Wu, C. Antiepileptic Effects of Protein-Rich Extract from Bombyx batryticatus on Mice and Its Protective Effects against H2O2-Induced Oxidative Damage in PC12 Cells via Regulating PI3K/Akt Signaling Pathways. Oxid. Med. Cell Longev. 2019, 2019, 7897584. [Google Scholar] [CrossRef]
- Xing, D.; Shen, G.; Li, Q.; Xiao, Y.; Yang, Q.; Xia, Q. Quality Formation Mechanism of Stiff Silkworm, Bombyx batryticatus Using UPLC-Q-TOF-MS-Based Metabolomics. Molecules 2019, 24, 3780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.; Yu, Z.; Wang, J.; Fan, W.; Liu, Y.; Li, J.; Xiao, H.; Li, Y.; Peng, W.; Wu, C. Traditional Uses, Origins, Chemistry and Pharmacology of Bombyx batryticatus: A Review. Molecules 2017, 22, 1779. [Google Scholar] [CrossRef]
- Wang, H.; Xu, L.; Dou, Y.; Wang, J. Bombyx batryticatus Cocoonase Inhibitor Separation, Purification, and Inhibitory Effect on the Proliferation of SMCC-7721 HeLa-Derived Cells. Evid. Based Complement. Alternat. Med. 2022, 2022, 4064829. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.P.; Cao, J.; Wu, J.Y.; Chen, H.; Wang, D. Anticancer activity of Bombyx batryticatus ethanol extract against the human tumor cell line HeLa. Genet. Mol. Res. 2015, 14, 79–88. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, J.S.; Moon, B.C.; Ryu, S.M.; Lee, J.; Park, G. Batryticatus Bombyx Protects Dopaminergic Neurons Against MPTP-Induced Neurotoxicity by Inhibiting Oxidative Damage. Antioxidants 2019, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shang, E.; Fan, X. Effect of San’ao decoction with scorpio and Bombyx batryticatus on CVA mice model via airway inflammation and regulation of TRPA1/TRPV1/TRPV5 channels. J. Ethnopharmacol. 2021, 264, 113342. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, Y.; Liu, H.; Jia, Q.; Liu, Q.; Tu, J. Purification and Identification of Pine Nut (Pinus yunnanensis Franch.) Protein Hydrolysate and Its Antioxidant Activity in Vitro and in Vivo. Chem. Biodivers. 2021, 18, e2000710. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, H.; Li, Q.; Gu, Q.; Zheng, Z.; Wu, H.; Ye, S.; Sun, X.; Xu, X.; Ho, P.C. Inhibition of pathologic retinal neovascularization by a small peptide derived from human apolipoprotein(a). Investig. Ophthalmol. Vis. Sci. 2009, 50, 5384–5395. [Google Scholar] [CrossRef]
- Katayama, H.; Nagasu, T.; Oda, Y. Improvement of in-gel digestion protocol for peptide mass fingerprinting by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom 2001, 15, 1416–1421. [Google Scholar] [CrossRef]
