Abstract
This paper presents a metamaterial biosensor composed of dual-cut wires (DCWs) and quadruple split-ring resonators (QSRs), achieving polarization-independent plasmon-induced transparency (PIT) effects in the terahertz range. By leveraging the coupling between bright and dark modes, we observe a transparent window with a minimal loss at 1.22 THz. We investigate the physical mechanism of the PIT effect by analyzing the surface current distribution and electric fields. Simulations reveal that the PIT transparency shows a peak shift of up to 146.7 GHz with an analyte thickness of 14 μm. Moreover, as the refractive index of the analyte increases from 1.0 to 1.6, the biosensor’s theoretical sensitivity is calculated to be 281.25 GHz/RIU. Furthermore, we explore the application of the proposed DCW/QSR biosensor for the detection of bacteriophage viruses. Our simulation results demonstrate that the DCW/QSR biosensor serves as an effective sensing platform for detecting viruses such as PRD1 and MS2. These findings underscore the potential of our high-sensitivity metamaterial biosensor, which holds great promise in the field of biosensing, offering a practical and cost-effective approach to label-free biomedical detection.
1. Introduction
Terahertz (THz) waves have garnered significant attention across diverse fields, including material science, physics, security, biology, and high-speed communication [1,2,3,4]. Their unique properties, encompassing non-conductivity, low photon energy, and broad bandwidth [5,6], make them particularly appealing. Their alignment with the vibrational frequencies of pivotal biomolecules, such as microorganisms, RNA, and DNA, accentuates their relevance in biological research [7,8,9]. Nevertheless, terahertz biomolecular detection presents intricacies, mainly due to challenges associated with diluted biological samples and feeble interactions with incident waves. Fortunately, metamaterials have emerged as a solution to the dearth of materials capable of robustly responding to THz waves [10,11]. Metamaterials, defined as engineered materials with controllable electromagnetic properties, have led to breakthroughs like perfect absorbers [12,13], invisibility cloaks [14], and enhancement lenses [15]. Moreover, they have demonstrated immense potential in the highly sensitive and label-free detection of minuscule quantities of biomolecules, including proteins [16], sugars [17], viruses [18], and RNA [19]. These metamaterials are well suited for sensing applications owing to their capacity to generate spoof surface plasmons characterized by localized electric field enhancement [20] and high-quality factor (Q factor) values. This unique combination intensifies the interaction between light and matter, resulting in the highly sensitive detection of even subtle environmental changes [21]. In comparison to current nanotechnology biosensing methods [22], metamaterial-based terahertz biosensors offer significant advantages for biological research, including in situ, cost-effective, and non-destructive detection.
Electromagnetically induced transparency (EIT) is a phenomenon observed in three-energy atomic systems, stemming from quantum interference effects that occur during the interaction of electromagnetic fields with dielectric atomic systems [23]. In contrast, the plasmon-induced transparency (PIT) effect can be realized under less stringent experimental conditions, eliminating the need for gaseous media, stable optical pumping, and low-temperature environments. PIT is a classical plasmon analog of the EIT effect. Notably, metamaterials founded on plasmon-induced transparency (PIT), such as split-ring resonators (SRRs) [24,25], can provoke asymmetric resonances, resulting in sharp narrow spectra with high-quality (Q) factors and heightened sensitivity to changes in the surrounding dielectric environment [26]. Consequently, PIT-like metamaterial biosensing in the THz band has attracted considerable research attention.
In this paper, we propose a high-sensitivity terahertz biosensor based on plasmon-induced transparency (PIT). The proposed DCW/QSR metamaterial biosensor consists of dual-cut wires (DCWs) and quadruple split-ring resonators (QSRs), which enables a polarization-independent transparency window to be generated at 1.22 THz, as a result of the proximate coupling of bright and dark modes. Furthermore, we analyze the sensing performance of the PIT metamaterial. Our simulations illustrate that, when a thickness at 14 um of analyte is covered on the surface, the frequency shift of the PIT transparent peak shift reaches 146.7 GHz. Moreover, theoretical analyses show that 281.25 GHz/RIU can be obtained at PIT transparent peak when the refractive index of analyte increases from 1.0 to 1.6. These results prove the potential of the PIT metamaterial biosensor as a promising tool for sensing in the terahertz domain.
2. Metamaterial Design and Simulation Method
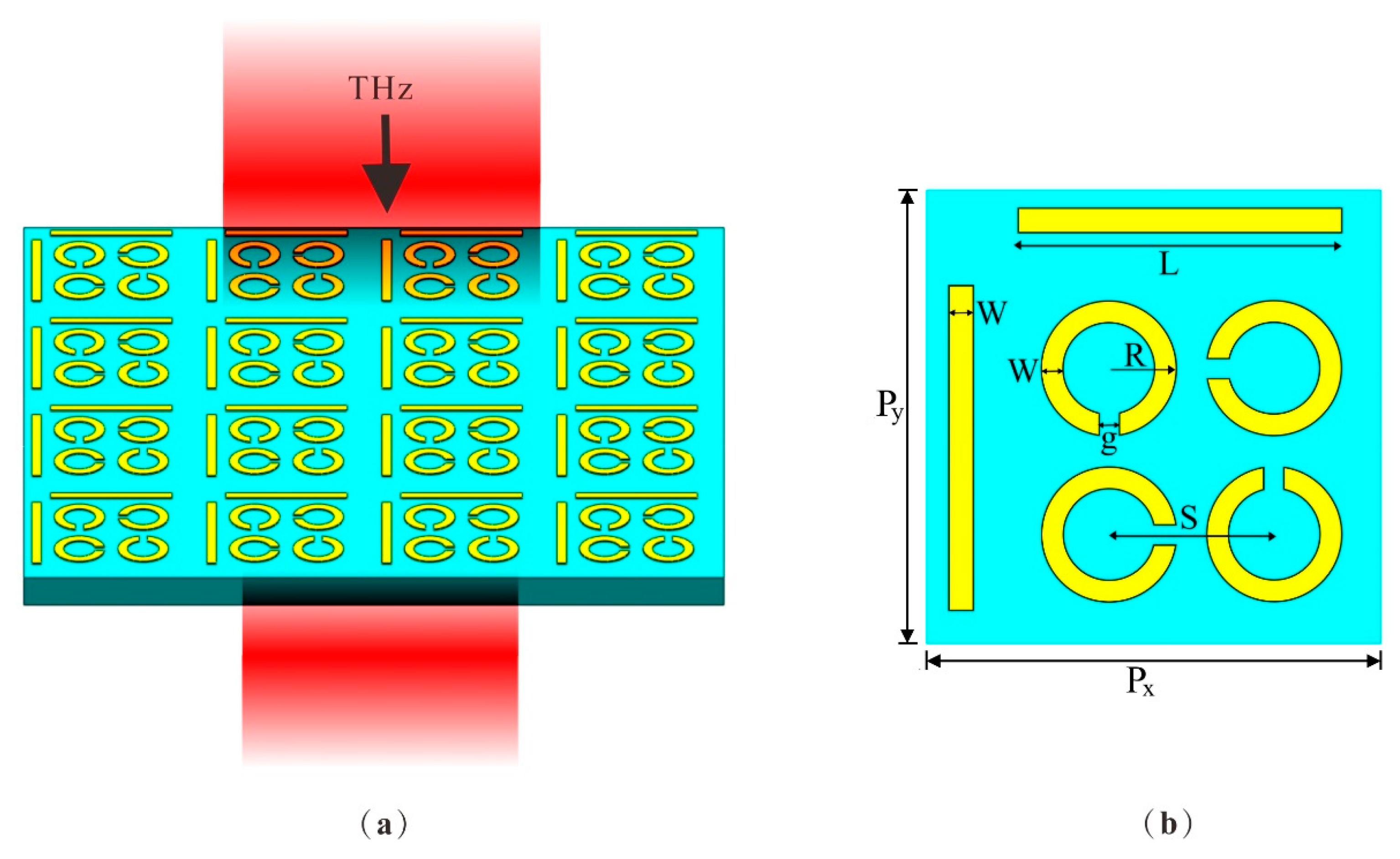
The schematic in Figure 1 illustrates the design of the DCW/QSR metamaterial biosensor. For the substrate material, we opted for flexible polyimide with a thickness of h = 50 µm, a dielectric constant (ε) of 3.5, and a loss angle of 0.0027, given its excellent terahertz band transmittance [27,28]. The upper layer comprised regularly patterned aluminum (Al), characterized by a conductance value of 3.56 × 107 S/m [29] and a thickness of 200 nm. Figure 1b shows a schematic outlining the parameters of a single-unit structure. Each unit cell within the array was composed of dual-cut wires (DCWs) and quadruple split-ring resonators (QSRs) with varying orientations. The period of the unit structure was Px = Py = 110 µm. The DCW length was L = 76.5 μm, the QSR outer radius was R = 16 μm, and both DCWs and QSRs had widths of W = 6 μm. The gap between them was g = 5 μm and the center-to-center distance of the QSR units was S = 40 μm. The gaps of the QSRs were sequentially positioned 90° apart.
