Abstract
The recovery of phenolic compounds from olive leaves (Olea europaea L.) has received special attention due to their significant potential for applications in food, nutraceuticals, cosmetics, and pharmaceuticals. In this work, the extraction of the phenolic compounds from olive leaves was examined by means of conventional extraction and microwave-assisted extraction (MAE) using nontoxic common solvents such as ethanol and water as well as using promising environmentally friendly, Deep Eutectic Solvents (DESs) and their mixtures with ethanol or water. The effects of the various parameters that likely govern the extractability of the bioactive compounds of olive leaves (OL), such as the solvent type, temperature, and biomass to solvent mass ratio, were studied and evaluated with regard to the oleuropein and hydroxytyrosol content, antioxidant activity, and total phenolic content of the extracts. The study also explores the effects of the microwave-assisted extraction parameters, namely irradiation power and time, on the total phenolic content and antioxidant activity of the extracts. The findings of this work suggest that among the solvents studied, the solvent mixture consisting of the DES choline chloride:acetic acid with a molar ratio of 1:2 and ethanol (80:20 w/w) is highly effective in recovering extracts rich in phenolic compounds and with significant antioxidant activity. Moreover, it is demonstrated that the MAE method allows for the recovery of bioactive compounds in a very short processing time.
1. Introduction
Bioactive compounds constitute “extra nutritional” substances that are commonly present in natural raw material, such as plant material. In plants, bioactive compounds are produced as secondary metabolites that appear to have beneficial effects on human health [1]. The bioactive compounds in plants can be categorized into three vast categories: (a) terpenes and terpenoids, (b) alkaloids, and (c) phenolic compounds [2]. A variety of extraction techniques, including non-conventional methods, have been developed and investigated in order to exploit the bioactive compounds present in natural plants and have led to high-added-value products [3]. In addition, the global growing interest in environmentally friendly products points to the crucial significance of “green” extraction techniques for the food, pharmaceutical, and cosmetic industries [4].
Olive tree (Olea europaea L.), mainly found in Mediterranean countries [5], has been recognized as a source of a wide variety of bioactive compounds that are able to act as antioxidants [6], prevent certain diseases [7,8], and play an important role in the human diet [9]. In particular, olive leaves, a part of olive trees and a significant by-product of olive oil production [10], contain a large number of bioactive compounds, such as oleuropein, hydroxytyrosol, tyrosol, caffeic acid, ferulic acid, coumaric acid, vanillic acid [6], ligstroside, glucosides of rutin, luteolin [11], etc. The significant biological activity of olive leaves has mainly been attributed to oleuropein [12,13] and its derivatives, hydroxytyrosol and tyrosol [12]. Oleuropein has been proven to have antioxidative, antimicrobial, antiviral, antiatherogenic, cardioprotective antihypertensive, and anti-inflammatory properties [13,14]. Research studies have shown that through using an appropriate extraction method, an extract rich in these beneficial bioactive compounds with a high total phenolic content as well as high antioxidant and antimicrobial activity can be obtained [15,16,17,18,19]. Although pure oleuropein exhibits strong biological activities, the use of the whole olive leaf extract is considered to achieve better biological activity due to the synergetic effects of all of the bioactive compounds that are present in it [20].