- Pakkianathan, B.C.; Singh, N.K.; Konig, S.; Krishnan, M. Antiapoptotic activity of 30 kDa lipoproteins family from fat body tissue of silkworm, Bombyx mori. Insect Sci. 2015, 22, 629–638. [Google Scholar] [CrossRef]
- Pietrzyk, A.J.; Bujacz, A.; Lochynska, M.; Jaskolski, M.; Bujacz, G. Crystal structure of Bombyx mori lipoprotein 6: Comparative structural analysis of the 30-kDa lipoprotein family. PLoS ONE 2014, 9, e108761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Shimada, M.; Nagaoka, S. Identification of the active protein in rice bran protein having an inhibitory activity of cholesterol micellar solubility. Biosci. Biotechnol. Biochem. 2017, 81, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Zhao, G.X.; Suo, S.K.; Wang, Y.M.; Chi, C.F.; Wang, B. Purification, Identification, Activity Evaluation, and Stability of Antioxidant Peptides from Alcalase Hydrolysate of Antarctic Krill (Euphausia superba) Proteins. Mar. Drugs 2021, 19, 347. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Song, H.; Huang, K.; Li, S.; Guan, X. Purification and characterization of antioxidant peptides from enzymatic hydrolysate of mungbean protein. J. Food Sci. 2020, 85, 1735–1741. [Google Scholar] [CrossRef]
- Yu, L.J.; Wall, B.A.; Wangari-Talbot, J.; Chen, S. Metabotropic glutamate receptors in cancer. Neuropharmacology 2017, 115, 193–202. [Google Scholar] [CrossRef]
- Mayor, D.; Tymianski, M. Neurotransmitters in the mediation of cerebral ischemic injury. Neuropharmacology 2018, 134, 178–188. [Google Scholar] [CrossRef]
- Yu, S.; Xin, W.; Jiang, Q.; Li, A. Propofol exerts neuroprotective functions by down-regulating microRNA-19a in glutamic acid-induced PC12 cells. Biofactors 2020, 46, 934–942. [Google Scholar] [CrossRef]
- Sarlo, G.L.; Holton, K.F. Brain concentrations of glutamate and GABA in human epilepsy: A review. Seizure 2021, 91, 213–227. [Google Scholar] [CrossRef]
- Guo, M.; Cui, C.; Song, X.; Jia, L.; Li, D.; Wang, X.; Dong, H.; Ma, Y.; Liu, Y.; Cui, Z.; et al. Deletion of FGF9 in GABAergic neurons causes epilepsy. Cell Death Dis. 2021, 12, 196. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Pang, X.; Zhang, J.; Guan, Y. Role of microRNA-155 in modifying neuroinflammation and gamma-aminobutyric acid transporters in specific central regions after post-ischaemic seizures. J. Cell Mol. Med. 2019, 23, 5017–5024. [Google Scholar] [CrossRef]