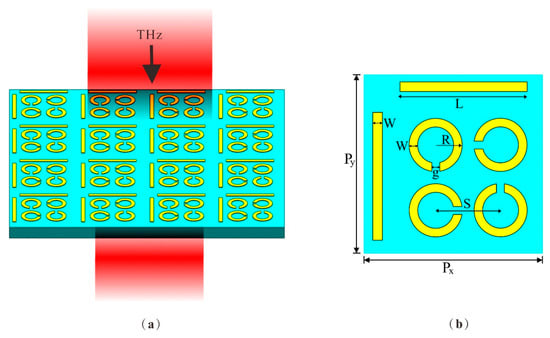
Figure 1.
(a) Schematic of the metamaterial biosensor. The substrate material was PI and the structure material was Al. (b) The top view of the unit structure, where Px = Py = 110 µm, length L = 76.5 μm, outer radius R = 16 μm, width W = 6 μm, gap g = 5 μm, and distance S = 40 μm.
We conducted a numerical analysis using the commercial software CST Microwave Studio 2020. In our simulations, we applied periodic boundary conditions with Floquet ports in the x and y directions, while open boundary conditions were employed along the z-direction to simulate the propagation of the incident plane wave.
3. Results and Discussion
3.1. Transmission Characteristics of the DCW/QSR Biosensor
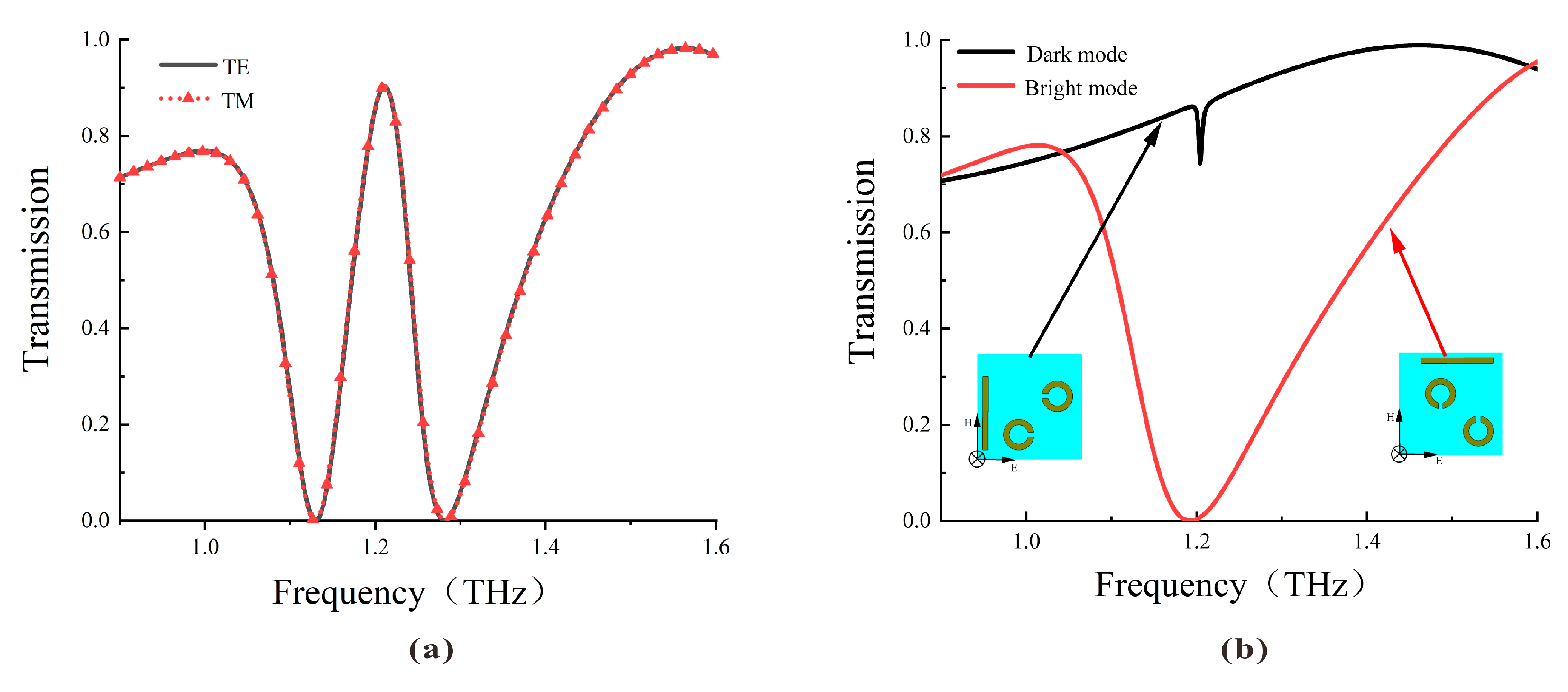
The transmission spectra of the DCW/QSR metamaterial biosensor are depicted in Figure 2. As illustrated in Figure 2a, the transmission spectra under TE or TM polarized incident waves overlap well, indicating the polarization independence of the proposed metamaterial due to its four-fold rotational symmetry. Figure 2b illustrates that the bright mode generated a robust resonance with a high Q factor, whereas the dark mode had limited interaction with the incident wave, generating a lower Q factor resonance. When these two substructures coexisted, the resonance that could not be directly excited by the incident light was excited by the bright mode. Similarly, the mode that could not be directly excited by the incident light but could be indirectly excited by other conditions was termed the dark mode. The destructive interference of scattering fields between these modes created a narrow transparent window at 1.22 THz.
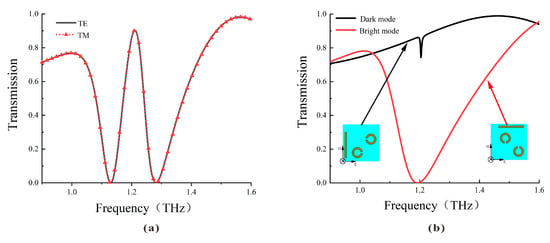
Figure 2.
(a) Simulated transmission curve of the metamaterial biosensor under TE and TM incident waves. (b) Simulated transmission curve of the bright mode (red line) and the dark mode (black line).
