Determination of the Loading Capacity and Recovery of Extracellular Vesicles Derived from Human Embryonic Kidney Cells and Urine Matrices on Capillary-Channeled Polymer (C-CP) Fiber Columns
Abstract
:1. Introduction
2. Experimental
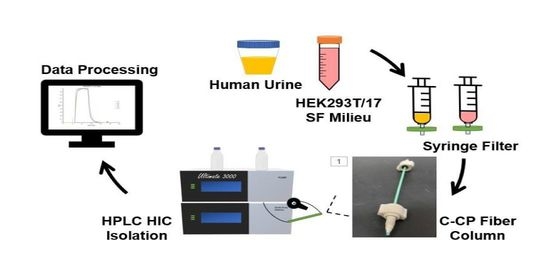
2.1. Chemicals and Sample Preparation
2.2. Chromatographic Columns
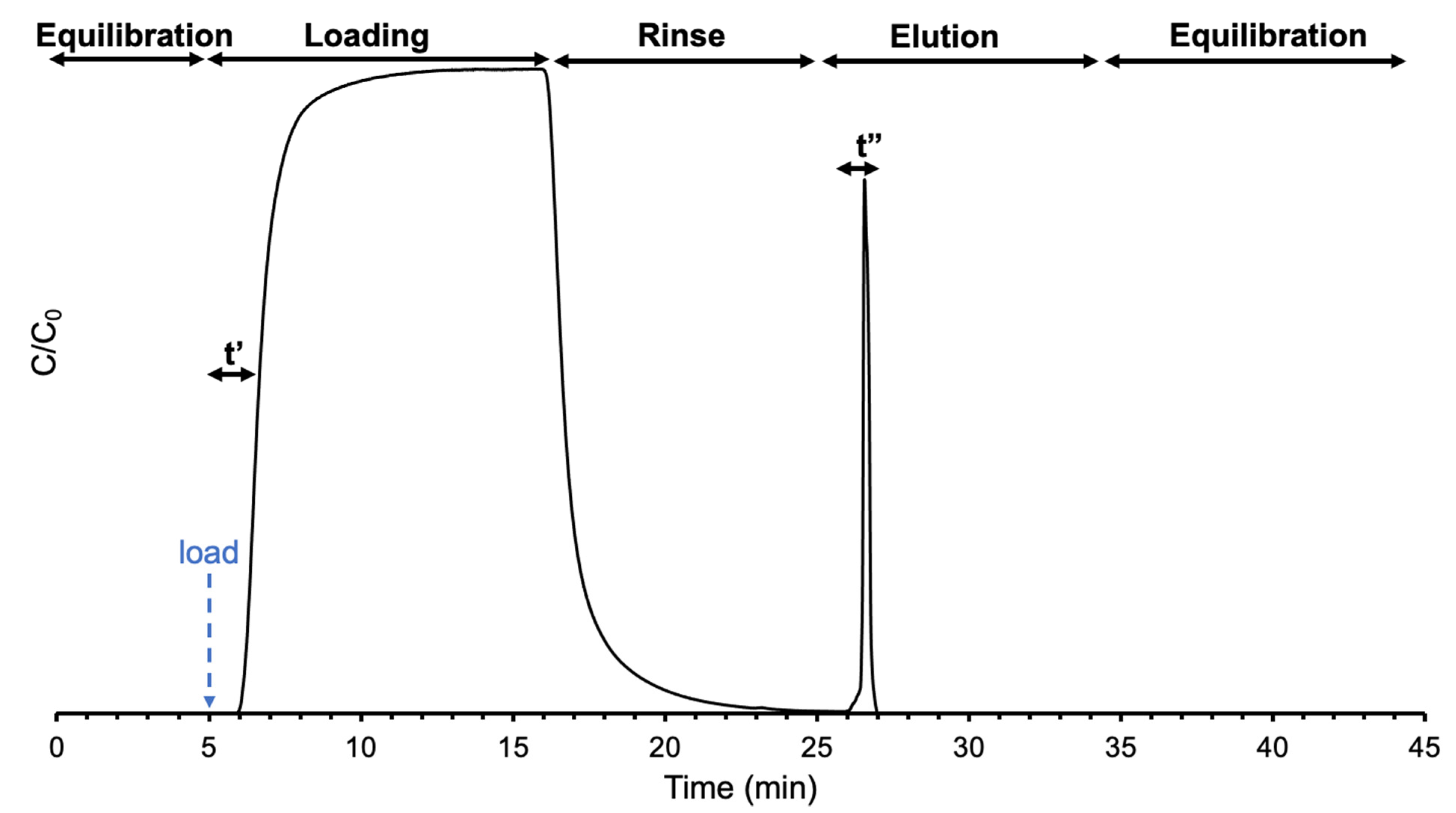
2.3. Instrumentation and Methods
3. Results and Discussion
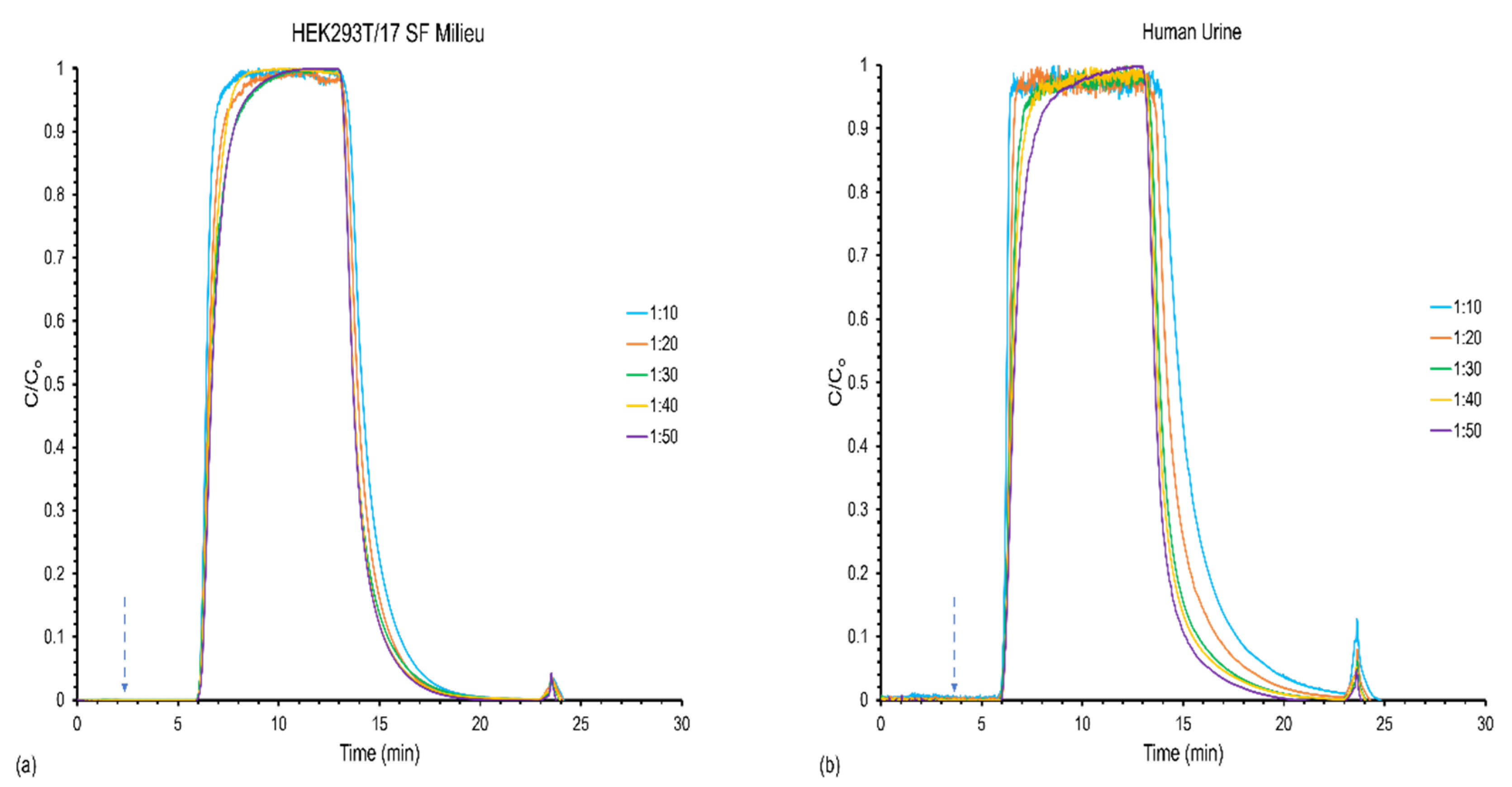
3.1. Effect of Feed Stock Concentration on Column Capacity, Recovery, and Yield
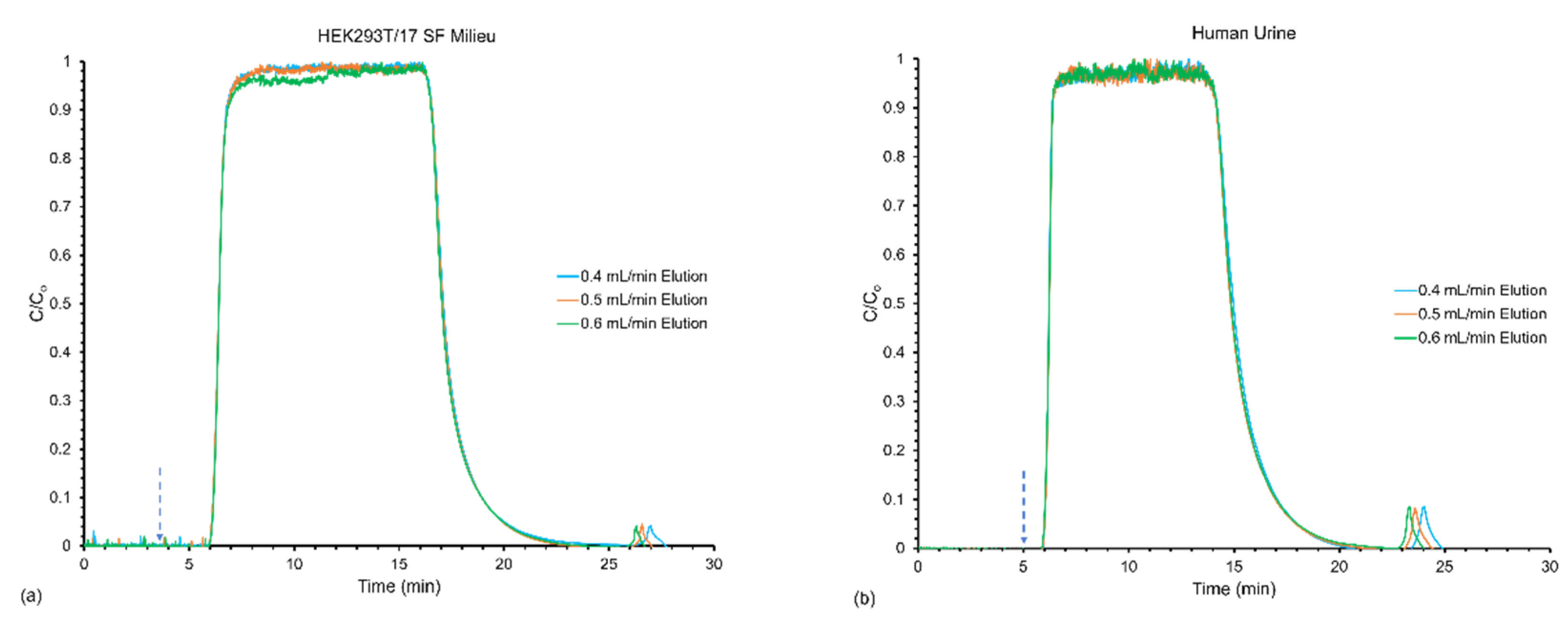
3.2. Effect of Flow Rates on Frontal Yields and Process Throughput
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutierrez-Millan, C.; Calvo Diaz, C.; Lanao, J.M.; Colino, C.I. Advances in Exosomes-Based Drug Delivery Systems. Macromol. Biosci. 2021, 21, e2000269. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Yan, H.; Li, Y.; Cheng, S.; Zeng, Y. Advances in Analytical Technologies for Extracellular Vesicles. Anal. Chem. 2021, 93, 4739–4774. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Watson, D.C.; Yung, B.C.; Bergamaschi, C.; Chowdhury, B.; Bear, J.; Stellas, D.; Morales-Kastresana, A.; Jones, J.C.; Felber, B.K.; Chen, X. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J. Extracell. Vesicles 2018, 7, 1442088. [Google Scholar] [CrossRef]
- Lamparski, H.G.; Metha-Damani, A.; Yao, J.-Y.; Patel, S.; Hsu, D.-H.; Ruegg, C.; Le Pecq, J.-B. Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods 2002, 270, 211–226. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef]
- Xitong, D.; Xiaorong, Z. Targeted therapeutic delivery using engineered exosomes and its applications in cardiovascular diseases. Gene 2016, 575, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Kothandan, V.K.; Oh, J.; Yoo, S.H.; Hwang, J.; Hwang, S.R. A pharmaceutical investigation into exosomes. J. Pharm. Investig. 2018, 48, 617–626. [Google Scholar] [CrossRef]
