Estimation of Anthocyanins in Heterogeneous and Homogeneous Bean Landraces Using Probabilistic Colorimetric Representation with a Neuroevolutionary Approach
Abstract
1. Introduction
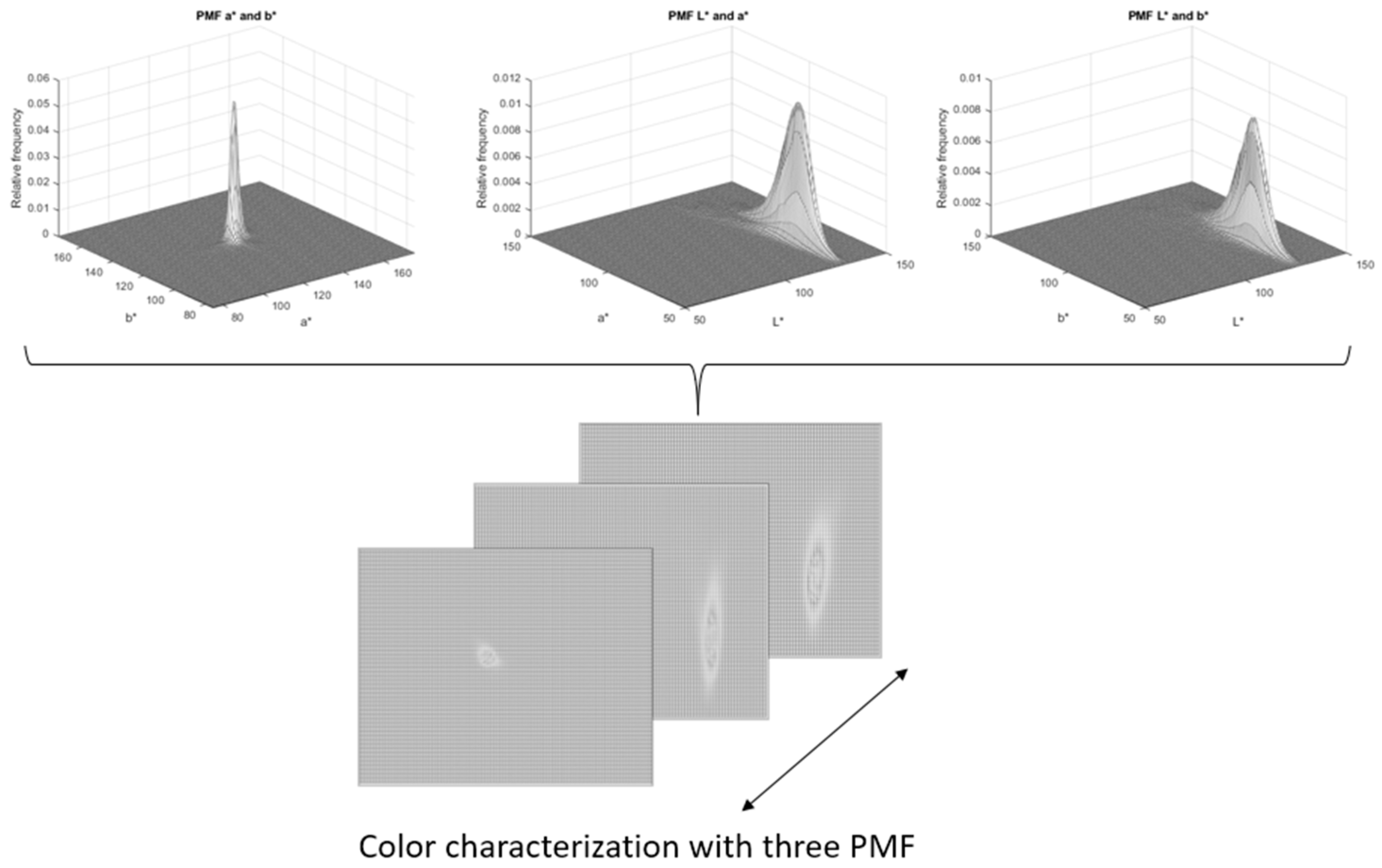
- An exploration of color characterization using two color spaces (HSI and CIE L*a*b*) to create two-dimensional histograms, using the probability mass function. Our main contribution is the color characterization represented with three two-dimensional histograms: the first color characterization (a* and b*, L* and a*, L* and b*) and the second color characterization (H and S, H and I, and S and I). We compared our color characterization and the color characterization created using only one two-dimensional histogram (chromaticity channel);
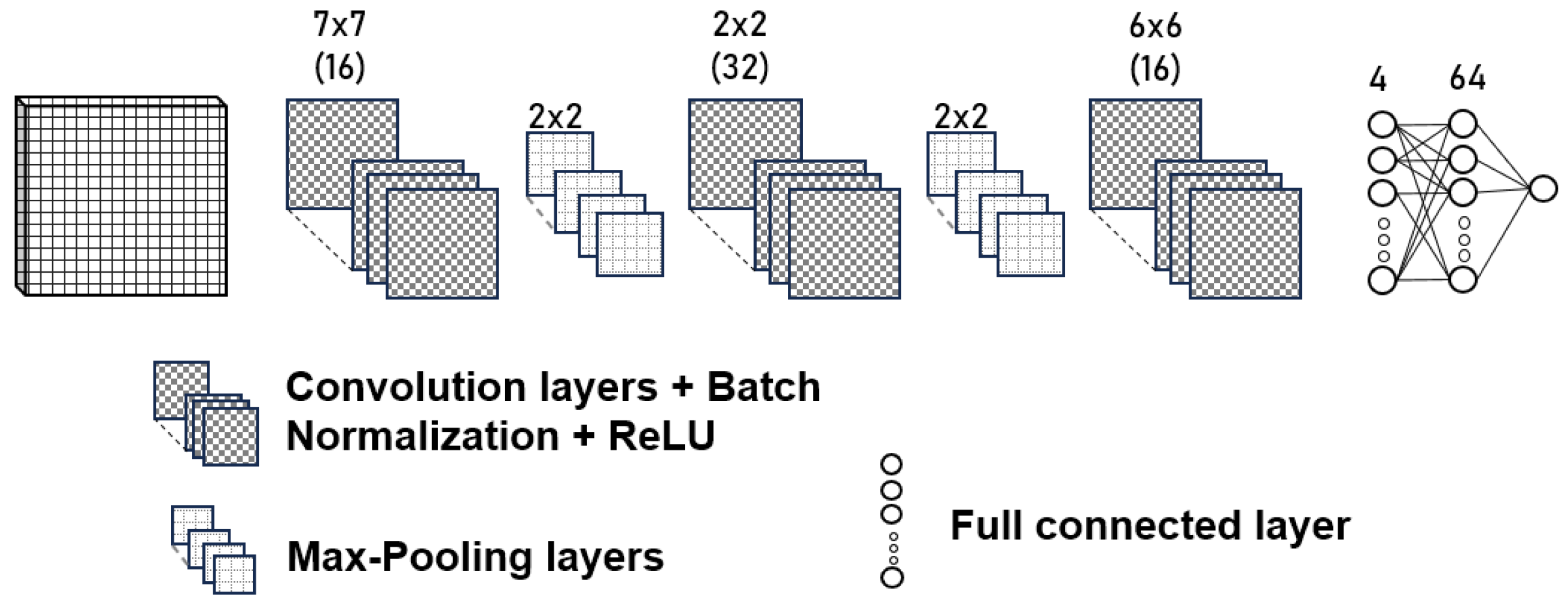
- It is a challenge to design convolutional neural network architecture for a specific task. Providing an architecture suitable for estimating anthocyanins in common bean landraces with homogeneous and heterogeneous coloring was considered using a neuroevolution approach. For this reason, the framework DeepGA was used to design the convolutional neural network architecture for our problem and to reduce the estimation error.