- Su, J.; Yin, J.; Qin, W.; Sha, S.; Xu, J.; Jiang, C. Role for pro-inflammatory cytokines in regulating expression of GABA transporter type 1 and 3 in specific brain regions of kainic acid-induced status epilepticus. Neurochem. Res. 2015, 40, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Oxley, D. Protein identification by MALDI-TOF mass spectrometry. Methods Mol. Biol. 2012, 800, 227–240. [Google Scholar] [PubMed]
- Gogichaeva, N.V.; Alterman, M.A. Amino Acid Analysis by Means of MALDI TOF Mass Spectrometry or MALDI TOF/TOF Tandem Mass Spectrometry. Methods Mol. Biol. 2019, 2030, 17–31. [Google Scholar] [PubMed]
- Cakir, B.; Gulseren, I. Identification of Novel Proteins from Black Cumin Seed Meals Based on 2D Gel Electrophoresis and MALDI-TOF/TOF-MS Analysis. Plant Foods Hum. Nutr. 2019, 74, 414–420. [Google Scholar] [CrossRef]
- Weng, C.Y.; Wang, C.E.; Xie, W.B.; Xu, S.Y.; Wang, Y.J.; Zheng, Y.G. Comparative proteome analysis of Actinoplanes utahensis grown on various saccharides based on 2D-DIGE and MALDI-TOF/TOF-MS. J. Proteom. 2021, 239, 104193. [Google Scholar] [CrossRef]
- Shi, X.F.; Li, Y.N.; Yi, Y.Z.; Xiao, X.G.; Zhang, Z.F. Identification and Characterization of 30 K Protein Genes Found in Bombyx mori (Lepidoptera: Bombycidae) Transcriptome. J. Insect Sci. 2015, 15, 71. [Google Scholar] [CrossRef]
- Pakkianathan, B.C.; Singh, N.K.; Krishnan, M.; Konig, S. A proteomic view on the developmental transfer of homologous 30 kDa lipoproteins from peripheral fat body to perivisceral fat body via hemolymph in silkworm, Bombyx mori. BMC. Biochem. 2012, 13, 5. [Google Scholar] [CrossRef]
- Yang, J.P.; Ma, X.X.; He, Y.X.; Li, W.F.; Kang, Y.; Bao, R.; Chen, Y.; Zhou, C.Z. Crystal structure of the 30 K protein from the silkworm Bombyx mori reveals a new member of the beta-trefoil superfamily. J. Struct. Biol. 2011, 175, 97–103. [Google Scholar] [CrossRef]
- Ujita, M.; Kimura, A.; Nishino, D.; Yokoyama, E.; Banno, Y.; Fujii, H.; Hara, A. Specific binding of silkworm Bombyx mori 30-kDa lipoproteins to carbohydrates containing glucose. Biosci. Biotechnol. Biochem. 2002, 66, 2264–2266. [Google Scholar] [CrossRef]
- Kim, E.J.; Rhee, W.J.; Park, T.H. Isolation and characterization of an apoptosis-inhibiting component from the hemolymph of Bombyx mori. Biochem. Biophys. Res. Commun. 2001, 285, 224–228. [Google Scholar] [CrossRef]
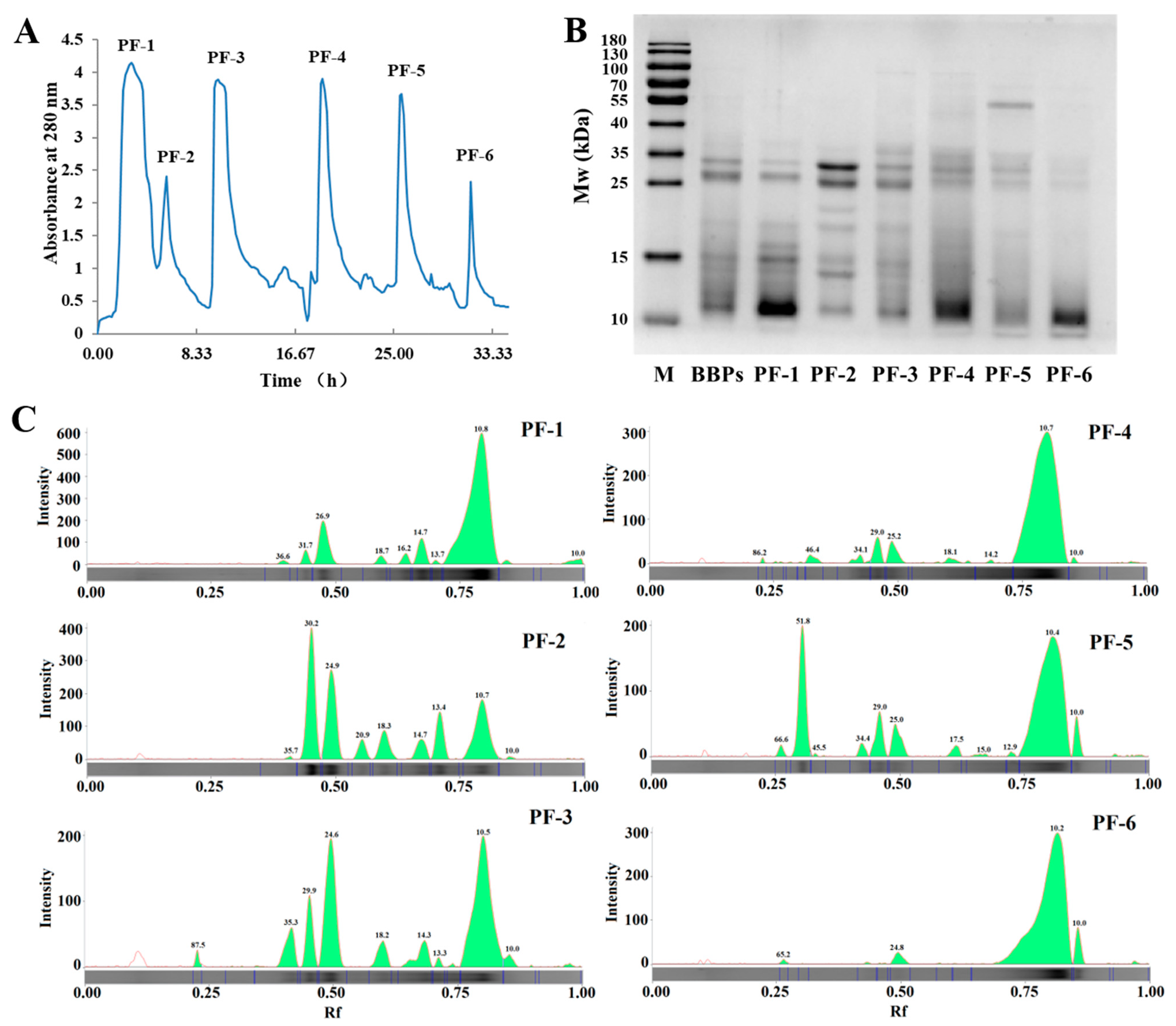
| Band No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | MW (kDa) | 180 | 130 | 100 | 70 | 55 | 40 | 35 | 25 | 15 | 10 |