In the past few years, several research works have been performed on the extraction of natural bioactive compounds from olive leaves [21,22,23,24,25,26,27,28]. Traditional extraction techniques such as maceration and Soxhlet, which use water or typical organic solvents such as ethanol, have been widely used [29]. Ultrasound-assisted extraction [30,31], microwave-assisted extraction [32], and supercritical fluid extraction [33,34] have been implemented as non-conventional technologies in order to optimize the extraction process, to maintain the chemical integrity and antioxidant activity of the bioactive compounds recovered from olive leaf, and, furthermore, to decrease the cost and the environmental footprint of the process. In addition, the need for non-toxic and biodegradable solvents has led to recent scientific efforts using Deep Eutectic Solvents (DES) as extraction agents of bioactive compounds, which generally come from bioresources [35] and, more specifically, from olive leaves [26,36,37]. DES constitute eutectic mixtures that are formed by combining one Hydrogen Bond Acceptor (HBA) and one Hydrogen Bond Donor (HBD) united by hydrogen bonding [38]. They present some remarkable physicochemical properties, such as a low melting temperature, negligible vapor pressure, non-flammability, low volatility, and water miscibility, and they are considered to be green and designer solvents [39,40]. The latter is a very important aspect of DES that enables the synthesis of solvents with the desired physicochemical properties by properly combining various HBAs with different HBDs. Owing to its low cost, biodegradability, and low toxicity, choline chloride (ChCl) has been widely used as an HBA to produce eutectic mixtures with inexpensive and sustainable HBDs such as urea and glycerol or renewably sourced carboxylic acids [26,35]. The main disadvantage of DES is their high viscosity. This demerit can be overcome in the extraction process by using a mixture of DES with a classical solvent such as ethanol or water in order to manipulate the viscosity and, moreover, to influence extraction yield and selectivity [26].
The objective of this work was to investigate the extraction of olive leaves using two different extraction methods: conventional extraction (maceration) and microwave-assisted extraction (MAE). To achieve this purpose, nontoxic conventional solvents such as ethanol, water, and an ethanol–water mixture as well as DES and mixtures of DES with conventional solvent mixtures, namely ChCl:urea (1:2), ChCl:acetic acid (1:2), a mixture of ChCl:urea (1:2) with water, a mixture of ChCl:acetic acid (1:2) with water, a mixture of ChCl:urea (1:2) with ethanol, and a mixture of ChCl:acetic acid (1:2) with ethanol, were assayed due to their potential for the effective extraction of phenolic compounds with high antioxidant activity. Parameters affecting the bioactive compound extraction, such as temperature and the ratio of biomass (olive leaves) to solvent mass for maceration as well as the irradiation power, the ratio of biomass to solvent mass, and extraction time for MAE were also investigated. The physical properties of the solvent, such as density and viscosity, can significantly affect mass transfer during the extraction process. Low solvent densities and viscosities lead to a higher solute solubility and higher diffusion from the solid to liquid phase, facilitating the penetration of the solvent to the cells of the biomass and the extraction of the target compounds. Therefore, the dynamic viscosity of ChCl:acetic acid (1:2) was experimentally determined in this work. As far as the density and dynamic viscosity of ChCl:urea (1:2) are concerned, they have already been included in a previous publication by our group [41].
2. Materials and Methods
2.1. Materials
Hydroxyphenethyl alcohol (tyrosol) (98+%; CAS Νο. 501-94-0; C8H10O2) was purchased from Carbosynth (Staad, Switzerland). Oleuropein (98+%; CAS Νο. 32619-42-4, C25H32O13), Folin–Ciocalteu reagent, DPPH (2,2-diphenyl-1-picrylhydrazyl, CAS Νο. 1898-66-4, C18H12N5O6), and anhydrous sodium carbonate (99.5%, CAS Νο. 497-19-8, Na2CO3) were obtained from Sigma Aldrich (St. Louis, MO, USA). Ethanol (99.9%; CAS No. 64-17-5; C2H6O), acetic acid (99.5%; CAS Νο. 64-19-7; C2H4O2), and urea (99%; CAS Νο. 57-13-6; CH4N2O) were obtained from Fisher Scientific (Hampton, NH, USA). Water (HPLC-gradient) (99.9%; CAS Νο. 7732-18-5; H2O) and gallic acid (98%, CAS Νο.149-91-7) were purchased from Honeywell (Charlotte, NC, USA) and Acros Organics (Geel, Belgium), respectively.
2.2. Plant Material
Fresh green olive leaves (Olea europaea L.) were collected from trees grown in the regional unit of Messinia, Greece. The collected leaves were double washed with distilled water, naturally dried at ambient temperature, and then milled using a laboratory mill. After that, they were sieved with a 100 and 425 μm sieve and were finally stored at 8–10 °C prior to extraction.