- Jiang, X.-C.; Gao, J.-Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Otta Oshiro, R.; Redruello, A.; López-Martín, S.; Gutiérrez-Vázquez, C.; Morato, E.; Marina, A.I.; Olivier Gómez, C.; Yáñez-Mó, M. Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur. J. Pharm. Sci. 2017, 98, 70–79. [Google Scholar] [CrossRef]
- Paganini, C.; Capasso Palmiero, U.; Pocsfalvi, G.; Touzet, N.; Bongiovanni, A.; Arosio, P. Scalable Production and Isolation of Extracellular Vesicles: Available Sources and Lessons from Current Industrial Bioprocesses. Biotechnol. J. 2019, 14, 1800528. [Google Scholar] [CrossRef] [PubMed]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef] [PubMed]
- Jong, A.Y.; Wu, C.H.; Li, J.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell. Vesicles 2017, 6, 1294368. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.-C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Wen, S.; Dooner, M.; Cheng, Y.; Papa, E.; Del Tatto, M.; Pereira, M.; Deng, Y.; Goldberg, L.; Aliotta, J.; Chatterjee, D.; et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016, 30, 2221–2231. [Google Scholar] [CrossRef]
- Didiot, M.-C.; Hall, L.M.; Coles, A.H.; Haraszti, R.A.; Godinho, B.M.D.C.; Chase, K.; Sapp, E.; Ly, S.; Alterman, J.F.; Hassler, M.R.; et al. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol. Ther. 2016, 24, 1836–1847. [Google Scholar] [CrossRef] [Green Version]
- Pi, F.; Binzel, D.W.; Lee, T.J.; Li, Z.; Sun, M.; Rychahou, P.; Li, H.; Haque, F.; Wang, S.; Croce, C.M.; et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat. Nanotechnol. 2018, 13, 82–89. [Google Scholar] [CrossRef]
- McNamara, R.P.; Caro-Vegas, C.P.; Costantini, L.M.; Landis, J.T.; Griffith, J.D.; Damania, B.A.; Dittmer, D.P. Large-scale, cross-flow based isolation of highly pure and endocytosis-competent extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1541396. [Google Scholar] [CrossRef]
