Photophysical Properties of Bright Luminescent Polyethyleneimine@Carbon Nanodots and Their Application in White Light-Emitting Diodes
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Characterization
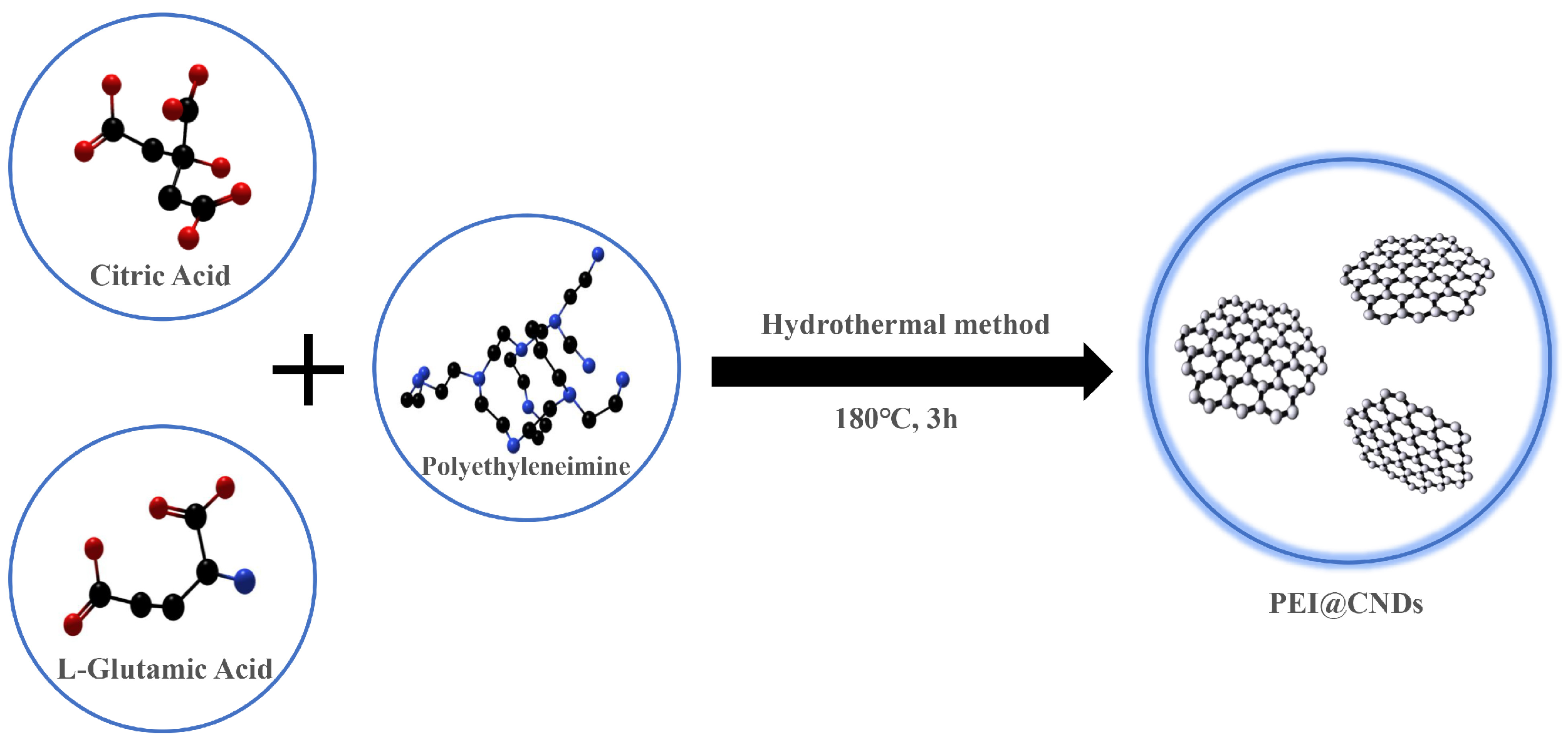
2.2. Preparation of PEI@CNDs