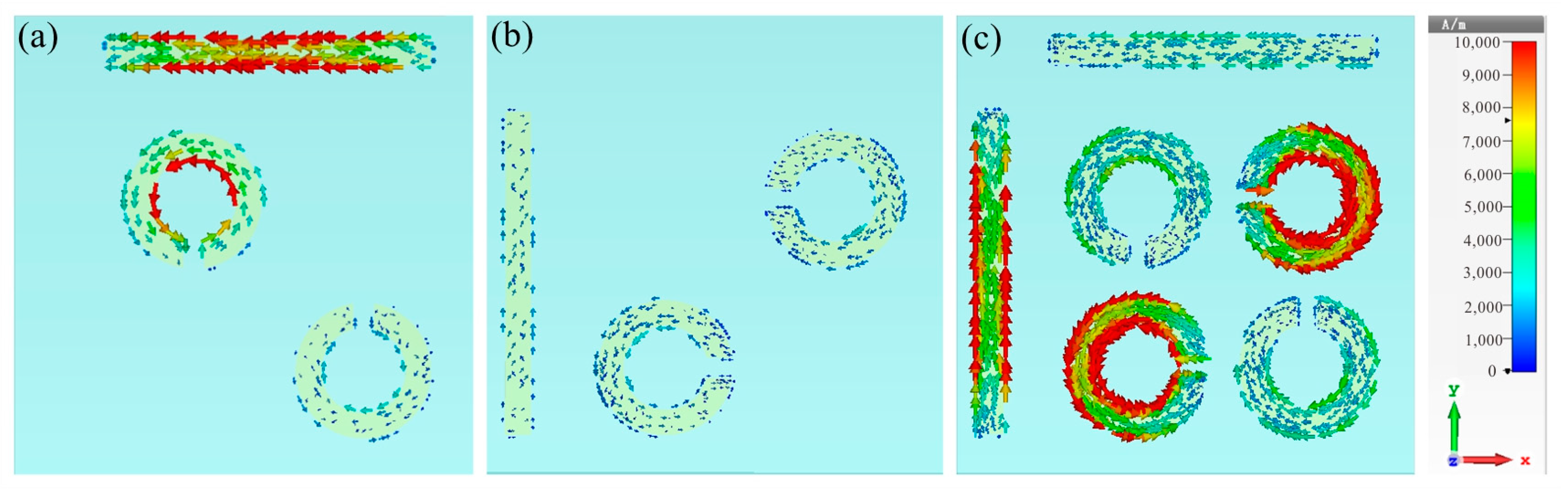
To determine the physical mechanism behind this plasmon-induced transparency (PIT), we further examined the surface current distribution of the bright mode (Figure 3a), dark mode (Figure 3b) at 1.19 THz, and the complete structure at 1.22 THz (Figure 3c). As shown in Figure 3a, when the TE wave impinged on the bright mode, it directly excited a significant enhancement of surface currents, mainly concentrated on the horizontal cut wire and the inner side of the SRRs, thus resulting in the generation of a significant transmission dip, as shown in the red curve of Figure 2b. Conversely, the vertical cut wire and the other two diagonal SRRs showed minimal response to the TE incidence; hence, it will not generate resonance, as shown in the black curve of Figure 2b. The combination of cut wires with the two sets of SRRs formed a complete metamolecule, transferring the intense enhancement of surface current entirely to the dark mode resonator through near-field coupling with the bright-mode resonator. Furthermore, conspicuous rotating surface charges appeared along the SRRs, with gaps oriented parallel to the direction of the incoming electric field, indicating the induction of a magnetic dipole on the dark mode, characterized by minimal radiative loss. The destructive interference of the dark mode’s indirect resonance intensity with the bright mode’s direct resonance intensity led to the appearance of a narrow transparent window at the frequency of 1.22 THz.
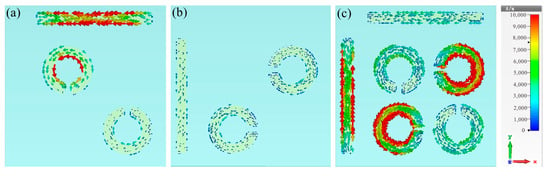
Figure 3.
Surface current distributions of (a) the bright mode, (b) the dark mode at 1.19 THz, and (c) the complete structure at 1.22 THz.
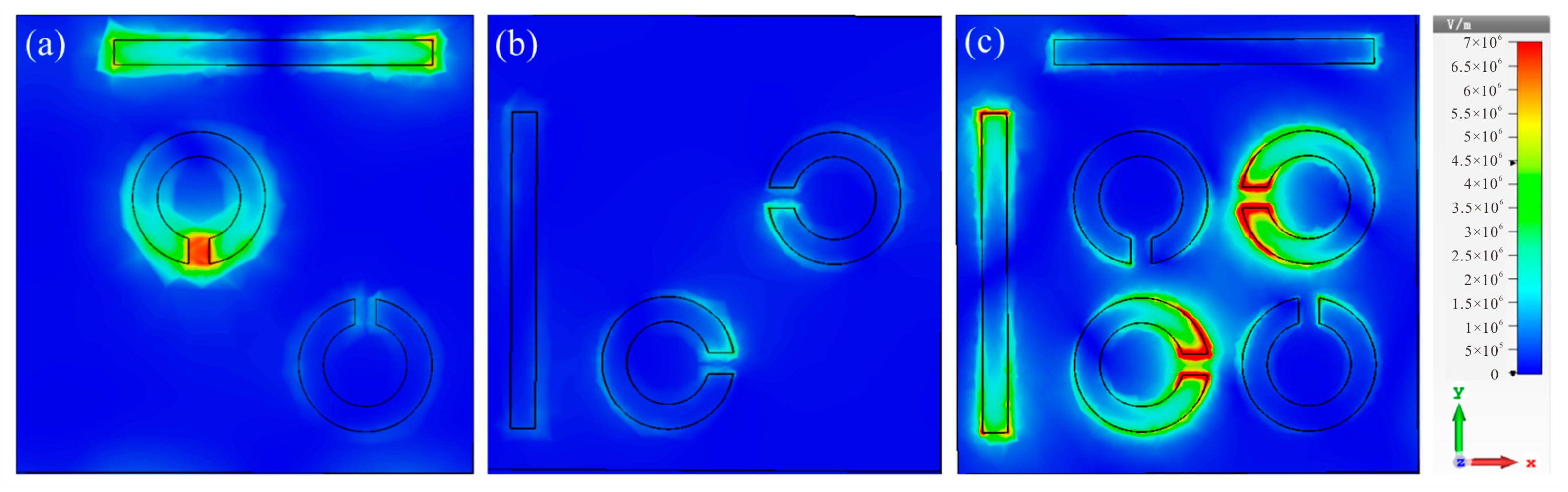
Corresponding electric field distributions for the bright mode (Figure 4a) and dark mode (Figure 4b) at 1.19 THz, as well as the complete structure at 1.22 THz, are depicted. As illustrated in Figure 4a, the electric field energy was primarily concentrated at the two ends of the horizontal cut wire and the gap of the SRRs, resulting in high-intensity resonance, as observed in the bright mode (Figure 2b). Conversely, the vertical cut wire and the other two diagonal SRRs remained unexcited independently, leading to a lack of resonance. Figure 4c reveals that when the two structures were coupled, the electric field energy from the bright mode was transferred to the dark mode, with the initial electric field distribution around the bright mode concentrating on the dark mode. This finding aligns with the surface current distribution for the complete structure illustrated in Figure 3.
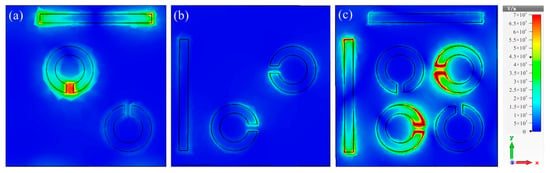
Figure 4.
Electric field distributions of (a) the bright mode, (b) the dark mode at 1.19 THz, and (c) the complete structure at 1.22 THz.