2. Materials and Methods
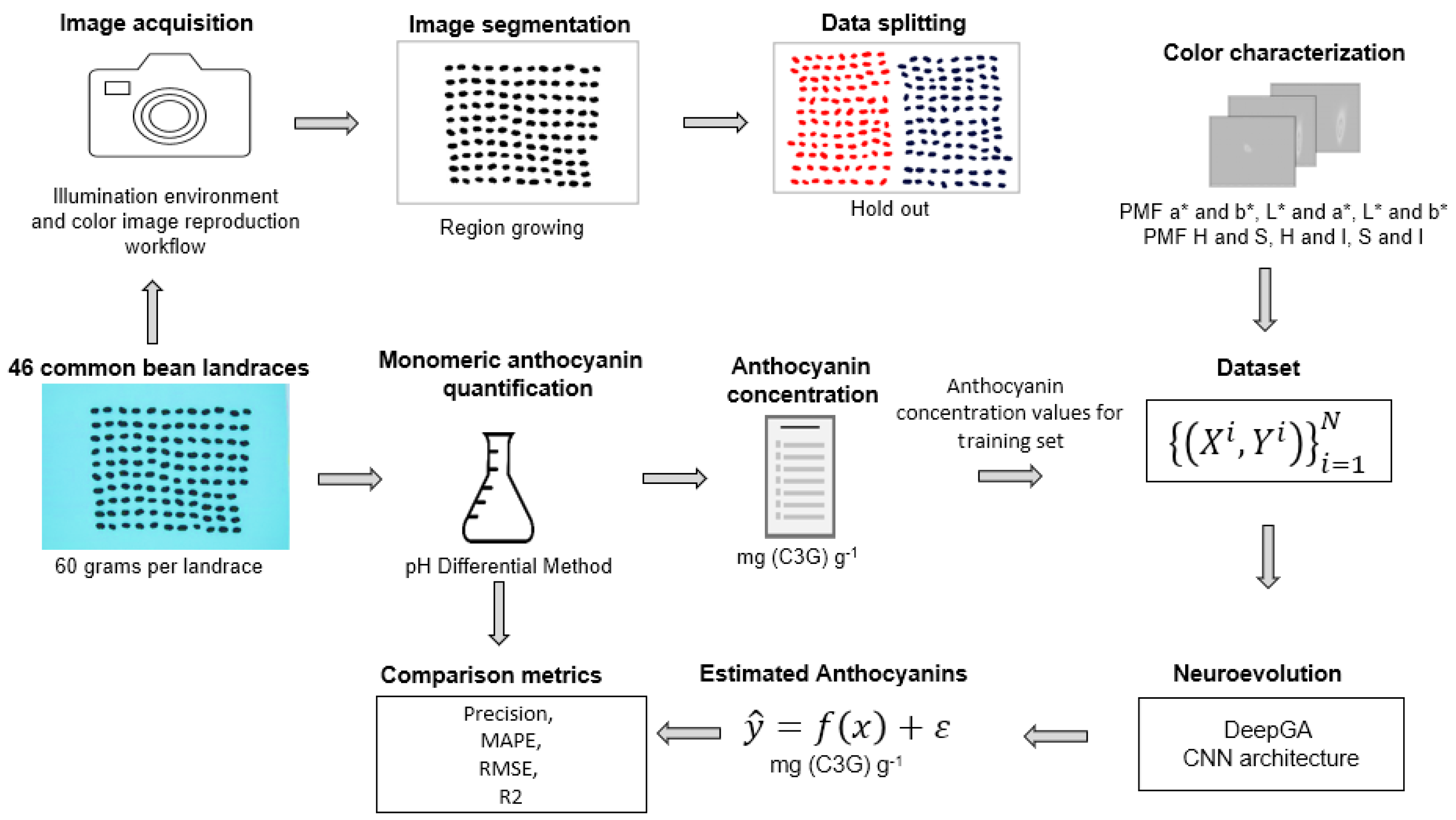
2.1. Workflow of the Study
- For the production of photographs of bean landraces, it was necessary to have the ideal lighting conditions and shooting settings to standardize the photographic acquisitions;
- Using a segmentation algorithm is pivotal in separating the background from the regions of interest. In this study, the color of the bean landrace seeds is the primary area of interest;
- Color characterization: after obtaining the colorimetric characteristics, these were represented as a joint probability distribution model. Histograms are appropriate for the color characterization of common bean landraces;
- Learning techniques play a significant role. Specifically, this study employed the neuroevolution technique to find the CNN architecture suitable for the estimation of anthocyanin concentrations;
- For a comparison of the methods, the anthocyanin estimation results were compared with the anthocyanin determination results from the pH differential method.