2.3. Preparation of Composite Films
2.4. Preparation of WLEDs
3. Results and Discussion
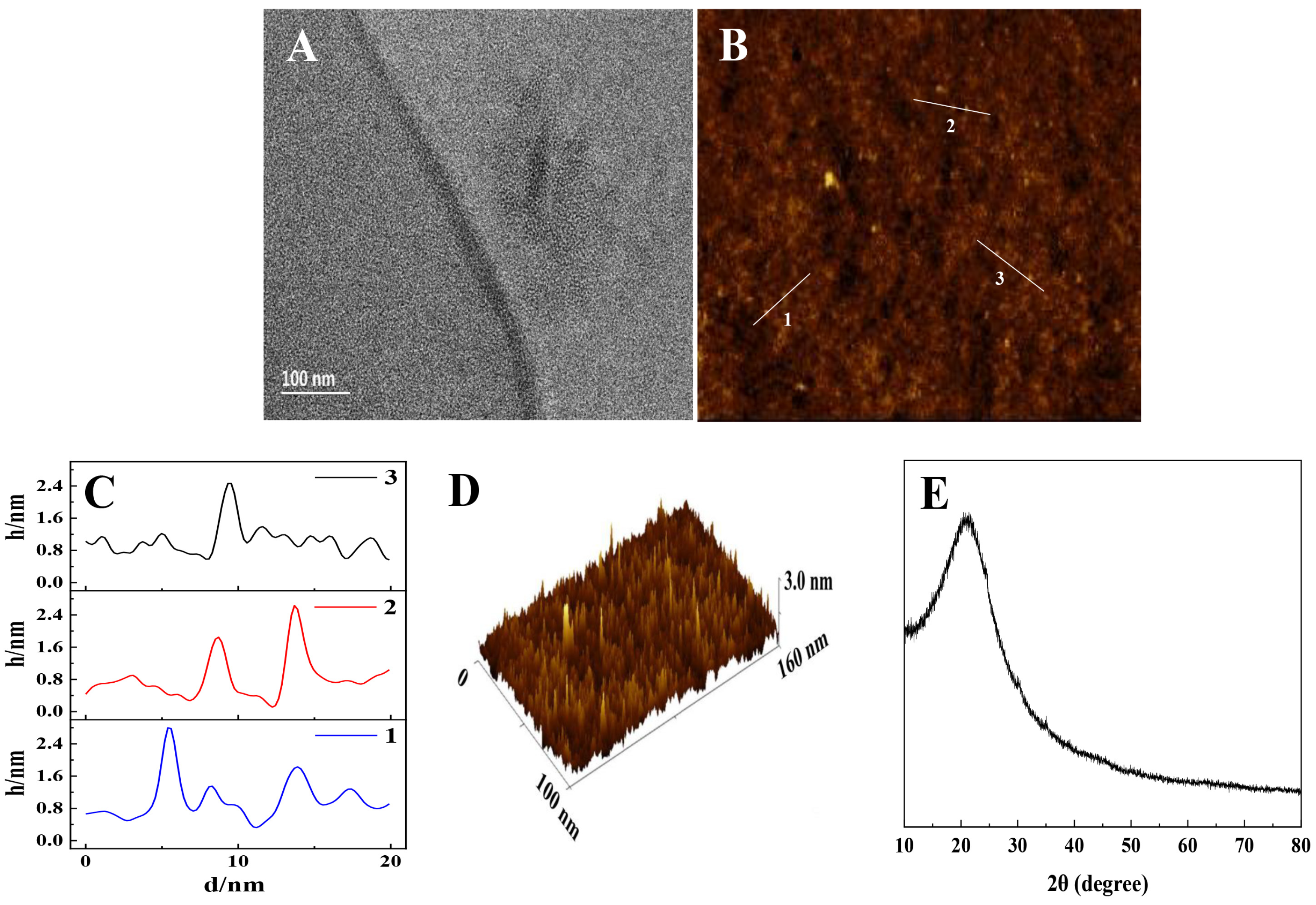