- Bruce, T.F.; Slonecki, T.J.; Wang, L.; Huang, S.; Powell, R.R.; Marcus, R.K. Exosome isolation and purification via hydrophobic interaction chromatography using a polyester, capillary-channeled polymer fiber phase. Electrophoresis 2019, 40, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.; Bruce, T.F.; Marcus, R.K. Isolation and quantification of human urinary exosomes by hydrophobic interaction chromatography on a polyester capillary-channeled polymer fiber stationary phase. Anal. Bioanal. Chem. 2019, 411, 6591–6601. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bruce, T.F.; Huang, S.; Marcus, R.K. Isolation and quantitation of exosomes isolated from human plasma via hydrophobic interaction chromatography using a polyester, capillary-channeled polymer fiber phase. Anal. Chim. Acta 2019, 1082, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.; Bruce, T.F.; Marcus, R.K. Evaluation of exosome loading characteristics in their purification via a glycerol-assisted hydrophobic interaction chromatography method on a polyester, capillary-channeled polymer fiber phase. Biotechnol. Prog. 2020, 36, e2998. [Google Scholar] [CrossRef]
- Jackson, K.K.; Powell, R.R.; Bruce, T.F.; Marcus, R.K. Solid-phase extraction of exosomes from diverse matrices via a polyester capillary-channeled polymer (C-CP) fiber stationary phase in a spin-down tip format. Anal. Bioanal. Chem. 2020, 412, 4713–4724. [Google Scholar] [CrossRef]
- Ji, X.; Huang, S.; Zhang, J.; Bruce, T.F.; Tan, Z.; Wang, D.; Zhu, J.; Marcus, R.K.; Lubman, D.M. A novel method of high-purity extracellular vesicle enrichment from microliter-scale human serum for proteomic analysis. Electrophoresis 2021, 42, 245–256. [Google Scholar] [CrossRef]
- Jackson, K.K.; Powell, R.R.; Bruce, T.F.; Marcus, R.K. Rapid isolation of extracellular vesicles from diverse biofluid matrices via capillary-channeled polymer fiber solid-phase extraction micropipette tips. Analyst 2021, 146, 4314–4325. [Google Scholar] [CrossRef]
- Huang, S.; Ji, X.; Jackson, K.K.; Lubman, D.M.; Ard, M.B.; Bruce, T.F.; Marcus, R.K. Rapid separation of blood plasma exosomes from low-density lipoproteins via a hydrophobic interaction chromatography method on a polyester capillary-channeled polymer fiber phase. Anal. Chim. Acta 2021, 1167, 338578. [Google Scholar] [CrossRef]