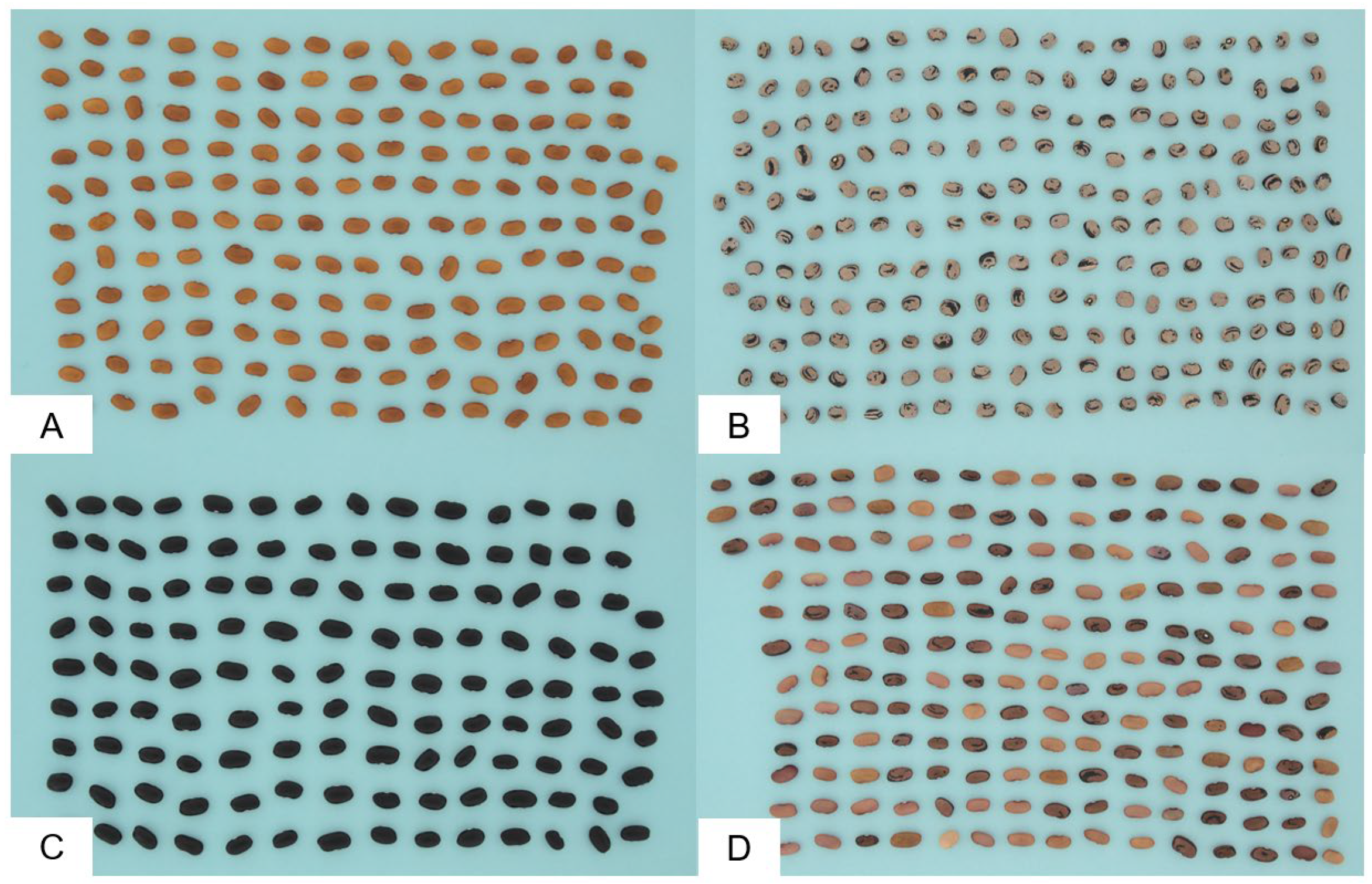
2.2. Common Bean Landraces
- Eighteen black bean landraces;
- Nine red bean landraces;
- Eight yellow bean landraces;
- Four white bean landraces;
- One brown bean landrace;
- Six heterogeneous colored bean landraces.
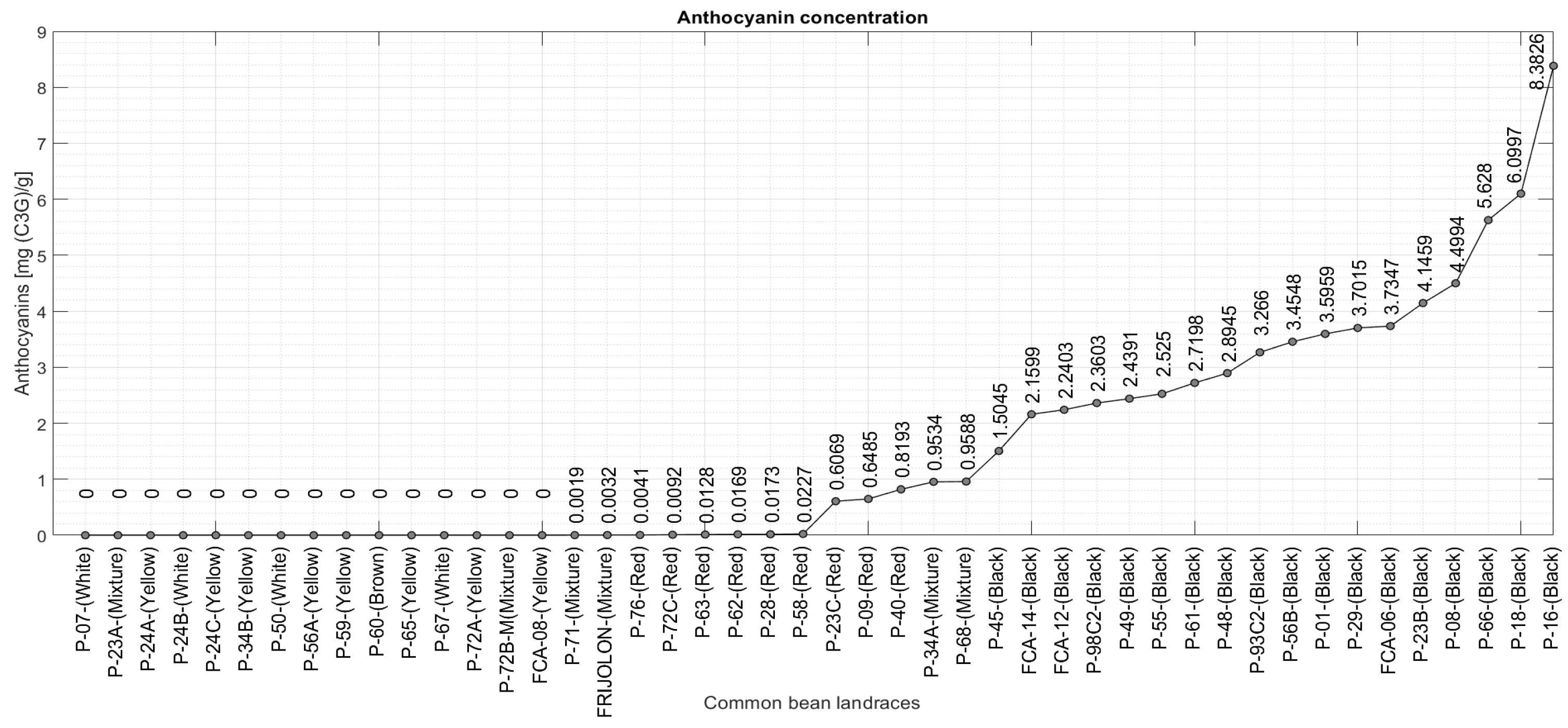
2.3. Quantification of Monomeric Anthocyanins
2.4. Acquisition System and Image Segmentation
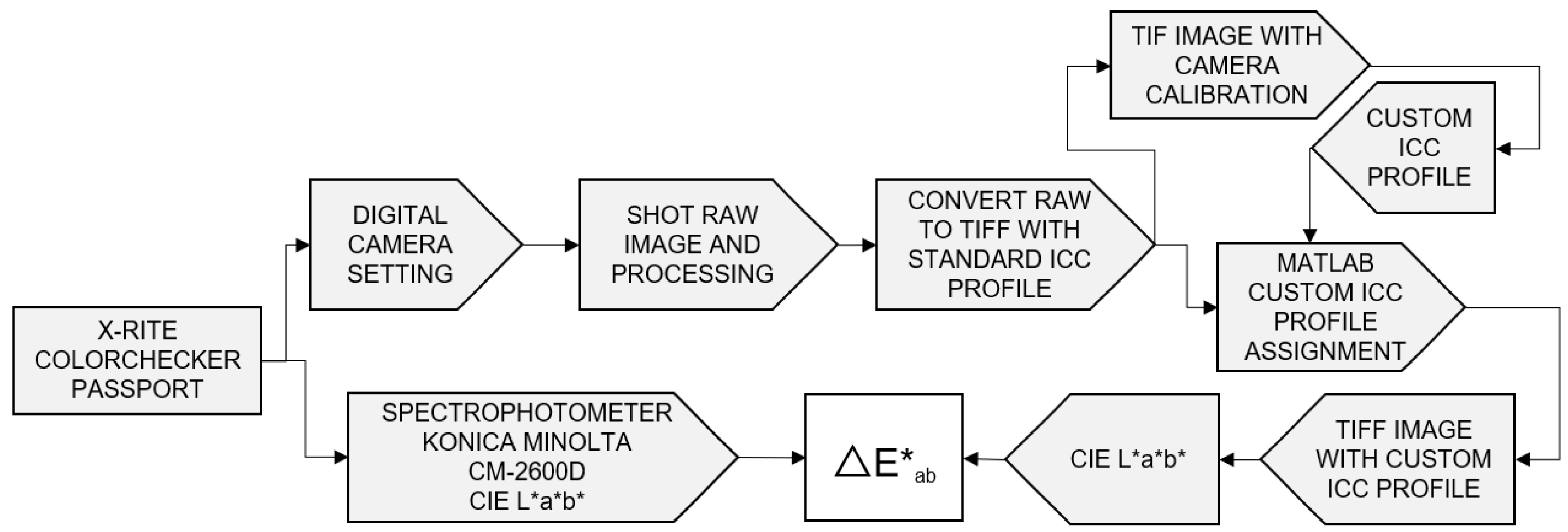
- The color management: First, for the image capture, a SONY ILSE 3500 digital camera was employed, with a focal length of 50 mm, the setting set at ISO 100, a shutter speed of 1/60, an aperture of f/8.0, and a white balance customized to using as a reference the X-Rite ColorChecker Passport’s white inner chart in a controlled environment. Second, the X-Rite ColorChecker Passport’s classic chart with 24 patches, model 2014, was used as a reference for shooting the RAW images; third, to convert the RAW images into TIFF images, the Darktable software 4.4 was used and the color space sRGB without compression and a standard ICC profile was established. Fourth, for the custom ICC profile calculation, X-Rite ColorChecker Camera Calibration v2.0 was utilized to create a custom ICC profile; next, to convert the color space sRGB into CIE L*a*b*, MATLAB software version 2023b and the Image Processing Toolbox were used to replace the standard ICC profile with a custom ICC profile. For a comparison between the laboratory equipment and the image reproduction workflow, a Konica Minolta CM-2600s spectrophotometer was used to measure the 24 patches in the X-Rite ColorChecker Passport’s classic chart; each patch was measured six times and the average of the values of the parameters L*, a*, and b* was determined. Finally, the color accuracy delta E was computed, achieving a maximum value of 11.8 and an average of 5.5.
2.5. Neuroevolution and DeepGA
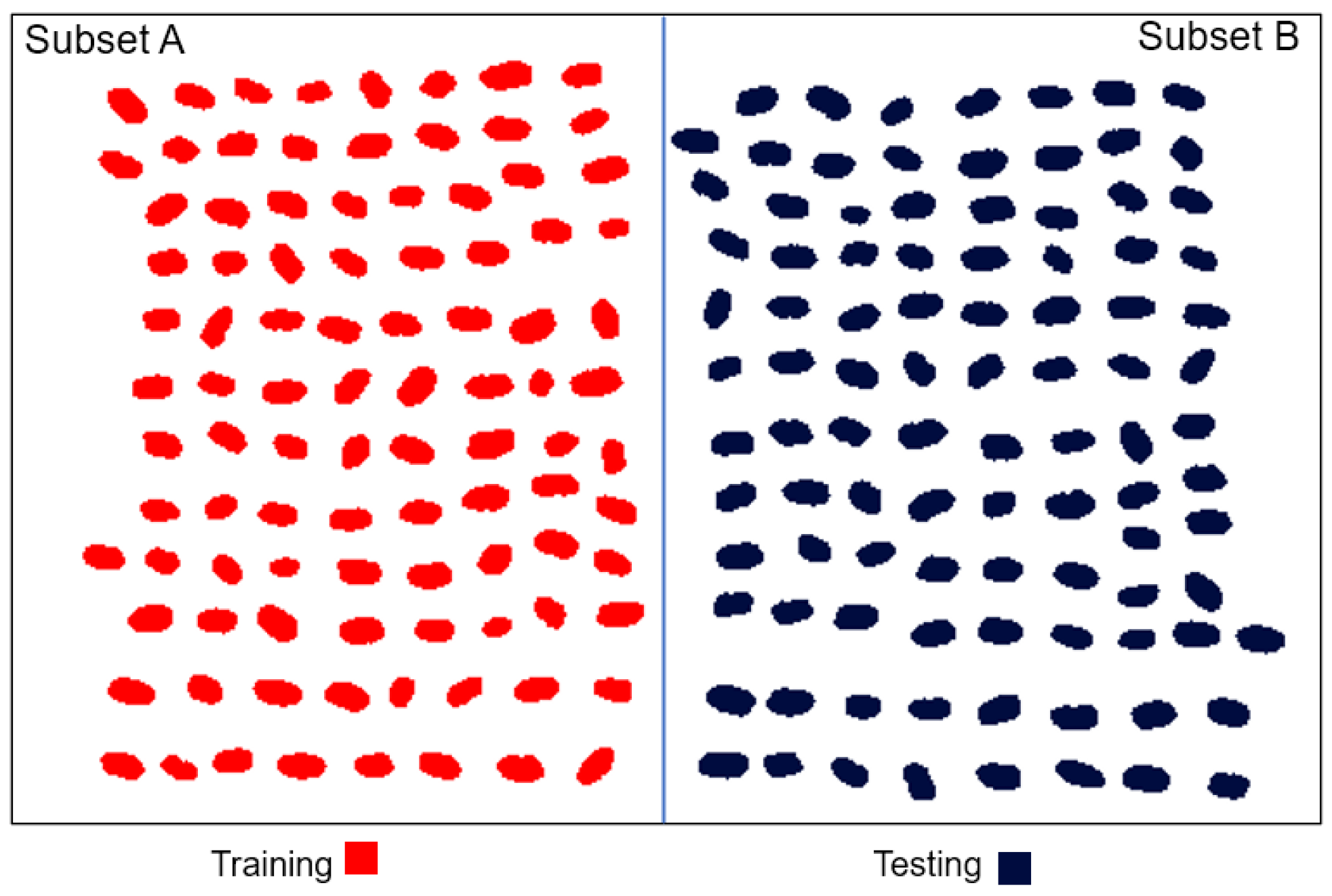
2.6. Data Splitting
2.7. Color Characterization Using a Probability Mass Function
2.7.1. Metric Performance
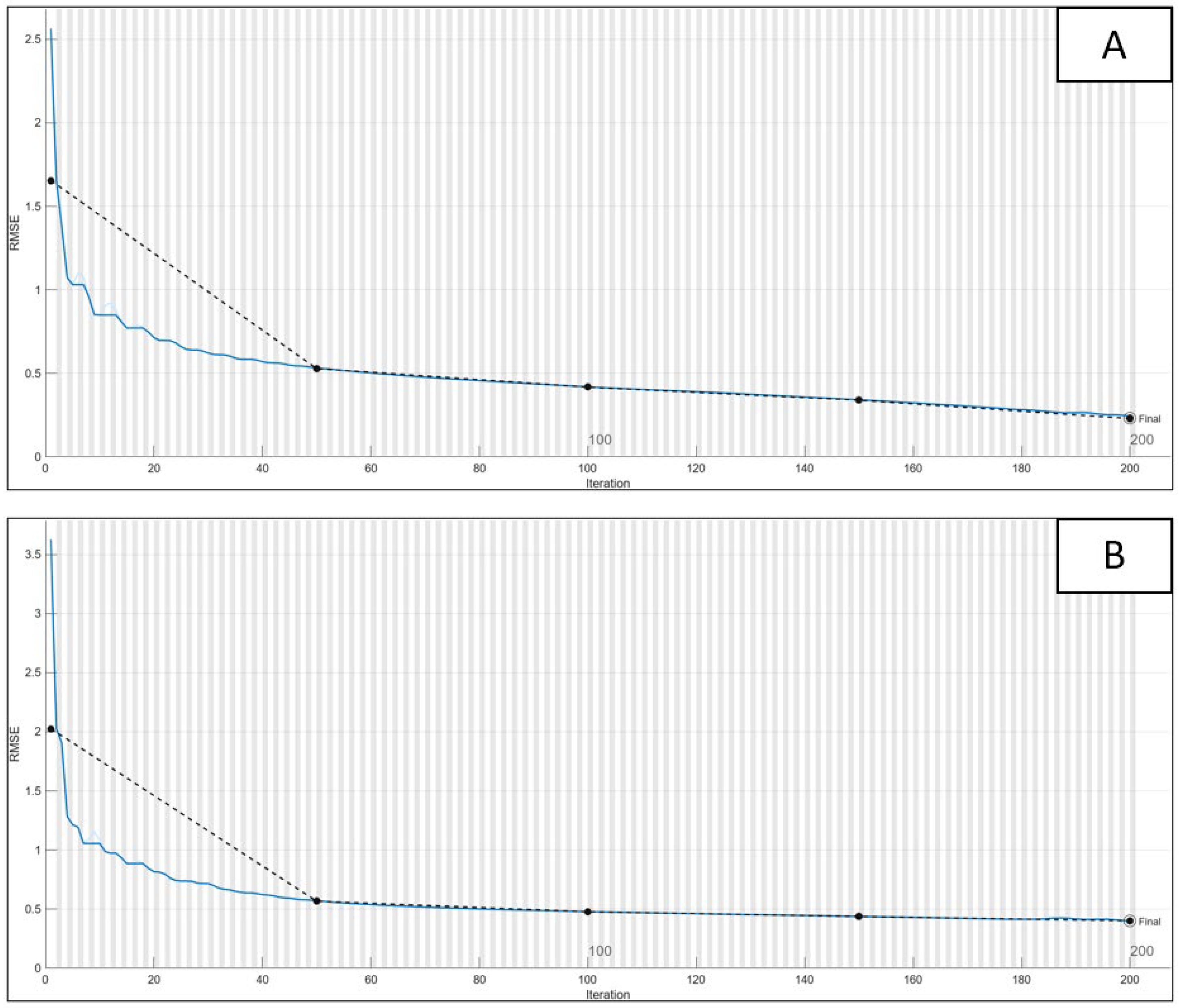
2.7.2. Experiment Design
2.7.3. CNN Optimized through DeepGA
3. Results
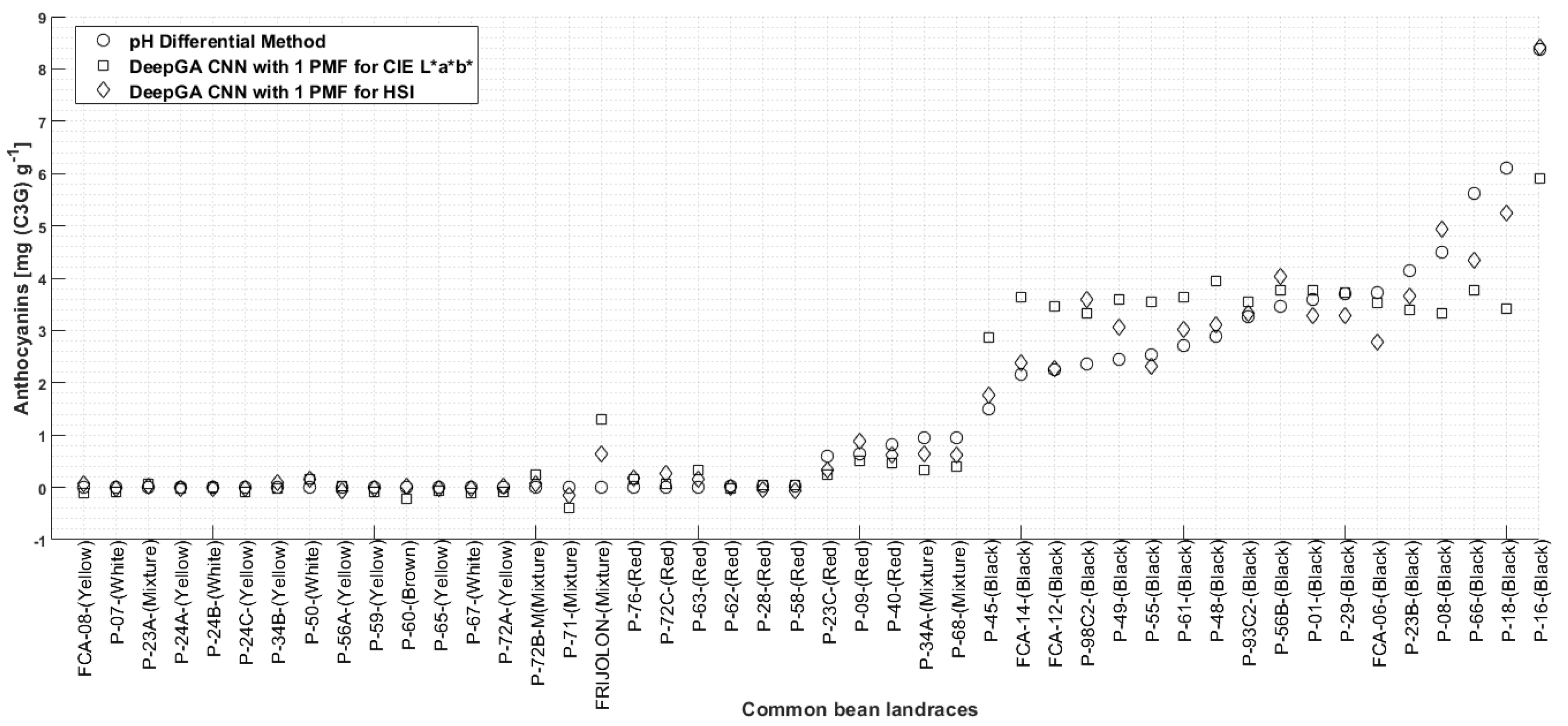
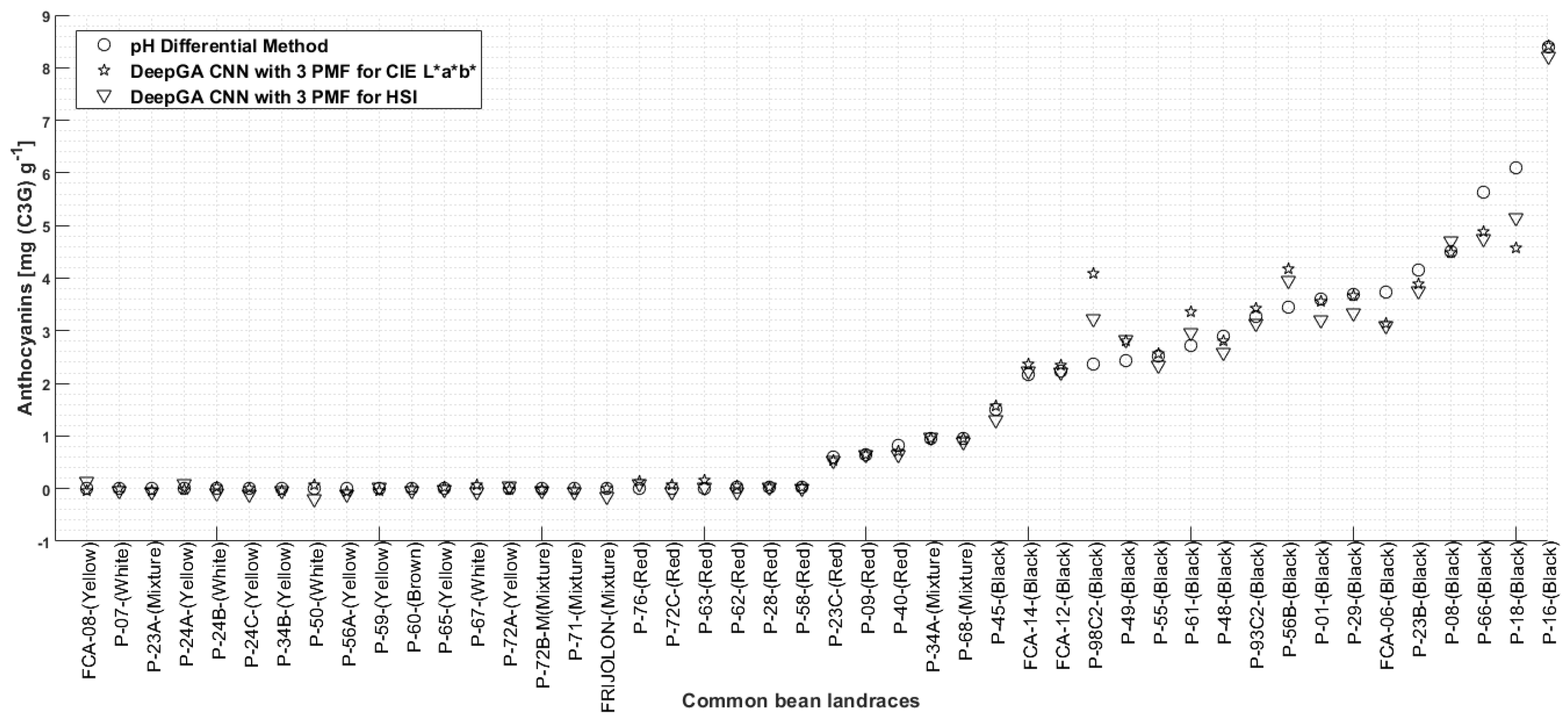
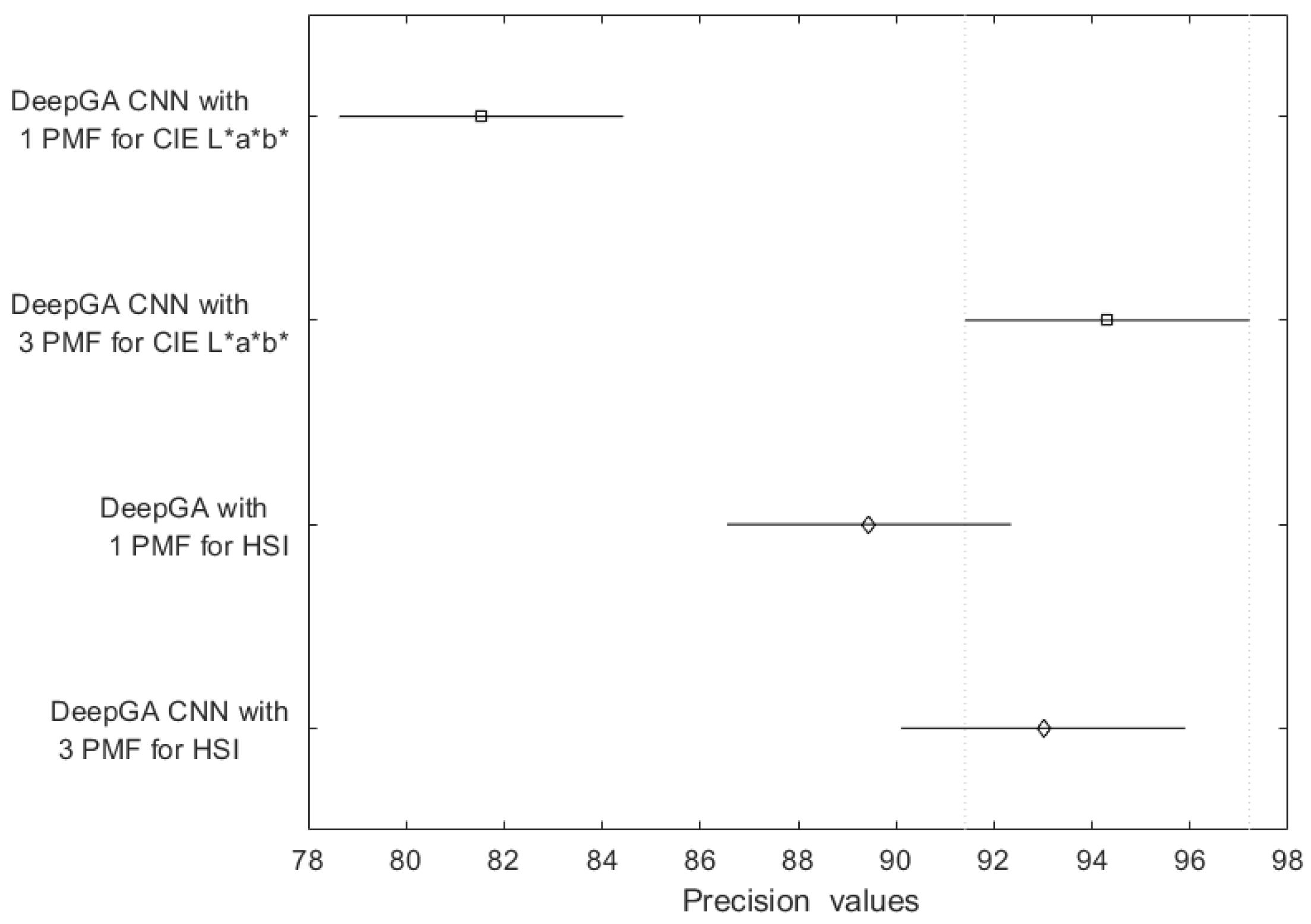