2.3. DES Synthesis
Choline chloride-based DES were prepared by mixing choline chloride with urea or acetic acid (AA) at a 1:2 molar ratio under continuous and vigorous stirring and heating at 80 °C until a homogeneous liquid was formed. The synthesis procedure was performed under an inert atmosphere. Due to their hygroscopic nature, the choline chloride and urea were dried under vacuum prior to synthesis, while the acetic acid was used without any further purification.
2.4. Density Measurements
Densities of ChCl:AA (1:2) were measured over a temperature range from 293.15 to 368.15 K at atmospheric pressure. A high-precision vibrating tube digital density meter (DA-640B, KEM, Kyoto, Japan) was used. The cell temperature was controlled automatically within 0.05 K. Calibration of the densimeter was performed using ultrapure water.
2.5. Viscosity Measurements
Dynamic viscosities of ChCl:AA (1:2) were measured over a temperature range from 293.15 to 363.15 K and following our previously published experimental method [42]. A Brookfield digital viscometer (LV-DVI-E, Toronto, ON, Canada) was used while the temperature was kept constant within ±0.01 °C by means of a Julabo F12 thermostated bath circulator (Seelbach, Germany). During the measurements, whether the viscosity demonstrated any time dependency was examined.
2.6. DPPH Free Radical Scavenging Activity
The determination of antioxidant activity (Trolox equivalent antioxidant capacity, TEAC) was based on the protocol described by Brand-Williams et al. [43]. Aliquots of extracts (0.1 mL) were reacted with 3.9 mL of DPPH solution. After agitation, the reaction mixture was incubated in the dark at room temperature for 30 min, and then, its absorbance at 515 nm was measured using a Shimadzu UV-1900i UV-Vis spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The DPPH solution served as a control, while a mixture of ethanol (3.9 mL) and extract sample (0.1 mL) served as a blank. Radical scavenging (SCA) was calculated using the following equation:
where Asample, Ablank, and Acontrol sare the absorbance of the sample, blank and control, respectively.
SCA = 100 × [1 − (Asample − Ablank)/Acontrol]
Results were expressed as the TEAC (mg Trolox per L of the extract) according to a standard curve. All measurements were performed in triplicate.
2.7. Determination of Total Phenolic Content (TPC)
The amount of total phenolic content in the extracts from the olive leaves was determined according to the Folin–Ciocalteu method [44]. Briefly, 0.1 mL of dissolved extract in methanol, 7.9 mL of deionized water, and 0.5 mL of Folin–Ciocalteu reagent were thoroughly mixed. After that, 1.5 mL of aqueous sodium carbonate (20% v/v) was added to the mixture, which was then vortexed and heated in a water bath at 40 °C for 30 min, and then, the mixture’s absorbance was measured. In the DES extracts, 3 mL of aqueous NaOH solution was added in order to avoid the precipitation of the DES. A mixture of water (8 mL), Folin–Ciocalteu reagent (0.5 mL), and sodium carbonate (1.5 mL) served as the control. All measurements were performed at 765 nm in a Shimadzu UV-1900i UV-Vis spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Phenolics were expressed as gallic acid equivalents according to a standard curve, and finally, the total phenolic content (TPC) was expressed as mg of gallic acid (GA) per L of extract (mg GA/L).