- Jackson, K.K.; Powell, R.R.; Bruce, T.F.; Marcus, R.K. Facile, Generic Capture and On-Fiber Differentiation of Exosomes via Confocal Immunofluorescence Microscopy using a Capillary-Channeled Polymer Fiber Solid-Phase Extraction Tip. Sens. Diagnost. 2022, 1, 525–533. [Google Scholar] [CrossRef]
- Jackson, K.K.; Powell, R.R.; Marcus, R.K.; Bruce, T.F. Comparison of the capillary-channeled polymer (C-CP) fiber spin-down tip approach to traditional methods for the isolation of extracellular vesicles from human urine. Anal. Bioanal. Chem. 2022, 414, 3813–3825. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.M.; Marcus, R.K. Characterization of Capillary-Channeled Polymer Fiber Stationary Phases for High-Performance Liquid Chromatography Protein Separations: Comparative Analysis with a Packed-Bed Column. Anal. Chem. 2006, 78, 8462–8471. [Google Scholar] [CrossRef] [PubMed]
- Randunu, K.M.; Dimartino, S.; Marcus, R.K. Dynamic evaluation of polypropylene capillary-channeled fibers as a stationary phase in high-performance liquid chromatography. J. Sep. Sci. 2012, 35, 3270–3280. [Google Scholar] [CrossRef] [PubMed]
- Randunu, K.M.; Marcus, R.K. Initial evaluation of protein throughput and yield characteristics on nylon 6 capillary-channeled polymer (C-CP) fiber stationary phases by frontal analysis. Biotechnol. Prog. 2013, 29, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Randunu, K.M.; Marcus, R.K. Microbore polypropylene capillary channeled polymer (C-CP) fiber columns for rapid reversed-phase HPLC of proteins. Anal. Bioanal. Chem. 2012, 404, 721–729. [Google Scholar] [CrossRef]
- Nelson, D.M.; Marcus, R.K. Potential for Ultrafast Protein Separations with Capillary-Channeled Polymer (C-CP) Fiber Columns. Protein Pept. Lett. 2006, 13, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.K. Use of polymer fiber stationary phases for liquid chromatography separations: Part I–physical and chemical rationale. J. Sep. Sci. 2008, 31, 1923–1935. [Google Scholar] [CrossRef]
- Jackson, K.K.; Mata, C.; Marcus, R.K. A Rapid Capillary-Channeled Polymer (C-CP) Fiber Spin-Down Tip Approach for the Isolation of Plant-Derived Extracellular Vesicles (PDEVs) from 20 Common Fruit and Vegetable Sources. Talanta 2022, in press. [Google Scholar] [CrossRef]