Results of Anthocyanin Estimation Using the Color Characterization Represented by One Two-Dimensional Histogram and Our Contribution on Color Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nadeem, M.A.; Yeken, M.Z.; Shahid, M.Q.; Habyarimana, E.; Yılmaz, H.; Alsaleh, A.; Hatipoğlu, R.; Çilesiz, Y.; Khawar, K.M.; Ludidi, N.; et al. Common Bean as a Potential Crop for Future Food Security: An Overview of Past, Current and Future Contributions in Genomics, Transcriptomics, Transgenics and Proteomics. Biotechnol. Biotechnol. Equip. 2021, 35, 759–787. [Google Scholar] [CrossRef]
- Bressani, R. Grain Quality of Common Beans. Food Rev. Int. 1993, 9, 237–297. [Google Scholar] [CrossRef]
- Singh, S.P. Broadening the Genetic Base of Common Bean Cultivars: A Review. Crop Sci. 2001, 41, 1659–1675. [Google Scholar] [CrossRef]
- Chávez-Servia, J.L.; Heredia-García, E.; Mayek-Pérez, N.; Aquino-Bolaños, E.N.; Hernández-Delgado, S.; Carrillo-Rodríguez, J.C.; Gill-Langarica, H.R.; Vera-Guzmán, A.M. Diversity of Common Bean (Phaseolus vulgaris L.) Landraces and the Nutritional Value of Their Grains. In Grain Legumes; Goyal, A.K., Ed.; InTech: Houston, TX, USA, 2016; ISBN 978-953-51-2720-8. [Google Scholar]
- Chacón S, M.I.; Pickersgill, B.; Debouck, D.G. Domestication Patterns in Common Bean (Phaseolus vulgaris L.) and the Origin of the Mesoamerican and Andean Cultivated Races. Theor. Appl. Genet. 2005, 110, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Bolaños, E.; Garca Daz, Y.; Chavez Servia, J.; Carrillo Rodrguez, J.; Vera Guzman, A.; Heredia Garcia, E. Anthocyanins, Polyphenols, Flavonoids and Antioxidant Activity in Common Bean (Phaseolus vulgaris L.) Landraces. Emir. J. Food Agric. 2016, 28, 581. [Google Scholar] [CrossRef]
- Chen, J.; Xu, B.; Sun, J.; Jiang, X.; Bai, W. Anthocyanin Supplement as a Dietary Strategy in Cancer Prevention and Management: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7242–7254. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ling, W. The Update of Anthocyanins on Obesity and Type 2 Diabetes: Experimental Evidence and Clinical Perspectives. Rev. Endocr. Metab. Disord. 2015, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, D.; Yang, D.; Li, X.; Sun, J.; Wang, G.; Tian, L.; Jiang, X.; Bai, W. Protective Effects of Anthocyanins on Neurodegenerative Diseases. Trends Food Sci. Technol. 2021, 117, 205–217. [Google Scholar] [CrossRef]