2.8. High-Performance Liquid Chromatography (HPLC) Analysis
The identification and quantification of oleuropein and hydroxytyrosol were performed by high-performance liquid chromatography (HPLC) (Shimadzu, Tokyo, Japan) using a μBondapack C18 column, particle size 10 μm, length 300 mm, diameter 3.9 mm, and a diode array UV detector. The method used was the same as the method previously described by Benavente-Garcia et al. [45] The mobile phases for the chromatographic analysis were as follows: (A) acetonitrile and (B) acetic acid/water (2.5:97.5 v/v). A linear gradient was run from 95% (A) and 5% (B) to 75% (A) and 25% (B) over 20 min; it changed to 50% (A) and (B) after 20 min (total time, 40 min); after 10 min, it changed to 20% (A) and 80% (B) (total time, 50 min); and after re-equilibration over 10 min (total time, 60 min), it returned to the initial composition. The elution was performed at 30 °C with a flow rate of 1 mL/min, and the absorbance changes were monitored at 280 nm. The oleuropein and hydroxytyrosol contents in the olive leaf extracts were identified according to their retention time and peak UV spectra in the extract chromatograms in comparison to the peaks of the external standards for oleuropein and hydroxytyrosol. The quantification was based on standard samples and calibration curves under the same conditions. Typical chromatograms of the standards and extracts can be found in the supplementary material.
2.9. Conventional Extraction (Maceration)
A conventional extraction procedure with heating was carried out by mixing and stirring a specific amount of dried and powdered OL with the following solvents: ethanol, water, ethanol–water mixture, ChCl:urea (1:2), ChCl:AA (1:2), ChCl:urea (1:2)–water mixture, ChCl:AA (1:2)–water mixture, ChCl:urea (1:2)–ethanol mixture, and ChCl:AA (1:2)–ethanol mixture. Extractions were performed at 55 and 70 °C for 24 h in a jacketed double glass vessel that was temperature controlled by means of a Julabo F12 thermostatic bath. The biomass to solvent mass ratios of 1:20 w/w and 1:30 w/w were tested. Following extraction, samples were centrifuged at 365–5835× g and were filtered under vacuum through a 0.45 μm cellulose membrane filter. Extracts were evaluated in terms of their total phenolic content (TPC), antioxidant radical scavenging (TEAC), and oleuropein and hydroxytyrosol content.
2.10. Microwave-Assisted Extraction (MAE)
MAE experiments were carried out using an MAS-II microwave extraction apparatus (Sineo Microwave Equipment Co., Ltd., Shanghai, China). Powdered and dried OL were extracted using ethanol as a solvent. The extraction parameters evaluated were the extraction temperature (40 and 70 °C), extraction time (5 and 30 min), irradiation power (500 and 750 Watt), and biomass to solvent mass ratios (1:10 w/w and 1:30 w/w). Following extraction, samples were centrifuged at 365× g for 10 min and were filtered under vacuum through a 0.45 μm cellulose membrane filter. Extracts were evaluated in terms of their total phenolic content (TPC) and antioxidant radical scavenging (TEAC).
3. Results and Discussion
3.1. Density and Viscosity Measurements
The experimental densities and viscosities of ChCl:AA (1:2) in the temperature range from 293.15 to 368.15 K and from 293.15 to 363.15 K, respectively, are reported in Table 1.

Table 1.
Experimental densities (ρ) as well as dynamic viscosities (η) of ChCl:AA (1:2) at atmospheric pressure.
For density, a linear equation was used to express its correlation with temperature:
In the above equation, ρ (g·cm−3) is the density, T is the temperature in K, and a and b are adjustable parameters. The values of these parameters were determined by minimizing the following objective function (OF1):
where ρexp and ρcal denote the experimental and calculated values of density, and NP is the number of experimental data points. The values of the adjustable parameters a and b are 1.297 g·cm−3 and −6.3·10−4 g·cm−3 K−1, respectively. A very low average absolute relative deviation (% AARD) equal to 0.07% was obtained, which indicates the linear correlation of density with temperature in the specific temperature range.