- Jurgielewicz, B.J.; Yao, Y.; Stice, S.L. Kinetics and Specificity of HEK293T Extracellular Vesicle Uptake using Imaging Flow Cytometry. Nanoscale Res. Lett. 2020, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.; Kim, S.; Lee, C.; Deci, M.; Nguyen, J. The phenotypic effects of exosomes secreted from distinct cellular sources: A comparative study based on miRNA composition. AAPS J. 2018, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta-Rev. Cancer 2014, 1846, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Faruqu, F.N.; Xu, L.; Al-Jamal, K.T. Preparation of exosomes for siRNA delivery to cancer cells. JoVE 2018, 142, e58814. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Liu, Z.; Zhou, Y.; Chu, D.; Li, X.; Jiang, X.; Hou, D.; Chen, X.; Chen, Y. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci. Rep. 2015, 517543. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.L. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]
- Zhou, H.; Yuen, P.S.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef]
- Merchant, M.L.; Powell, D.W.; Wilkey, D.W.; Cummins, T.D.; Deegens, J.K.; Rood, I.M.; McAfee, K.J.; Fleischer, C.; Klein, E.; Klein, J.B. Microfiltration isolation of human urinary exosomes for characterization by MS. PROTEOMICS–Clin. Appl. 2010, 4, 84–96. [Google Scholar] [CrossRef]
- Jackson, K.K.; Kenneth Marcus, R. Rapid Isolation and Quantification of Extracellular Vesicles from Suspension-Adapted Human Embryonic Kidney Cells using Capillary-Channeled Polymer Fiber Spin-Down Tips. Electrophoresis 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Lehrich, B.M.; Liang, Y.; Khosravi, P.; Federoff, H.J.; Fiandaca, M.S. Fetal Bovine Serum-Derived Extracellular Vesicles Persist within Vesicle-Depleted Culture Media. Int. J. Mol. Sci. 2018, 19, 3538. [Google Scholar] [CrossRef]
- Eitan, E.; Zhang, S.; Witwer, K.W.; Mattson, M.P. Extracellular vesicle–depleted fetal bovine and human sera have reduced capacity to support cell growth. J. Extracell. Vesicles 2015, 4, 26373. [Google Scholar] [CrossRef]