- Wallace, T.C. Anthocyanins in Cardiovascular Disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Regulation of Adipocyte Function by Anthocyanins; Possibility of Preventing the Metabolic Syndrome. J. Agric. Food Chem. 2008, 56, 642–646. [Google Scholar] [CrossRef]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry Anthocyanins as Novel Antioxidants in Human Health and Disease Prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, C.; Calvini, R.; Nigro, G.; Tessarin, P.; Bossio, D.; Calderisi, M.; Ferrari, V.; Foca, G.; Ulrici, A. Design and Application of a Smartphone-Based Device for in Vineyard Determination of Anthocyanins Content in Red Grapes. Microchem. J. 2023, 191, 108811. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Franco, C.; Mendes-Ferreira, A.; Mendes-Faia, A.; Costa, P.L.D.; Melo-Pinto, P. Brix, pH and Anthocyanin Content Determination in Whole Port Wine Grape Berries by Hyperspectral Imaging and Neural Networks. Comput. Electron. Agric. 2015, 115, 88–96. [Google Scholar] [CrossRef]
- Grimm, E.; Kuhnke, F.; Gajdt, A.; Ostermann, J.; Knoche, M. Accurate Quantification of Anthocyanin in Red Flesh Apples Using Digital Photography and Image Analysis. Horticulturae 2022, 8, 145. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Al-Obeed, R.S.; Aboukarima, A.M.; Eshra, D.H. Development of an Artificial Neural Network as a Tool for Predicting the Chemical Attributes of Fresh Peach Fruits. PLoS ONE 2021, 16, e0251185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, L.; Wang, M.; Wu, M.; Gao, W. Prediction of Chlorophyll and Anthocyanin Contents in Purple Lettuce Based on Image Processing; ASABE: St. Joseph, MI, USA, 2020; p. 1. [Google Scholar]
- Amoriello, T.; Ciccoritti, R.; Ferrante, P. Prediction of Strawberries’ Quality Parameters Using Artificial Neural Networks. Agronomy 2022, 12, 963. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Nakayama, M.; Noguchi, Y.; Horie, H. Use of Image Analysis to Estimate Anthocyanin and UV-Excited Fluorescent Phenolic Compound Levels in Strawberry Fruit. Breed. Sci. 2013, 63, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Pusty, K.; Kumar Dash, K.; Giri, S.; Raj, G.V.S.B.; Tiwari, A.; Shaikh, A.M.; Béla, K. Ultrasound Assisted Phytochemical Extraction of Red Cabbage by Using Deep Eutectic Solvent: Modelling Using ANFIS and Optimization by Genetic Algorithms. Ultrason. Sonochemistry 2024, 102, 106762. [Google Scholar] [CrossRef]