As far as viscosity is concerned, in order to investigate if ChCl:AA (1:2) is a Newtonian fluid or not, each measurement was repeated several times by applying different shear rates (γ). Viscosity remained constant regardless of the shear rates, indicating Newtonian behavior. As shown in Table 1, in the temperature range studied, the viscosity drastically decreases as the temperature increases. The Vogel–Tammann–Fulcher (VFT) equation was applied to describe the variation in viscosity in mPa·s with respect to temperature in K as follows:
where A, B, and T0 are adjustable parameters determined by minimizing the following objective function (OF2):
where ηexp and ηcal denote the experimental and calculated values of dynamic viscosity, and NP is the number of experimental data points. The values of the adjustable parameters A, B, and T0 are 0.5318 (mPa·s), 447.05 K, and 208.3 K, respectively. A considerably small % AARD value is obtained (1.48%), indicating that the Vogel–Tammann–Fulcher equation provides an excellent description of the temperature dependence of viscosity.
3.2. Conventional Extraction (Maceration)
The OL extracts obtained via maceration extraction were evaluated with regard to the oleuropein and hydroxytyrosol content since oleuropein is one of the most abundant phenolic compound present in OL, and hydroxytyrosol is its main derivative. Their concentrations in the extracts are presented in Table 2. The total phenolic content and antioxidant activity were also measured, and the results are shown in Table 3.

Table 2.
Oleuropein and hydroxytyrosol concentrations of the extracts obtained by conventional extraction.

Table 3.
Antioxidant activity and total phenolic content of the extracts obtained by conventional extraction.
Table 2 shows that ethanol is a good solvent for oleuropein, especially at higher temperatures and solvent to biomass mass ratios. The maximum value of oleuropein (29,580 mg/L) was obtained at a temperature of 70 °C and a biomass to solvent mass ratio of 1:30. On the other hand, water is not a good candidate for the extraction of oleuropein from OL, which is due to oleuropein being more soluble in solvents with medium polarity, such as ethanol, than in strongly polar ones, such as water. These findings are in line with other literature data [46]. As for non-conventional solvents, although pure DES provide extracts with a relatively lower oleuropein content compared to ethanol, their mixtures with ethanol show a very high oleuropein extraction capacity. If the extracts obtained at 55 °C and at the biomass to solvent mass ratio equal to 1:20 are compared, then it can be observed that the solvent mixture ChCl:AA (1:2)–ethanol (80:20 w/w) is the best candidate for oleuropein. This implies that the addition of ethanol leads to reinforced interactions with oleuropein and improved mass transfer due to the decrease in the viscosity. The addition of water as a co-solvent either to ethanol or to DES negatively affects oleuropein recovery compared to that extracted with pure solvents. The increase in the temperature and solvent to biomass mass ratio resulted in a positive impact on the extraction efficiency of ethanol and ChCl:urea (1:2). The opposite case is true for ChCl:AA (1:2), whose best performance was obtained at lower temperatures and solvent to biomass mass ratios.
As far as hydroxytyrosol is concerned, most of the extracts contained no detectable traces. The highest hydroxytyrosol concentration (351 mg/L) was achieved using a ChCl:urea (1:2)–water (70:30 w/w) mixture at 70 °C and at a biomass to solvent mass ratio of 1:20.
Regarding the antioxidant activity of the OL extracts (Table 3), it is shown that among all of the extracts, those obtained by the solvent ChCl:AA (1:2)–ethanol (80:20 w/w) have the strongest antioxidant activity and the highest amount of phenolics. It is shown that higher temperatures promote an increase in both antioxidant activity and phenolic content (Figure 1a,b), which is due to the higher molecular motion, which increases solubility. The increasing temperature may also cause increases in intra-cellular pressure that may lead to cell rupture and thus increased extraction rates. One the other hand, no considerable impact of the biomass to solvent mass ratio on the antioxidant activity and phenolic content of the extracts was observed.
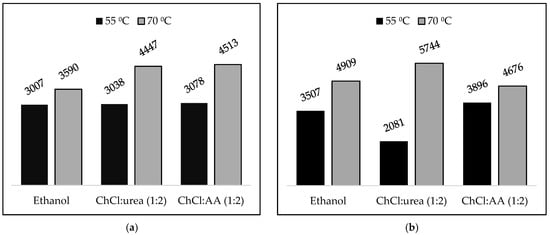
Figure 1.