3.1. Morphology and Structure of the PEI@CNDs
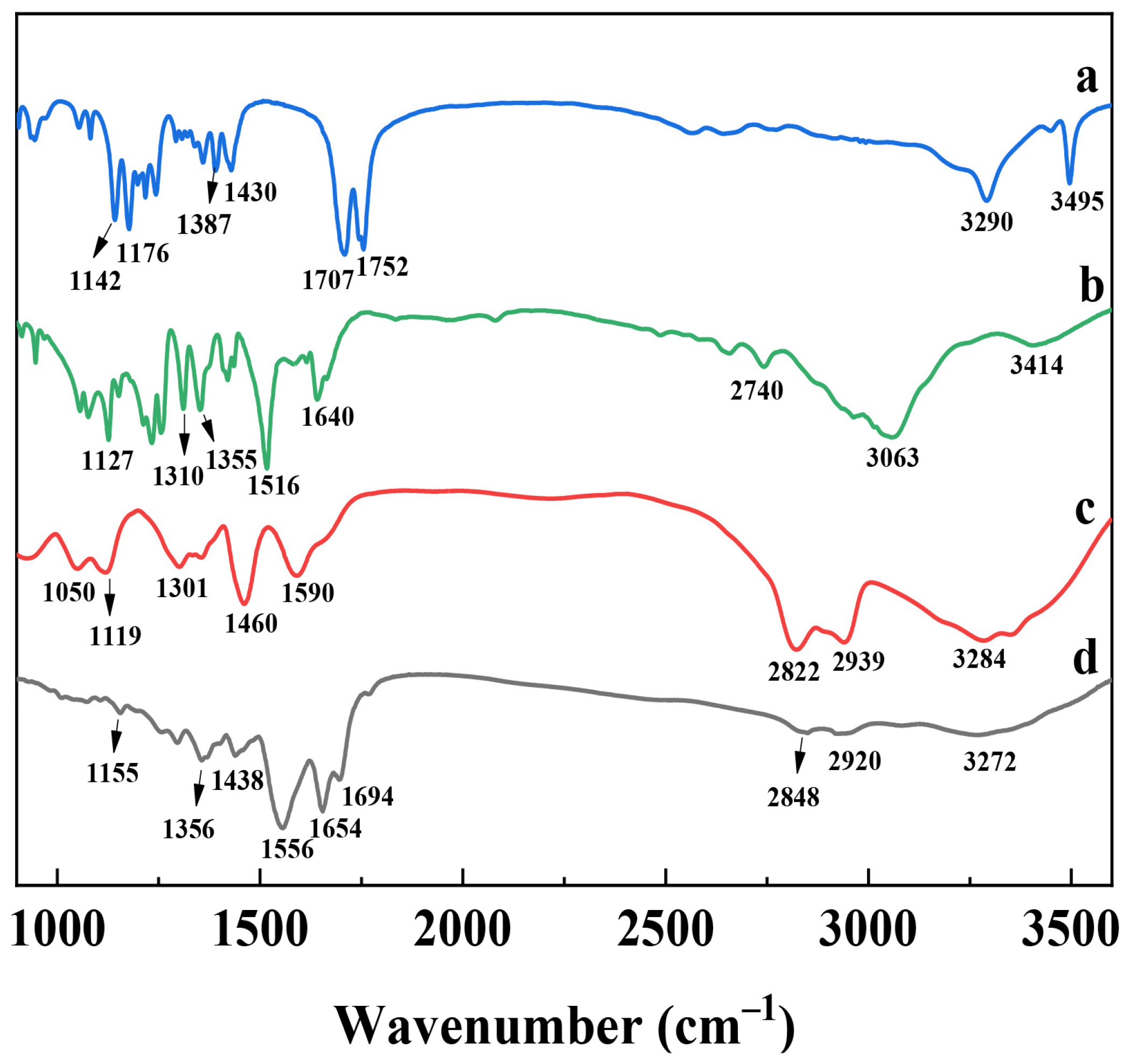
3.2. Surface Chemistry Property of the PEI@CNDs