- Qi, H.; Li, H.; Chen, L.; Chen, F.; Luo, J.; Zhang, C. Hyperspectral Imaging Using a Convolutional Neural Network with Transformer for the Soluble Solid Content and pH Prediction of Cherry Tomatoes. Foods 2024, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, W.; Zhou, L.; Cheng, H.; Ye, X.; He, Y. Developing Deep Learning Based Regression Approaches for Determination of Chemical Compositions in Dry Black Goji Berries (Lycium ruthenicum Murr.) Using near-Infrared Hyperspectral Imaging. Food Chem. 2020, 319, 126536. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, J.C.; Gallardo-López, A.; Buide, M.L.; Whittall, J.B.; Narbona, E. Digital Photography Provides a Fast, Reliable, and Noninvasive Method to Estimate Anthocyanin Pigment Concentration in Reproductive and Vegetative Plant Tissues. Ecol. Evol. 2018, 8, 3064–3076. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.; Fernandes, A.; Martins-Lopes, P.; Pereira, L.; Mendes Faia, A.; Melo-Pinto, P. Characterization of Neural Network Generalization in the Determination of pH and Anthocyanin Content of Wine Grape in New Vintages and Varieties. Food Chem. 2017, 218, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Prilianti, K.R.; Anam, S.; Brotosudarmo, T.H.P.; Suryanto, A. Real-Time Assessment of Plant Photosynthetic Pigment Contents with an Artificial Intelligence Approach in a Mobile Application. J. Agric. Eng. 2020, 51, 220–228. [Google Scholar] [CrossRef]
- Mu, C.; Yuan, Z.; Ouyang, X.; Sun, P.; Wang, B. Non-destructive Detection of Blueberry Skin Pigments and Intrinsic Fruit Qualities Based on Deep Learning. J Sci Food Agric 2021, 101, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Prilianti, K.R.; Setiyono, E.; Kelana, O.H.; Brotosudarmo, T.H.P. Deep Chemometrics for Nondestructive Photosynthetic Pigments Prediction Using Leaf Reflectance Spectra. Inf. Process. Agric. 2021, 8, 194–204. [Google Scholar] [CrossRef]
- Nofrizal, A.Y.; Sonobe, R.; Yamashita, H.; Seki, H.; Mihara, H.; Morita, A.; Ikka, T. Evaluation of a One-Dimensional Convolution Neural Network for Chlorophyll Content Estimation Using a Compact Spectrometer. Remote Sens. 2022, 14, 1997. [Google Scholar] [CrossRef]
- Morales-Reyes, J.L.; Acosta-Mesa, H.-G.; Aquino-Bolaños, E.-N.; Herrera Meza, S.; Márquez Grajales, A. Anthocyanins Estimation in Homogeneous Bean Landrace (Phaseolus vulgaris L.) Using Probabilistic Representation and Convolutional Neural Networks. J. Agric. Eng. 2023, 54, 1–12. [Google Scholar] [CrossRef]