Effect of temperature οn (a) antioxidant activity (mg Trolox/L) and (b) total phenolic content (mgGA/L) in extracts obtained by conventional extraction at the biomass to solvent mass ratio of 1:20 w/w.
3.3. Microwave-Assisted Extraction
The total phenolic content and antioxidant activity of the extracts obtained with MAE using ethanol as a solvent are provided in Table 4.

Table 4.
Antioxidant activity and total phenolic content of the extracts obtained from OL using MAE.
As shown in Figure 2a, under MAE, stronger antioxidant activity and higher amounts of phenolics were obtained at high temperatures. Additionally, Figure 2b demonstrates a decrease in the amount of phenolics recovered and in the antioxidant activity as the biomass to solvent mass ratio decreased. In addition to this, the lower irradiation power was proven to be most effective in obtaining extracts with stronger antioxidant activity and a higher phenolic content, as seen in Figure 2c. As for the extraction time, the results do not allow for any evident conclusions.
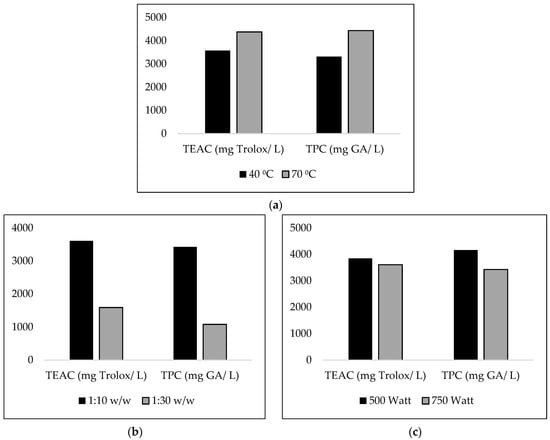
Figure 2.
Effect of (a) temperature (1:10 w/w, 30 min, 500 Watt), (b) biomass to solvent mass ratio (40 °C, 5 min, 750 Watt), and (c) irradiation power (1:10 w/w, 40 °C, 5 min) on antioxidant activity (TEAC) and total phenolic content (TPC).
3.4. Comparison between Conventional and Microwave-Assisted Extraction
The comparison of the results obtained for the antioxidant activity and total phenolic content of the extracts obtained by conventional extraction (maceration) with ethanol as the solvent (Table 3) to those obtained by microwave-assisted extraction also using ethanol as a solvent (Table 4) suggests that both methods produce comparable values. Temperature affects both maceration and MAE in the same way, i.e., as the temperature increases, both the antioxidant activity and phenolic content increase. On the other hand, although no significant impact of the biomass to solvent mass ratio on the antioxidant activity and phenolic content of the maceration extracts was observed, for the MAE extracts, an increase in the amount of phenolics recovered and in the antioxidant activity was observed as the solvent to biomass mass ratio decreased. In conclusion, it is worth mentioning that MAE can provide extracts with a comparable phenolic and antioxidant profile to those obtained via conventional extraction and has the advantage of a much shorter extraction time and lower solvent consumption.
4. Conclusions
In the present study, the extraction of olive leaves using conventional and microwave-assisted extraction was investigated using classical (ethanol, water) and alternative (DES) solvents. The extracts recovered using both methods revealed that olive leaves are a good source of phenolic compounds and have high antioxidant activity. The results of conventional extraction showed that ethanol is the most effective solvent for the recovery of oleuropein. The DES investigated in this work, although not the best solvents when used alone, were demonstrated to be highly efficient during oleuropein extraction, especially when mixed with ethanol. The ChCl:AA (1:2)–ethanol (80:20 w/w) solvent mixture possessed the strongest phenolic content and antioxidant activity of the extracts obtained by conventional extraction. Regarding the operational parameters for conventional extraction, it was shown that the higher the temperature, the higher the oleuropein and phenolic content and antioxidant activity, while, generally, lower biomass to solvent mass ratios favored the recovery of oleuropein. As far as the MAE of olive leaves is concerned, it was found that high temperatures, high biomass to solvent mass ratios, and low irradiation power lead to extracts with a high phenolic content and high antioxidant activity. Compared to the conventional method, it can be stated that MAE can be used as an alternative effective time-saving extraction method.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/separations9090255/s1, Figure S1: HPLC chromatograms of tyrosol (7.8 min), hydroxytyrosol (11.02 min), and oleuropein (24.78 min) standards; Figure S2: HPLC chromatogram of OL extract (solvent: ChCl:urea (1:2), T = 55 °C, mass ratio 1:30 w/w, t = 24 h); Figure S3: HPLC chromatogram of OL extract (solvent: ChCl:AA (1:2), T = 55 °C, mass ratio 1:30 w/w); Figure S4: HPLC chromatogram of OL extract (solvent: ethanol = 55 °C, mass ratio 1:20 w/w).