- Huang, S.; Bruce, T.F.; Ding, H.; Wei, Y.; Marcus, R.K. Rapid isolation of lentivirus particles from cell culture media via a hydrophobic interaction chromatography method on a polyester, capillary-channeled polymer fiber stationary phase. Anal. Bioanal Chem. 2021, 413, 2985–2994. [Google Scholar] [CrossRef] [PubMed]
- Ladisch, M.R. Bioseparations Engineering: Principles, Practice, and Economics; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Carta, G.; Jungbauer, A. Protein Chromatography: Process. Development and Scale-Up; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Hearn, M.T.; Anspach, B. Chemical, physical, and biochemical concepts in isolation and purification of proteins. Sep. Purif. Methods 2001, 30, 221–263. [Google Scholar] [CrossRef]
- Guiochon, G.; Felinger, A.; Shirazi, D.G. Fundamentals of Preparative and Nonlinear Chromatography; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Vera-Avila, L.E.; Gallegos-Perez, J.L.; Camacho-Frias, E. Frontal analysis of aqueous phenol solutions in amberlite XAD-4 columns: Implications on the operation and design of solid phase extraction systems. Talanta 1999, 50, 509–526. [Google Scholar] [CrossRef]
- Singh, A.; Pinto, N.G. Polymeric short-fiber chromatographic supports for downstream processing of biomolecules. React. Polym. 1995, 24, 229–242. [Google Scholar] [CrossRef]
- Wang, Z.; Marcus, R.K. Determination of pore size distributions in capillary-channeled polymer fiber stationary phases by inverse size-exclusion chromatography and implications for fast protein separations. J. Chromatogr. A 2014, 1351, 82–89. [Google Scholar] [CrossRef]
- Wang, L.; Stevens, K.A.; Haupt-Renaud, P.; Marcus, R.K. Dynamic evaluation of a trilobal capillary-channeled polymer fiber shape for reversed phase protein separations and comparison to the eight-channeled form. J. Sep. Sci. 2018, 41, 1063–1073. [Google Scholar] [CrossRef]