3.2. Sensing Capabilities of the DCW/QSR Biosensor
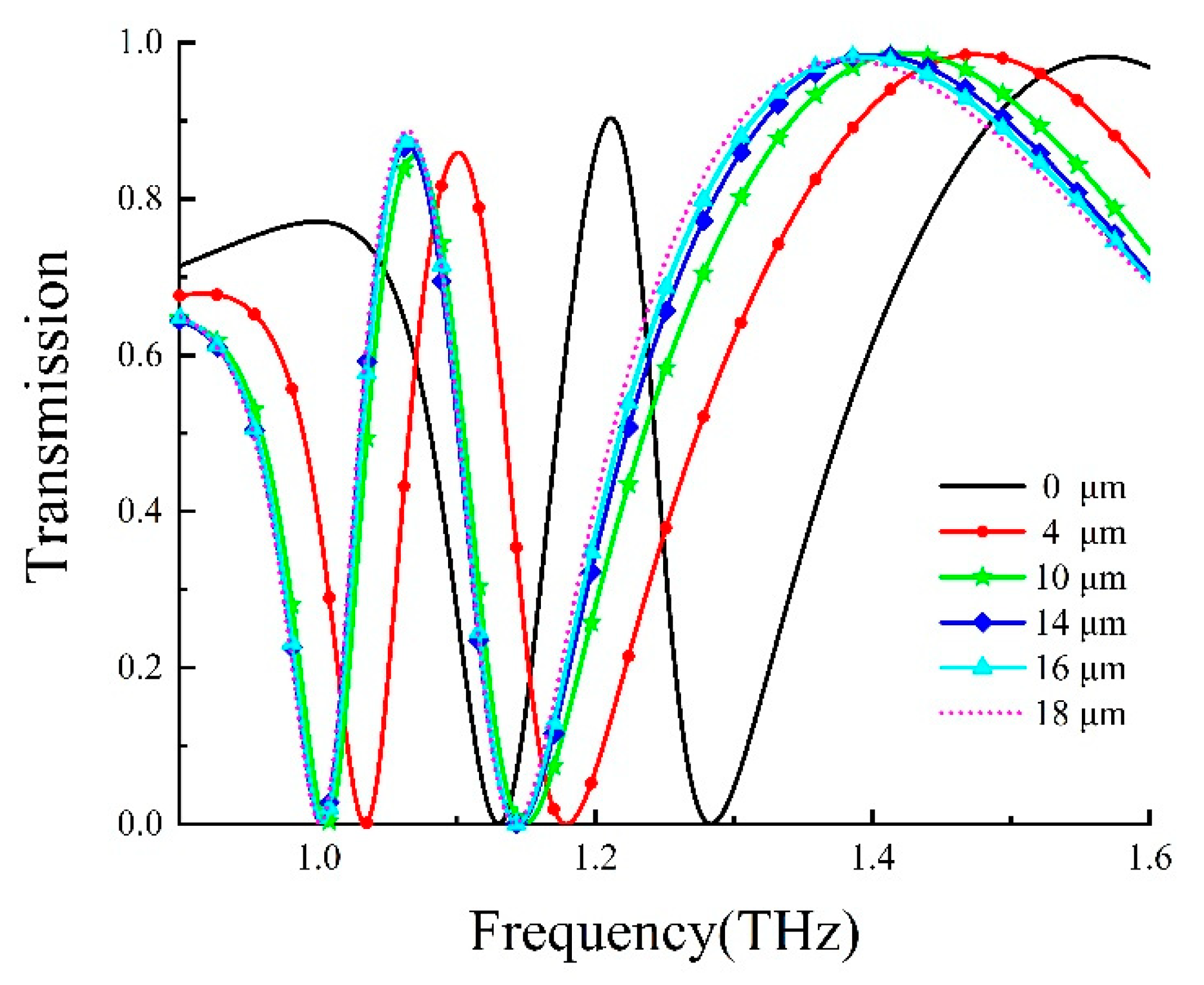
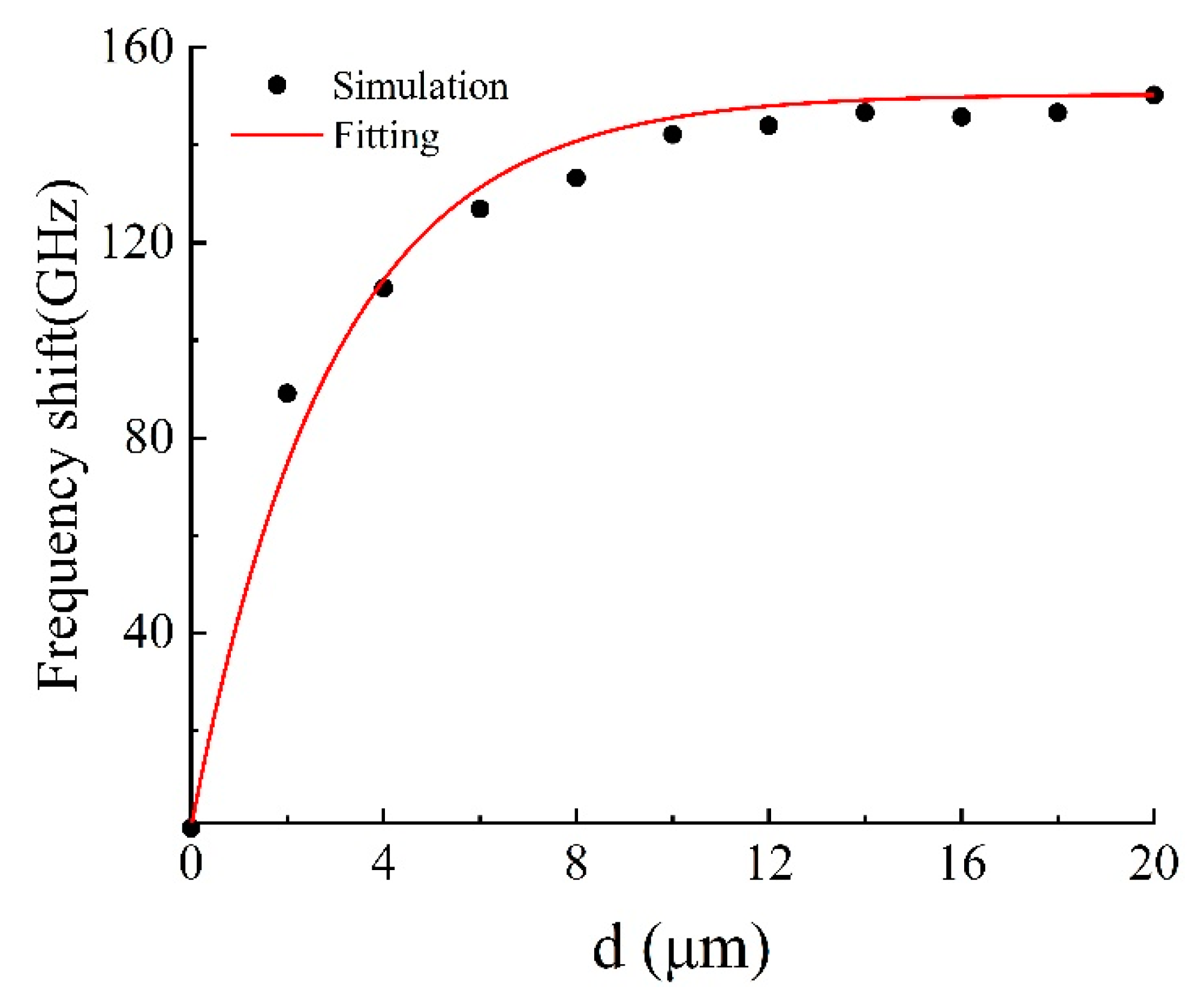
To explore the sensing properties of the DCW/QSR metamaterial, we initially analyzed the influence of analyte thickness on the proposed metamaterial biosensor through numerical simulations. Figure 5 displays the transmission spectra of the DCW/QSR metamaterial biosensor at various analyte thicknesses, ranging from 0 μm to 18 μm. The transparent peak frequency redshift (Δf) increased with growing analyte thickness. As shown in Figure 6, the red curve represents the fitting curve, with R2 = 0.9917, indicating an excellent linear relationship between frequency shift and analyte thickness. Additionally, when the analyte thickness surpassed 12 μm, the transparent peak frequency remained nearly constant. This is because excessive analyte thickness reduced the near-field coupling effect on the metamaterial structure’s surface, resulting in the saturation of a frequency shift.