Author Contributions
Conceptualization, E.V., K.M., H.S., G.P. and V.L.; methodology, E.B.; validation, V.L. and G.P.; formal analysis, E.B.; investigation, E.B. and N.P.; writing—original draft preparation, E.B. and N.P., writing—review and editing E.V., V.L. and G.P.; visualization, E.B.; supervision, E.V., K.M. and H.S.; project administration, E.V. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T1EDK-01716).
Acknowledgments
Authors would like to thank M. Nikolaou for her contributions to the experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bernhoft, A. A brief review on bioactive compounds in plants. In Bioactive Compounds in Plants-Benefits and Risks for Man and Animals; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010; Volume 50, pp. 11–17. [Google Scholar]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites). Biochem. Mol. Biol. Plants 2000, 24, 1250–1319. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Ansari, M.; Kazemipour, M.; Fathi, S. Development of a simple green extraction procedure and HPLC method for determination of oleuropein in olive leaf extract applied to a multi-source comparative study. J. Iran. Chem. Soc. 2011, 8, 38–47. [Google Scholar] [CrossRef]
- Yuan, J.-J.; Wang, C.-Z.; Ye, J.-Z.; Tao, R.; Zhang, Y.-S. Enzymatic hydrolysis of oleuropein from Olea europea (olive) leaf extract and antioxidant activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef] [PubMed]
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. LWT-Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Lins, P.G.; Marina Piccoli Pugine, S.; Scatolini, A.M.; de Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef]
- Acar-Tek, N.; Ağagündüz, D. Olive Leaf (Olea europaea L. folium): Potential Effects on Glycemia and Lipidemia. Ann. Nutr. Metab. 2020, 76, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Le Tutour, B.; Guedon, D. Antioxidative activities of Olea europaea leaves and related phenolic compounds. Phytochemistry 1992, 31, 1173–1178. [Google Scholar] [CrossRef]
- Lee, O.-H.; Lee, B.-Y.; Lee, J.; Lee, H.-B.; Son, J.-Y.; Park, C.-S.; Shetty, K.; Kim, Y.-C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Bilgin, M.; Dramur, M.U. Investigation of Oleuropein Content in Olive Leaf Extract Obtained by Supercritical Fluid Extraction and Soxhlet Methods. Sep. Sci. Technol. 2011, 46, 1829–1837. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.; Masson, L.; Barriga, A.; Chávez, J.; Robert, P. Oxidative stability of oils containing olive leaf extracts obtained by pressure, supercritical and solvent-extraction. Eur. J. Lipid Sci. Technol. 2011, 113, 497–505. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. Olive Leaves through the Evaluation of Their Extracts: Antioxidant and Antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Papoti, V.T. Chapter 39—Bioactive Ingredients in Olive Leaves. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 349–356. [Google Scholar] [CrossRef]
- Bisignano, G.; Tomaino, A.; Lo Cascio, R.; Crisafi, G.; Uccella, N.; Saija, A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Oleuropein and related secoiridoids. Antioxidant activity and sources other than Olea europaea L. (olive tree). Recent Prog. Med. Plants 2009, 25, 53–74. [Google Scholar]
- Lee, O.-H.; Lee, B.-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef]
- Xynos, N.; Papaefstathiou, G.; Gikas, E.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.-L. Design optimization study of the extraction of olive leaves performed with pressurized liquid extraction using response surface methodology. Sep. Purif. Technol. 2014, 122, 323–330. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; del Mar Contreras, M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef]