- Wang, Z.; Marcus, R.K. Roles of Interstitial Fraction and Load Linear Velocity on the Dynamic Biding Capacity of Proteins on Capillary Channeled Polymer Fiber Columns. Biotechnol. Progr. 2015, 15, 97–109. [Google Scholar] [CrossRef]
- Leveque, M. The Laws of Convective Heat Transfer. Ann. Mines 1928, 13, 284. [Google Scholar]
- Bird, B.R.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; Wiley: New York, NY, USA, 1960. [Google Scholar]

| Sample | Loading and Elution Flow Rate (mL min−1) | Linear Velocity (mm s−1) | EVs Loaded (Q′) (Particles) (%RSD) | EVs Recovered (Q) (Particles) (%RSD) | Yield (%) (%RSD) | Throughput (T) (Particles min−1) (%RSD) |
|---|---|---|---|---|---|---|
| HEK293T/17 SF Milieu | 0.4 | 24.9 | 4.15 × 1012 (0.19) | 2.00 × 1012 (15) | 48.0 (15) | 5.92 × 1010 (15) |
| 0.5 | 31.1 | 4.88 × 1012 (0.93) | 3.19 × 1012 (24) | 65.0 (24) | 9.67 × 1010 (24) | |
| 0.6 | 37.4 | 5.69 × 1012 (0.09) | 2.85 × 1012 (10) | 50.0 (10) | 8.77 × 1010 (10) | |
| Human Urine | 0.4 | 24.9 | 3.19 × 1012 (0.16) | 2.81 × 1012 (20) | 70.0 (21) | 9.16 × 1010 (19) |
| 0.5 | 31.1 | 3.82 × 1012 (0.08) | 3.33 × 1012 (16) | 87.0 (14) | 1.11 × 1011 (16) | |
| 0.6 | 37.4 | 4.43 × 1012 (0.06) | 4.47 × 1012 (6.0) | 100 (5.0) | 1.52 × 1011 (6.0) |
| Sample | Loading and Elution Flow Rate (mL min−1) | Linear Velocity (mm s−1) | EVs Loaded (Q′) (Particles) (%RSD) | EVs Recovered (Q) (Particles) (%RSD) | Yield (%) (%RSD) | Throughput (T) (Particles min−1) (%RSD) |
|---|---|---|---|---|---|---|
| HEK293T/17 SF Milieu | 0.4 | 24.9 | 4.15 × 1012 (0.19) | 2.00 × 1012 (15) | 48.0 (15) | 5.92 × 1010 (15) |
| 0.5 | 31.1 | 4.88 × 1012 (0.93) | 3.19 × 1012 (24) | 65.0 (24) | 9.67 × 1010 (24) | |
| 0.6 | 37.4 | 5.69 × 1012 (0.09) | 2.85 × 1012 (10) | 50.0 (10) | 8.77 × 1010 (10) | |
| Human Urine | 0.4 | 24.9 | 3.19 × 1012 (0.16) | 2.81 × 1012 (20) | 70.0 (21) | 9.16 × 1010 (19) |
| 0.5 | 31.1 | 3.82 × 1012 (0.08) | 3.33 × 1012 (16) | 87.0 (14) | 1.11 × 1011 (16) | |
| 0.6 | 37.4 | 4.43 × 1012 (0.06) | 4.47 × 1012 (6.0) | 100 (5.0) | 1.52 × 1011 (6.0) |