- Morales-Reyes, J.-L.; Aquino-Bolaños, E.-N.; Acosta-Mesa, H.-G.; Márquez-Grajales, A. Estimation of Anthocyanins in Homogeneous Bean Landraces Using Neuroevolution. In Advances in Computational Intelligence. MICAI 2023 International Workshops; Calvo, H., Martínez-Villaseñor, L., Ponce, H., Zatarain Cabada, R., Montes Rivera, M., Mezura-Montes, E., Eds.; Lecture Notes in Computer Science; Springer Nature: Cham, Switzerland, 2024; Volume 14502, pp. 373–384. ISBN 978-3-031-51939-0. [Google Scholar]
- Xu, B.J.; Yuan, S.H.; Chang, S.K.C. Comparative Analyses of Phenolic Composition, Antioxidant Capacity, and Color of Cool Season Legumes and Other Selected Food Legumes. J. Food Sci. 2007, 72, S167–S177. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Reyes, J.L.M.; Mesa, H.G.A.; Bolanos, E.N.A.; Meza, S.H.; Ramirez, N.C.; Servia, J.L.C. Classification of Bean (Phaseolus vulgaris L.) Landraces with Heterogeneous Seed Color Using a Probabilistic Representation. In Proceedings of the 2021 IEEE International Autumn Meeting on Power, Electronics and Computing (ROPEC), Ixtapa, Mexico, 10 November 2021; pp. 1–7. [Google Scholar]
- Tang, J. A Color Image Segmentation Algorithm Based on Region Growing. In Proceedings of the 2010 2nd International Conference on Computer Engineering and Technology, Chengdu, China, 16–18 April 2010; pp. V6-634–V6-637. [Google Scholar]
- Woods, R.E.; Gonzalez, R.C. Digital Image Processing, 3rd ed.; Pearson Education India: Tamil Nadu, India, 2021. [Google Scholar]
- Chandra, R.; Tiwari, A. Distributed Bayesian Optimisation Framework for Deep Neuroevolution. Neurocomputing 2022, 470, 51–65. [Google Scholar] [CrossRef]
- Lehman, J.; Miikkulainen, R. Neuroevolution. Scholarpedia 2013, 8, 30977. [Google Scholar] [CrossRef]
- Stanley, K.O.; Clune, J.; Lehman, J.; Miikkulainen, R. Designing Neural Networks through Neuroevolution. Nat. Mach. Intell. 2019, 1, 24–35. [Google Scholar] [CrossRef]
- Vargas-Hákim, G.-A.; Mezura-Montes, E.; Acosta-Mesa, H.-G. Hybrid Encodings for Neuroevolution of Convolutional Neural Networks: A Case Study. In Proceedings of the Genetic and Evolutionary Computation Conference Companion, Lille, France, 7 July 2021; pp. 1762–1770. [Google Scholar]
- Namatēvs, I. Deep Convolutional Neural Networks: Structure, Feature Extraction and Training. Inf. Technol. Manag. Sci. 2017, 20, 40–47. [Google Scholar] [CrossRef]