3.3. Photophysical Properties of PEI@CNDs at Low Concentration
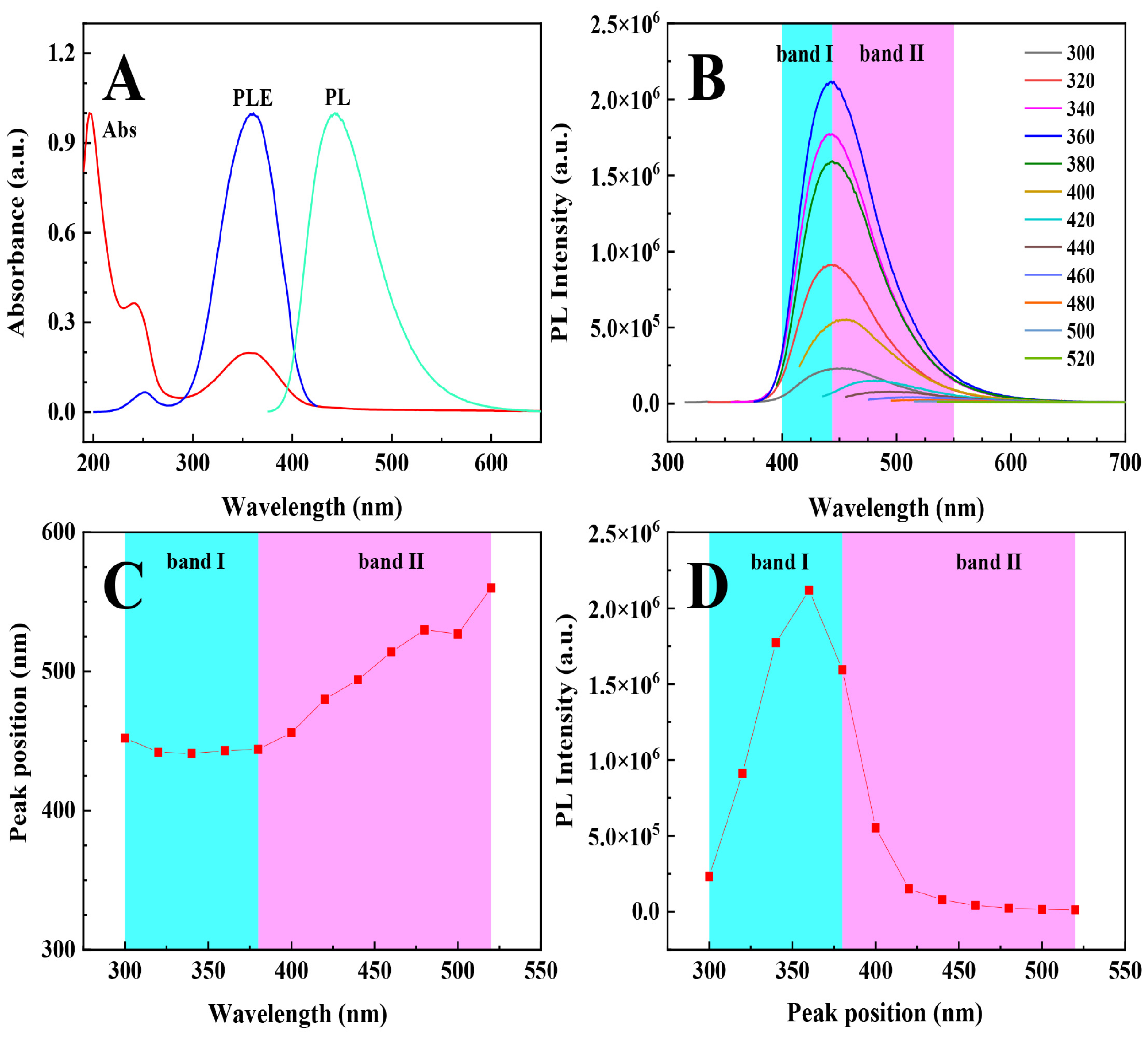
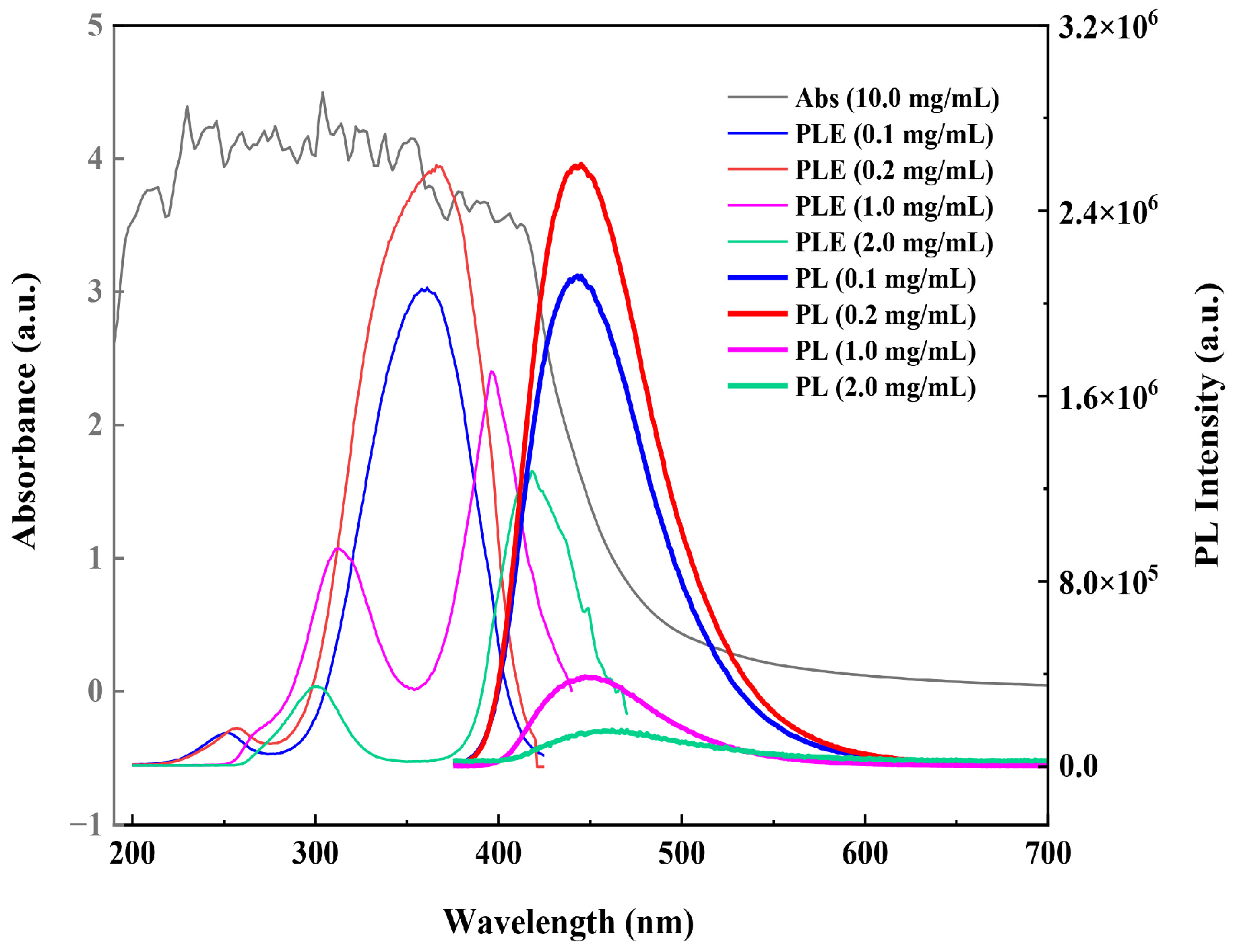