- Xie, P.-J.; Huang, L.-X.; Zhang, C.-H.; You, F.; Zhang, Y.-L. Reduced pressure extraction of oleuropein from olive leaves (Olea europaea L.) with ultrasound assistance. Food Bioprod. Process. 2015, 93, 29–38. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times. Foods 2014, 3, 66–81. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Pontes, P.V.; Ayumi Shiwaku, I.; Maximo, G.J.; Caldas Batista, E.A. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Mkaouar, S.; Bahloul, N.; Gelicus, A.; Allaf, K.; Kechaou, N. Instant controlled pressure drop texturing for intensifying ethanol solvent extraction of olive (Olea europaea) leaf polyphenols. Sep. Purif. Technol. 2015, 145, 139–146. [Google Scholar] [CrossRef]
- Abi-Khattar, A.-M.; Rajha, H.N.; Abdel-Massih, R.M.; Maroun, R.G.; Louka, N.; Debs, E. Intensification of Polyphenol Extraction from Olive Leaves Using Ired-Irrad®, an Environmentally-Friendly Innovative Technology. Antioxidants 2019, 8, 227. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- Şahin, S.; Şamlı, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef]
- Şahin, S.; Samli, R.; Tan, A.S.B.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-Free Microwave-Assisted Extraction of Polyphenols from Olive Tree Leaves: Antioxidant and Antimicrobial Properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef]
- Baldino, L.; Della Porta, G.; Osseo, L.S.; Reverchon, E.; Adami, R. Concentrated oleuropein powder from olive leaves using alcoholic extraction and supercritical CO2 assisted extraction. J. Supercrit. Fluids 2018, 133, 65–69. [Google Scholar] [CrossRef]
- de Lucas, A.; Martinez de la Ossa, E.; Rincón, J.; Blanco, M.A.; Gracia, I. Supercritical fluid extraction of tocopherol concentrates from olive tree leaves. J. Supercrit. Fluids 2002, 22, 221–228. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S. Choline chloride–based deep eutectic solvents (Ch-DESs) as promising green solvents for phenolic compounds extraction from bioresources: State-of-the-art, prospects, and challenges. Biomass Convers. Biorefinery 2020, 12, 2949–2962. [Google Scholar] [CrossRef]
- Ünlü, A.E. Green and Non-conventional Extraction of Bioactive Compounds from Olive Leaves: Screening of Novel Natural Deep Eutectic Solvents and Investigation of Process Parameters. Waste Biomass Valorization 2021, 12, 5329–5346. [Google Scholar] [CrossRef]
- Şahin, S.; Kurtulbaş, E.; Bilgin, M. Special designed deep eutectic solvents for the recovery of high added-value products from olive leaf: A sustainable environment for bioactive materials. Prep. Biochem. Biotechnol. 2021, 51, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Prinos, N.; Boli, E.; Louli, V.; Pappa, G.; Magoulas, K.; Voutsas, E. Solubilities of Caffeic acid and Tyrosol in two Protic Ionic Liquids and one Deep Eutectic Solvent. Fluid Phase Equilibria 2022, 559, 113462. [Google Scholar] [CrossRef]
- Boli, E.; Katsavrias, T.; Voutsas, E. Viscosities of pure protic ionic liquids and their binary and ternary mixtures with water and ethanol. Fluid Phase Equilibria 2020, 520, 112663. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Cifá, D.; Skrt, M.; Pittia, P.; Di Mattia, C.; Poklar Ulrih, N. Enhanced yield of oleuropein from olive leaves using ultrasound-assisted extraction. Food Sci. Nutr. 2018, 6, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).