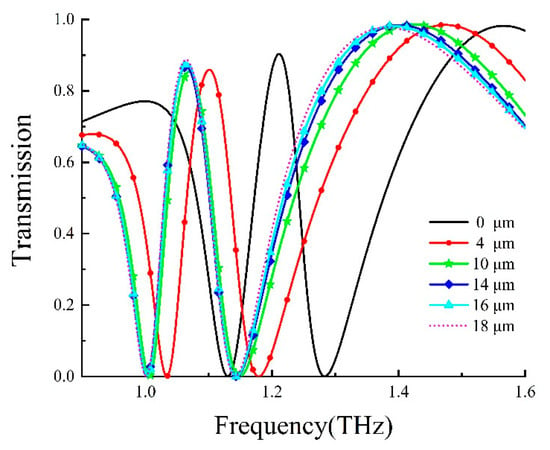
Figure 5.
Transmission curve of the metamaterial biosensor at different analyte thicknesses.
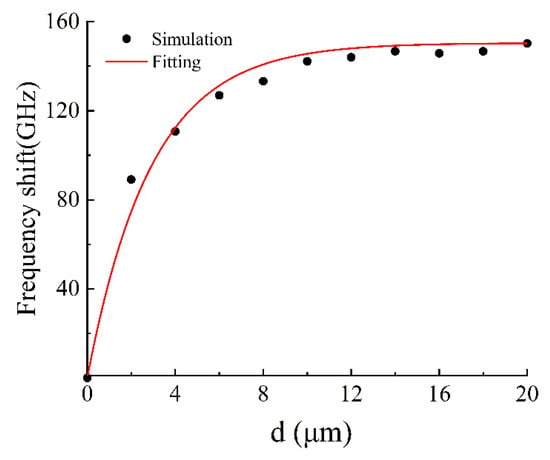
Figure 6.
Relationship between transparent peak frequency shift and analyte thickness.
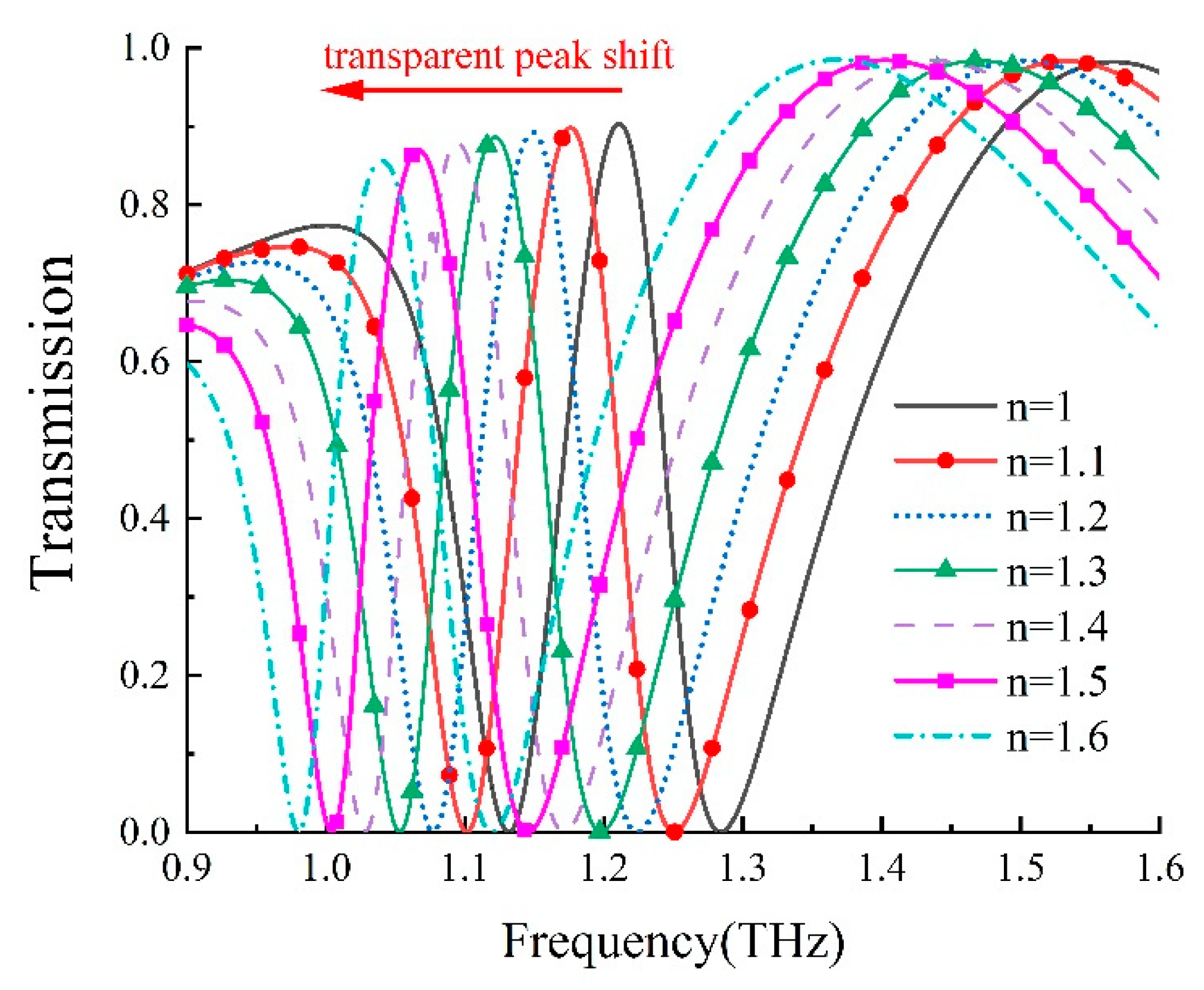
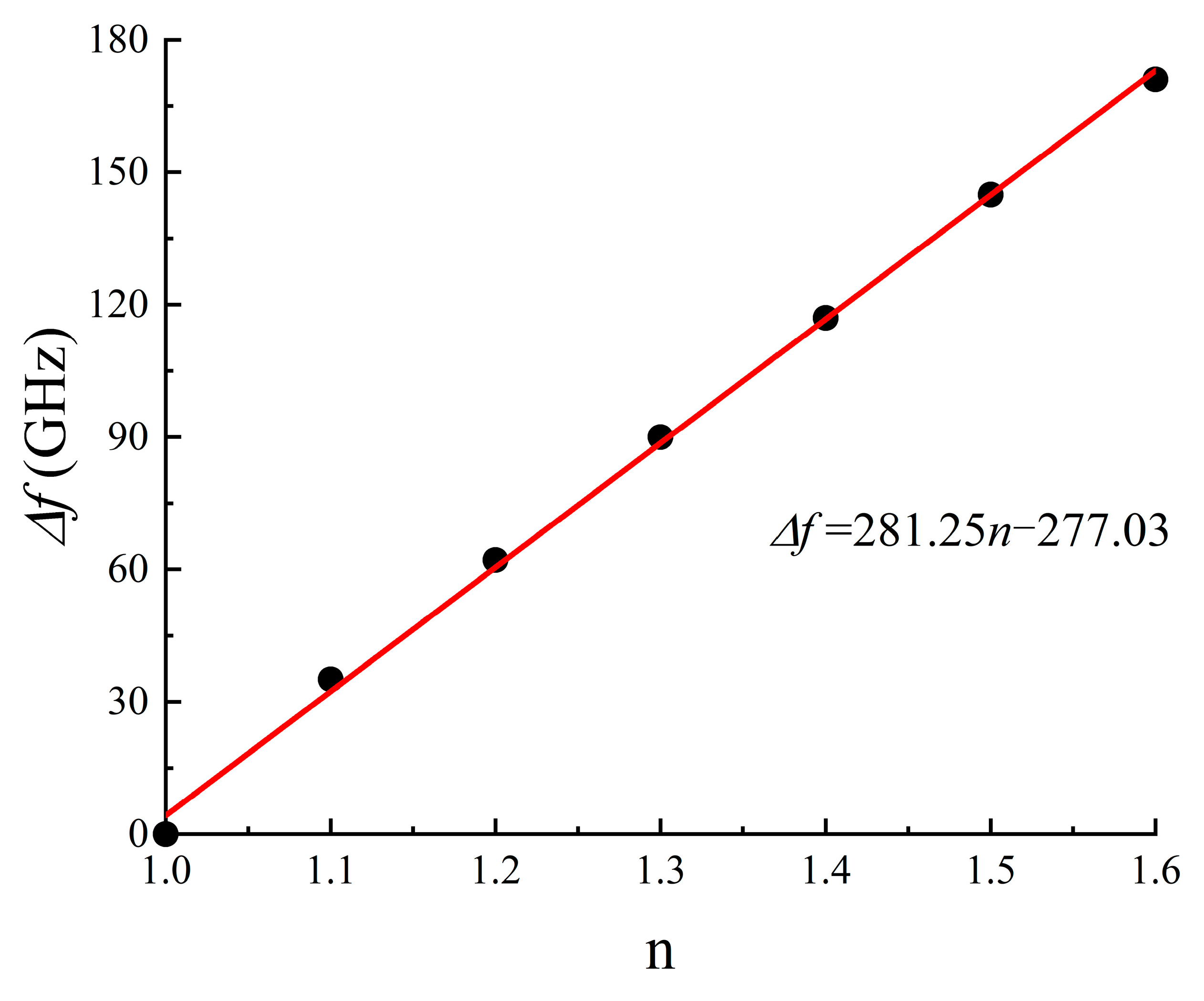
We further delved into the refractive index sensitivity of our metamaterial biosensor. The refractive index sensitivity, denoted as S [30], can be typically defined as S = Δf/Δn; to characterize the sensor’s sensitivity, where Δn and Δf; represent variations in the analyte’s refractive index and resonance frequency, respectively. By altering the analyte’s refractive index from 1.0 to 1.6 while maintaining a thickness of 12 μm, we established the theoretical relationships between a transparent peak shift and the analyte’s refractive index, as presented in Figure 7. Furthermore, as shown in Figure 8, there