| Sample | Loading Flow Rate (mL min−1) | Elution Flow Rate (mL min−1) | Linear Velocity (mm s−1) | EVs Loaded (Q′) (Particles) (%RSD) | EVs Recovered (Q) (Particles) (%RSD) | Yield (Y) (%) (%RSD) | Throughput (T) (Particles min−1) (%RSD) |
|---|---|---|---|---|---|---|---|
| HEK293T/17 SF Milieu | 0.5 | 0.4 | 24.9 | 4.90 × 1012 (0.20) | 7.79 × 1011 (27) | 16.0 (27) | 2.33 × 1010 (27) |
| 0.5 | 0.5 | 31.1 | 4.88 × 1012 (0.93) | 1.53 × 1012 (48) | 31.0 (49) | 4.64 × 1010 (48) | |
| 0.5 | 0.6 | 37.4 | 4.91 × 1012 (0.09) | 1.65 × 1012 (58) | 34.0 (58) | 5.04 × 1010 (58) | |
| Human Urine | 0.5 | 0.4 | 24.9 | 3.80 × 1012 (0.12) | 2.15 × 1012 (4.0) | 57.0 (5.0) | 7.12 × 1010 (4.0) |
| 0.5 | 0.5 | 31.1 | 3.80 × 1012 (0.15) | 2.60 × 1012 (5.0) | 68.0 (5.0) | 8.70 × 1010 (5.0) | |
| 0.5 | 0.6 | 37.4 | 3.80 × 1012 (0.08) | 3.63 × 1012 (12) | 96.0 (12) | 1.23 × 1011 (12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billotto, L.S.; Jackson, K.K.; Marcus, R.K. Determination of the Loading Capacity and Recovery of Extracellular Vesicles Derived from Human Embryonic Kidney Cells and Urine Matrices on Capillary-Channeled Polymer (C-CP) Fiber Columns. Separations 2022, 9, 251. https://doi.org/10.3390/separations9090251
Billotto LS, Jackson KK, Marcus RK. Determination of the Loading Capacity and Recovery of Extracellular Vesicles Derived from Human Embryonic Kidney Cells and Urine Matrices on Capillary-Channeled Polymer (C-CP) Fiber Columns. Separations. 2022; 9(9):251. https://doi.org/10.3390/separations9090251
Chicago/Turabian StyleBillotto, Lacey S., Kaylan K. Jackson, and R. Kenneth Marcus. 2022. "Determination of the Loading Capacity and Recovery of Extracellular Vesicles Derived from Human Embryonic Kidney Cells and Urine Matrices on Capillary-Channeled Polymer (C-CP) Fiber Columns" Separations 9, no. 9: 251. https://doi.org/10.3390/separations9090251
APA StyleBillotto, L. S., Jackson, K. K., & Marcus, R. K. (2022). Determination of the Loading Capacity and Recovery of Extracellular Vesicles Derived from Human Embryonic Kidney Cells and Urine Matrices on Capillary-Channeled Polymer (C-CP) Fiber Columns. Separations, 9(9), 251. https://doi.org/10.3390/separations9090251