| Hyperparameters Evolutionary Algorithm | Value | Hyperparameters CNN | Value |
|---|---|---|---|
| Population size | 20 | Epoch number | 30 |
| Generation number | 50 | Learning rate | 0.001 |
| Objective function | MAPE | Optimization method | ADAM |
| Loss function | MSE |
| Color Space | HSI | CIE L*a*b* | |
|---|---|---|---|
| Models | DeepGA | DeepGA | |
| Color Characterization Technique | PMF of H and S | PMF of a* and b* | |
| Train | Precision | ||
| RMSE | |||
| R2 | |||
| Test | Precision | ||
| RMSE | 0.40 | 0.84 | |
| R2 | 0.95 | 0.82 | |
| Color Space | HSI | CIE L*a*b* | |
|---|---|---|---|
| Models | DeepGA | DeepGA | |
| Color characterization technique | PMF of H and S | PMF of a* and b* | |
| PMF of H and I | PMF of L* and a* | ||
| PMF of S and I | PMF of L* and b* | ||
| Train | Precision | ||
| RMSE | |||
| R2 | |||
| Test | Precision | ||
| RMSE | 0.30 | 0.40 | |
| R2 | 0.97 | 0.95 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Reyes, J.-L.; Aquino-Bolaños, E.-N.; Acosta-Mesa, H.-G.; Márquez-Grajales, A. Estimation of Anthocyanins in Heterogeneous and Homogeneous Bean Landraces Using Probabilistic Colorimetric Representation with a Neuroevolutionary Approach. Math. Comput. Appl. 2024, 29, 68. https://doi.org/10.3390/mca29040068
Morales-Reyes J-L, Aquino-Bolaños E-N, Acosta-Mesa H-G, Márquez-Grajales A. Estimation of Anthocyanins in Heterogeneous and Homogeneous Bean Landraces Using Probabilistic Colorimetric Representation with a Neuroevolutionary Approach. Mathematical and Computational Applications. 2024; 29(4):68. https://doi.org/10.3390/mca29040068
Chicago/Turabian StyleMorales-Reyes, José-Luis, Elia-Nora Aquino-Bolaños, Héctor-Gabriel Acosta-Mesa, and Aldo Márquez-Grajales. 2024. "Estimation of Anthocyanins in Heterogeneous and Homogeneous Bean Landraces Using Probabilistic Colorimetric Representation with a Neuroevolutionary Approach" Mathematical and Computational Applications 29, no. 4: 68. https://doi.org/10.3390/mca29040068
APA StyleMorales-Reyes, J.-L., Aquino-Bolaños, E.-N., Acosta-Mesa, H.-G., & Márquez-Grajales, A. (2024). Estimation of Anthocyanins in Heterogeneous and Homogeneous Bean Landraces Using Probabilistic Colorimetric Representation with a Neuroevolutionary Approach. Mathematical and Computational Applications, 29(4), 68. https://doi.org/10.3390/mca29040068