was a linear relationship between the refractive index change and the transparent peak shift. Our findings indicate that our DCW/QSR metamaterial can provide a refractive index sensitivity of 281.25 GHz/RIU. Additionally, we compared the performance of the proposed sensor with various types of terahertz metamaterial sensors, as summarized in Table 1. The proposed sensor exhibited superior sensitivity compared to the reported sensors. Therefore, the proposed plasmon-induced transparency (PIT) metamaterial holds significant promise in applications such as bio-sensing and chemical sensing.
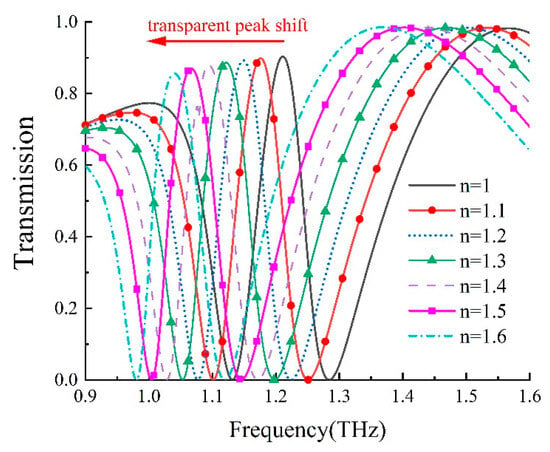
Figure 7.
Dependence of transmission on the refractive index of the analyte, ranging from 1.0 to 1.6.
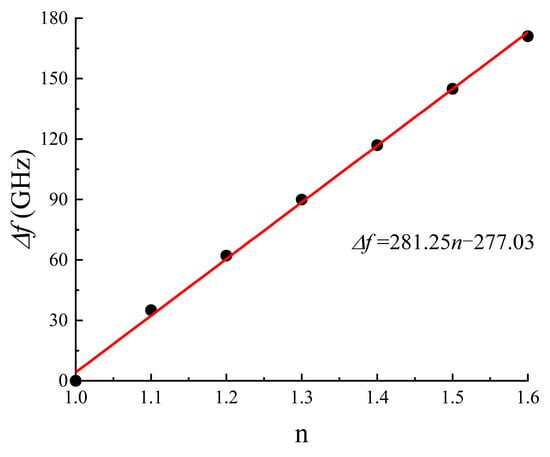
Figure 8.
Relationship between transparent peak frequency shift and the refractive index of the analyte.

Table 1.
Performance comparison of various terahertz metamaterial sensors.