| RQ | 0.22 | 0.37 | 0.68 | 0.88 | 0.99 | 0.62 | 0.82 | 0.66 | 1.00 | 1.04 | |
| BBPs | MW (kDa) | 95.64 | 57.83 | 36.51 | 32.24 | 27.14 | 19.17 | 15.00 | 13.88 | 10.81 | 10.00 |
| RQ | 0.01 | 0.01 | 0.01 | 0.14 | 0.43 | 0.01 | 0.07 | 0.02 | 0.29 | 0.19 | |
| PF-1 | MW (kDa) | 36.63 | 31.72 | 26.92 | 18.70 | 16.17 | 14.73 | 13.69 | 10.76 | 10.00 | |
| RQ | 0.02 | 0.07 | 0.34 | 0.05 | 0.06 | 0.17 | 0.02 | 2.19 | 0.05 | ||
| PF-2 | MW (kDa) | 35.69 | 30.19 | 24.88 | 20.88 | 18.33 | 14.73 | 13.39 | 10.71 | ||
| RQ | 0.01 | 0.57 | 0.47 | 0.09 | 0.15 | 0.11 | 0.21 | 0.47 | |||
| PF-3 | MW (kDa) | 87.48 | 35.34 | 29.95 | 24.63 | 18.24 | 14.33 | 13.32 | 10.51 | 10.00 | |
| RQ | 0.02 | 0.10 | 0.13 | 0.37 | 0.07 | 0.08 | 0.01 | 0.61 | 0.03 | ||
| PF-4 | MW (kDa) | 86.19 | 46.44 | 34.15 | 28.98 | 25.21 | 18.05 | 10.66 | 10.00 | ||
| RQ | 0.01 | 0.03 | 0.03 | 0.09 | 0.09 | 0.03 | 1.64 | 0.01 | |||
| PF-5 | MW (kDa) | 66.57 | 51.81 | 34.43 | 28.98 | 25.00 | 17.52 | 15.00 | 10.37 | 10.00 | |
| RQ | 0.02 | 0.26 | 0.02 | 0.09 | 0.08 | 0.02 | 0.01 | 0.83 | 0.06 | ||
| PF-6 | MW (kDa) | 65.25 | 24.75 | 10.18 | 10.00 | ||||||
| RQ | 0.01 | 0.05 | 1.67 | 0.10 |
| Protein Name | Amino Acid Sequence |
|---|---|
| Low molecular mass 30 kDa lipoprotein 21G1 | 1 MKFLVVFASC VLAVSAGVTE MSAGSMSSSN KELEEKLYNS ILTGDYDSAV RQSLEYENQG 61 KGSIIQNVVN NLIIDKSRNT MEYCYKLWVG NGQHIVRKYF PYNFRLIMAG NFVKLIYRNY 121 NLALKLGPTL DPANERLAYG DGKEKNSDLI SWKFITLWEN NRVYFKIHNT KYNQYLKLSS 181 TTDCNTQDRV IFGTNTADTT REQWFLQPTK YENDVLFFIY NREYNDALKL GRIVDASGDR 241 MAFGHDGEVA GLPDIFSWFV TPF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.-B.; Meng, X.-L.; Wang, P.; Zhang, S.-S.; Wu, C.-J.; Liu, Y.-J. Purification, Identification and Neuroprotective Effects of Proteins from Bombyx batryticatus in Glu-Stimulated PC12 Cells. Separations 2022, 9, 236. https://doi.org/10.3390/separations9090236
Hu M-B, Meng X-L, Wang P, Zhang S-S, Wu C-J, Liu Y-J. Purification, Identification and Neuroprotective Effects of Proteins from Bombyx batryticatus in Glu-Stimulated PC12 Cells. Separations. 2022; 9(9):236. https://doi.org/10.3390/separations9090236
Chicago/Turabian StyleHu, Mei-Bian, Xiang-Long Meng, Pu Wang, Shuo-Sheng Zhang, Chun-Jie Wu, and Yu-Jie Liu. 2022. "Purification, Identification and Neuroprotective Effects of Proteins from Bombyx batryticatus in Glu-Stimulated PC12 Cells" Separations 9, no. 9: 236. https://doi.org/10.3390/separations9090236