3.3. Sensing Viruses with the DCW/QSR Biosensor
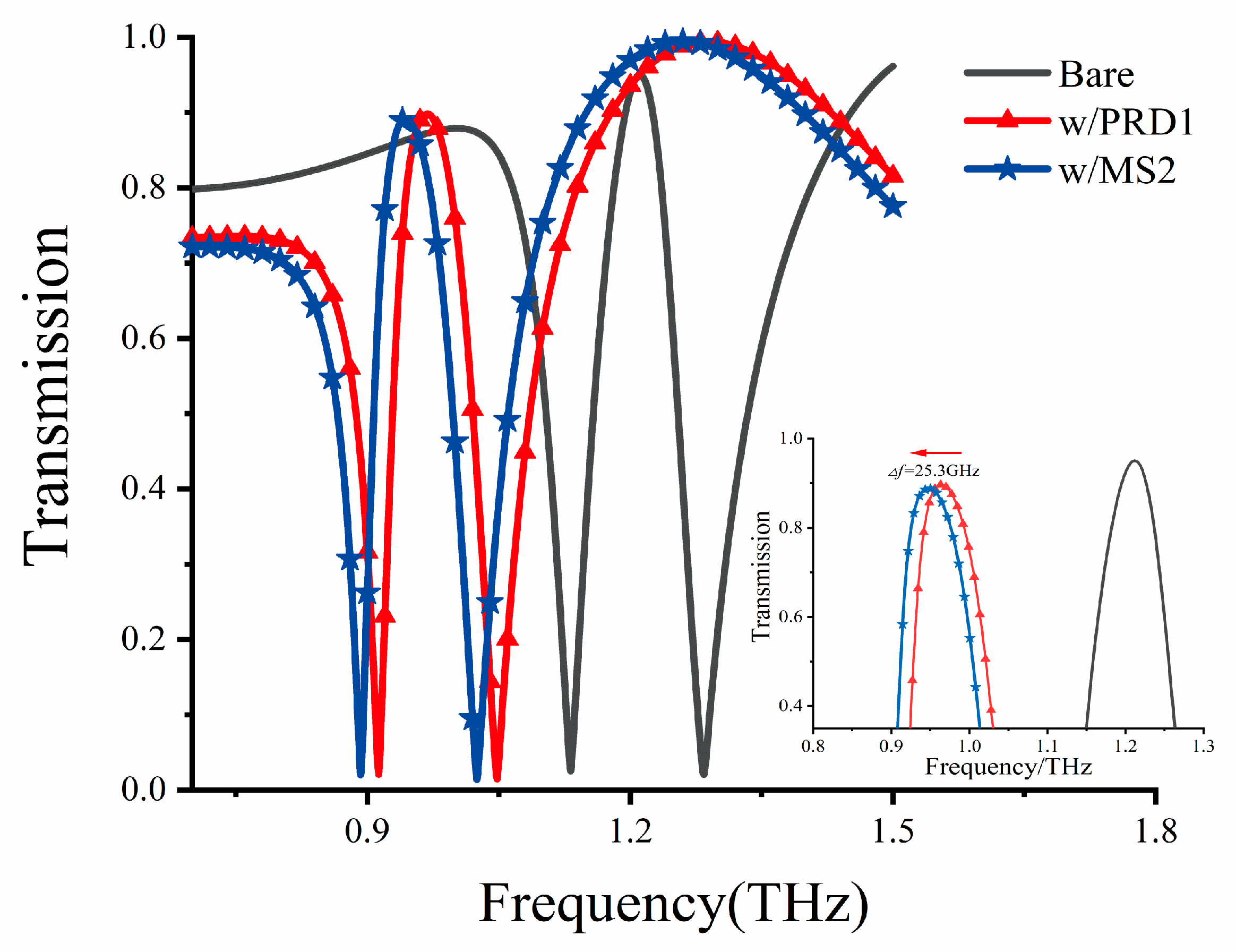
In light of the heightened demand for rapid and accurate diagnostic methods for highly pathogenic viruses like COVID-19, Middle East respiratory syndrome (MERS), and influenza A (H1N1 virus subtype), we explored alternative detection methods. Over the years, techniques like polymerase chain reaction (PCR) and branched-chain DNA (bDNA) tests, commonly used for virus detection purposes, have been plagued by issues of detection sensitivity and speed [34,35]. Notably, many intra/intermolecular vibration modes of biomaterials, including proteins [16] and nucleic acids [9], fall within the THz spectral range. This has spurred considerable interest in utilizing THz sensing technology for biomaterials. Consequently, we focused on the biosensing capabilities of our proposed DCW/QSR biosensor for a range of viruses. Specifically, we explored the use of our metamaterial sensors for detecting bacteriophage viruses, namely MS2 (30 nm) and PRD1 (60 nm), representing single-strand RNA and double-stranded DNA viruses, respectively. The dielectric constants of bacteriophage viruses can be measured by creating a thick and dense virus layer [36]. At 1.0 THz, the dielectric constants of PRD1 and MS2 viruses were found to be 3.57 and 3.92, respectively [36]. Therefore, we established a virus analysis layer with a thickness of 12 μm and the dielectric constant values of the respective viruses to assess the potential of our sensor to detect different bacteriophage viruses. The corresponding simulation results are presented in Figure 9, where a significant redshift of the PIT transparent peak can be observed towards lower frequencies. Additionally, as shown in the inset of Figure 8, the MS2 virus, with a higher dielectric constant, exhibited a resonant redshift of 25.3 GHz compared to PRD1. This biosensing detection method holds promise for the cost-effective label-free detection and identification of viruses.
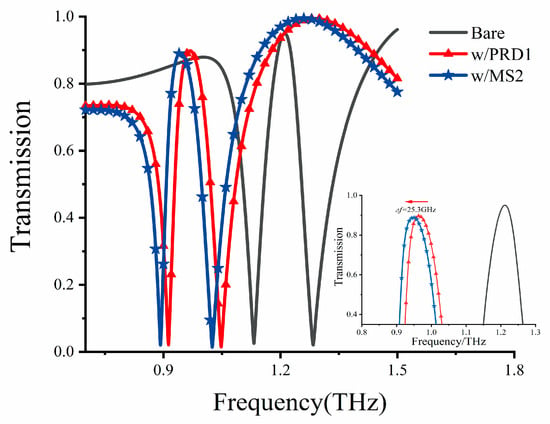
Figure 9.
Transmission curve of the metamaterial biosensor for different viruses. Inset indicates the transparent peak before and after the deposition of PRD1 and MS2 viruses.
4. Conclusions
In this study, we introduce a novel metamaterial biosensor based on the plasmon-induced transparency (PIT) effect, demonstrating high sensitivity. Our metamaterial biosensor comprises dual-cut wires (DCWs) and quadruple split-ring resonators (QSRs), enabling polarization-independent PIT effects at 1.22 THz. Our simulations reveal a strong linear relationship between the thickness of the analyte and the transparent peak frequency shift. Moreover, the theoretical refractive index sensitivity of the biosensor was calculated to be as high as 281.25 GHz/RIU. We explored the application of our DCW/QSR biosensor for the detection of bacteriophage viruses, showcasing its effectiveness in detecting various viruses, such as PRD1 and MS2. Therefore, the proposed metamaterial biosensor holds significant potential in the field of biosensing, offering a practical and cost-effective approach to label-free biomedical detection.
Author Contributions
M.G.: investigation, software, writing—original draft, and methodology. X.S.: investigation and validation. J.W.: investigation and data curation. X.J.: formal analysis and supervision. X.C.: supervision and funding acquisition. R.C.: conceptualization, supervision, writing—review and editing, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Key Research and Development Program of Jiangxi Academy of Sciences (grant numbers 2021YSBG22036, 2022YSBG22022, and 2022YSBG21017), the Key Research and Development Program of Jiangxi Province (grant number 20201BBE51015), and the Emerging Interdisciplinary Cultivation Program of the Jiangxi Academy of Sciences (grant number 2022YXXJC0102).
Data Availability Statement
The data presented in this paper are available on reasonable request from the corresponding author.
Acknowledgments
All authors contributed extensively to the work presented in this article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sarieddeen, H.; Alouini, M.S.; Al-Naffouri, T.Y. An overview of signal processing techniques for terahertz communications. Proc. IEEE 2021, 109, 1628–1665. [Google Scholar] [CrossRef]
- Li, B.; Bai, J.; Zhang, S. Low concentration noroxin detection using terahertz spectroscopy combined with metamaterial. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119101. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, F.; Xu, Y.; Mauk, M.G.; Qiu, X.; Tian, Z.; Zhang, L. Recent progress in terahertz biosensors based on artificial electromagnetic subwavelength structure. TrAC Trends Anal. Chem. 2022, 158, 116888. [Google Scholar] [CrossRef]
- Feng, C.H.; Otani, C. Terahertz spectroscopy technology as an innovative technique for food: Current state-of-the-Art research advances. Crit. Rev. Food Sci. Nutr. 2021, 61, 2523–2543. [Google Scholar] [CrossRef]
- Tonouchi, M. Cutting-edge terahertz technology. Nat. Photonics 2007, 1, 97–105. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, H.; Sun, D.W. Hyperspectral imaging technique for evaluating food quality and safety during various processes: A review of recent applications. Trends Food Sci. Technol. 2017, 69, 25–35. [Google Scholar] [CrossRef]
- Yoon, S.A.; Cha, S.H.; Jun, S.W.; Park, S.J.; Park, J.-Y.; Lee, S.; Kim, H.S.; Ahn, Y.H. Identifying different types of microorganisms with terahertz spectroscopy. Biomed. Opt. Express 2020, 11, 406–416. [Google Scholar] [CrossRef]
- Ryder, M.R.; Van de Voorde, B.; Civalleri, B.; Bennett, T.D.; Mukhopadhyay, S.; Cinque, G.; Fernandez-Alonso, F.; De Vos, D.; Rudić, S.; Tan, J.-C. Detecting molecular rotational dynamics complementing the low-frequency terahertz vibrations in a zirconium-based metal-organic framework. Phys. Rev. Lett. 2017, 118, 255502. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical applications of terahertz spectroscopy and imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef]
- Kowerdziej, R.; Olifierczuk, M.; Parka, J.; Wróbel, J. Terahertz characterization of tunable metamaterial based on electrically controlled nematic liquid crystal. Appl. Phys. Lett. 2014, 105, 022908. [Google Scholar] [CrossRef]
- Degl’Innocenti, R.; Lin, H.; Navarro-Cía, M. Recent progress in terahertz metamaterial modulators. Nanophotonics 2022, 11, 1485–1514. [Google Scholar] [CrossRef]
- Tao, H.; Landy, N.I.; Bingham, C.M.; Zhang, X.; Averitt, R.D.; Padilla, W.J. A metamaterial absorber for the terahertz regime: Design, fabrication and characterization. Opt. Express 2008, 16, 7181–7188. [Google Scholar] [CrossRef]
- Yang, C.; Chang, H.; Xiao, L.; Qu, Y. Visible and NIR transparent broadband microwave absorption metamaterial based on silver nanowires. Opt. Mater. 2022, 131, 112464. [Google Scholar] [CrossRef]
- Cai, W.; Chettiar, U.K.; Kildishev, A.V.; Shalaev, V.M. Optical cloaking with metamaterials. Nat. Photonics 2007, 1, 224–227. [Google Scholar] [CrossRef]
- Zhou, L.; Chan, C.T. Relaxation mechanisms in three-dimensional metamaterial lens focusing. Opt. Lett. 2005, 30, 1812–1814. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Xu, L.; Yu, X.; Zou, L.; Shen, Y.; Deng, X. High-sensitivity biosensor for identification of protein based on terahertz Fano resonance metasurfaces. Opt. Commun. 2020, 473, 125850. [Google Scholar] [CrossRef]
- Lee, D.-K.; Kang, J.-H.; Lee, J.-S.; Kim, H.-S.; Kim, C.; Kim, J.H.; Lee, T.; Son, J.-H.; Park, Q.-H.; Seo, M. Highly sensitive and selective sugar detection by terahertz nano-antennas. Sci. Rep. 2015, 5, 15459. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, A.; Gerislioglu, B.; Tomitaka, A.; Manickam, P.; Kaushik, A.; Bhansali, S.; Nair, M.; Pala, N. Extreme sensitive metasensor for targeted biomarkers identification using colloidal nanoparticles-integrated plasmonic unit cells. Biomed. Opt. Express 2018, 9, 373–386. [Google Scholar] [CrossRef]
- Yang, K.; Li, J.; de la Chapelle, M.L.; Huang, G.; Wang, Y.; Zhang, J.; Xu, D.; Yao, J.; Yang, X.; Fu, W. A terahertz metamaterial biosensor for sensitive detection of microRNAs based on gold-nanoparticles and strand displacement amplification. Biosens. Bioelectron. 2021, 175, 112874. [Google Scholar] [CrossRef]
- Cetin, A.E.; Turkmen, M.; Aksu, S.; Etezadi, D.; Altug, H. Multi-resonant compact nanoaperture with accessible large nearfields. Appl. Phys. B 2015, 118, 29–38. [Google Scholar] [CrossRef]
- Xu, W.; Xie, L.; Ying, Y. Mechanisms and applications of terahertz metamaterial sensing: A review. Nanoscale 2017, 9, 13864–13878. [Google Scholar] [CrossRef] [PubMed]
- Dastgeer, G.; Shahzad, Z.M.; Chae, H.; Kim, Y.H.; Ko, B.M.; Eom, J. Bipolar junction transistor exhibiting excellent output characteristics with a prompt response against the selective protein. Adv. Funct. Mater. 2022, 32, 2204781. [Google Scholar] [CrossRef]
- Boller, K.J.; Imamoğlu, A.; Harris, S.E. Observation of electromagnetically induced transparency. Phys. Rev. Lett. 1991, 66, 2593. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Zhao, H.W.; Han, J.G. Terahertz metamaterial sensor based on electromagnetically induced transparency effect. J. Electron. Sci. Technol. 2015, 13, 117–121. [Google Scholar]
- Yan, X.; Yang, M.; Zhang, Z.; Liang, L.; Wei, D.; Wang, M.; Zhang, M.; Wang, T.; Liu, L.; Xie, J.; et al. The terahertz electromagnetically induced transparency-like metamaterials for sensitive biosensors in the detection of cancer cells. Biosens. Bioelectron. 2019, 126, 485–492. [Google Scholar] [CrossRef]
- He, X.; Yang, X.; Lu, G.; Yang, W.; Wu, F.; Yu, Z.; Jiang, J. Implementation of selective controlling electromagnetically induced transparency in terahertz graphene metamaterial. Carbon 2017, 123, 668–675. [Google Scholar] [CrossRef]
- Jung, H.; Jo, H.; Lee, W.; Kim, B.; Choi, H.; Kang, M.S.; Lee, H. Electrical control of electromagnetically induced transparency by terahertz metamaterial funneling. Adv. Opt. Mater. 2019, 7, 1801205. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Liang, L.; Zhou, G.; Zheng, F.; Li, C.; Zhang, C.; Jin, B. Tailoring electromagnetically induced transparency effect of terahertz metamaterials on ultrathin substrate. Sci. China Inf. Sci. 2016, 59, 1–6. [Google Scholar] [CrossRef]
- Lang, T.; Yu, Z.; Zhang, J.; Hong, Z.; Liu, J.; Wang, P. Bovine serum albumin detection based on electromagnetically induced transparency in terahertz metamaterial. Sens. Actuators A Phys. 2023, 360, 114522. [Google Scholar] [CrossRef]
- Tavakoli, F.; Zarrabi, F.B.; Saghaei, H. Modeling and analysis of high-sensitivity refractive index sensors based on plasmonic absorbers with Fano response in the near-infrared spectral region. Appl. Opt. 2019, 58, 5404–5414. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, R.; Tan, S.; Cong, L.; Singh, R.; Yan, F.; Zhang, W. Multispectral terahertz sensing with highly flexible ultrathin metamaterial absorber. J. Appl. Phys. 2015, 118, 083103. [Google Scholar] [CrossRef]
- Xiong, Z.; Shang, L.; Yang, J.; Chen, L.; Guo, J.; Liu, Q.; Akwasi Danso, S.; Li, J. Terahertz Sensor With Resonance Enhancement Based on Square Split-Ring Resonators. IEEE Access 2021, 9, 59211–59221. [Google Scholar] [CrossRef]
- Fan, F.; Zhong, C.; Zhang, Z.; Li, S.; Chang, S. Terahertz chiral sensing and magneto-optical enhancement for ferromagnetic nanofluids in the chiral metasurface. Nanoscale Adv. 2021, 3, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Erlich, H.A. Principles and Applications for DNA Amplification. In PCR Technology; Stockton Press: New York, NY, USA, 2015; pp. 1–246. [Google Scholar]
- Pachl, C.; Todd, J.A.; Kern, D.G.; Sheridan, P.J.; Fong, S.J.; Stempien, M.; Bradley, H.; Diana, B.; Torange, Y.; Bruce, I.; et al. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. JAIDS J. Acquir. Immune Defic. Syndr. 1995, 8, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Cha, S.H.; Shin, G.A.; Ahn, Y.H. Sensing viruses using terahertz nano-gap metamaterials. Biomed. Opt. Express 2017, 8, 3551–3558. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).