3.3.1. UV–Vis Absorption and Photoluminescence
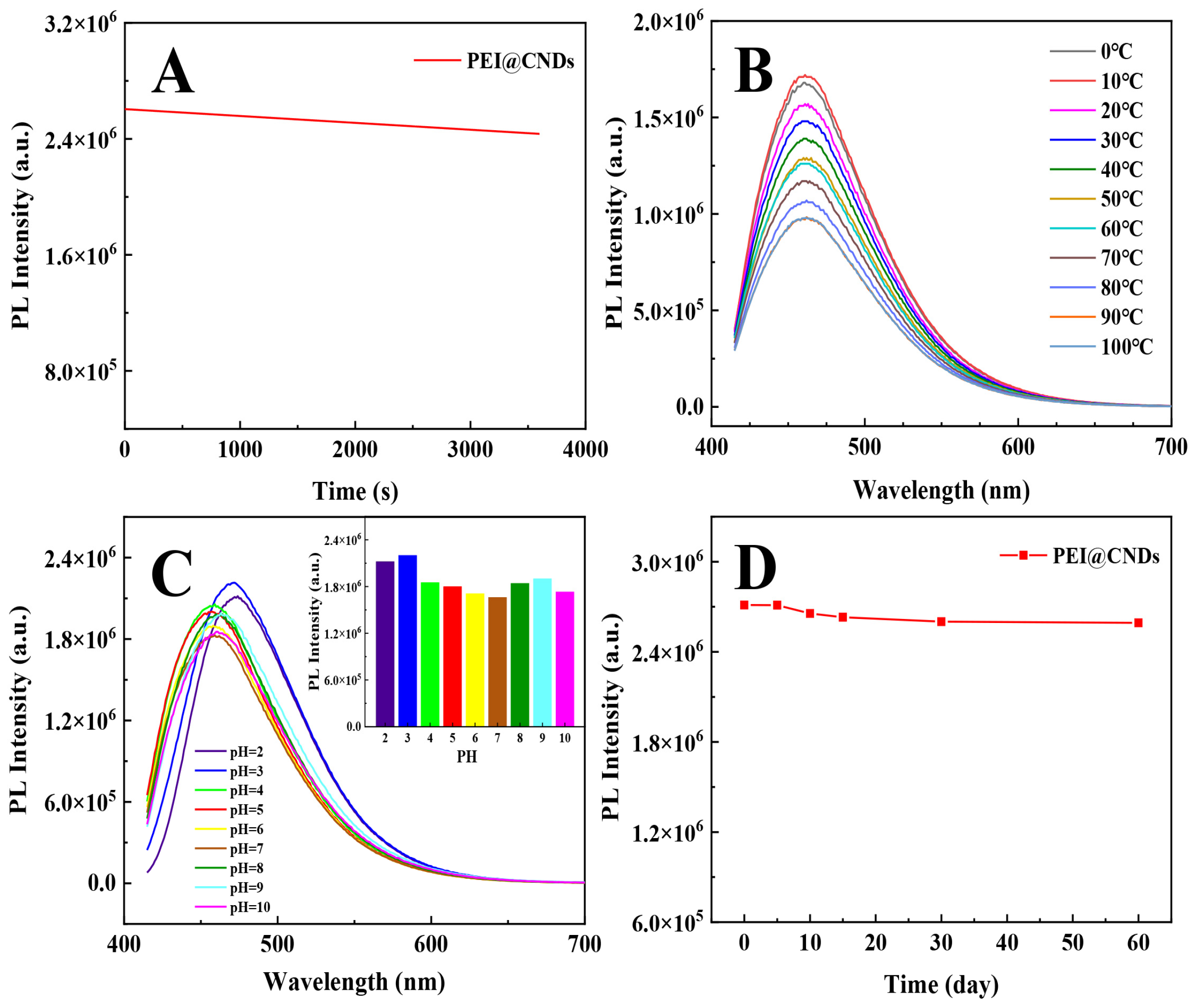
3.3.2. Optical Stability
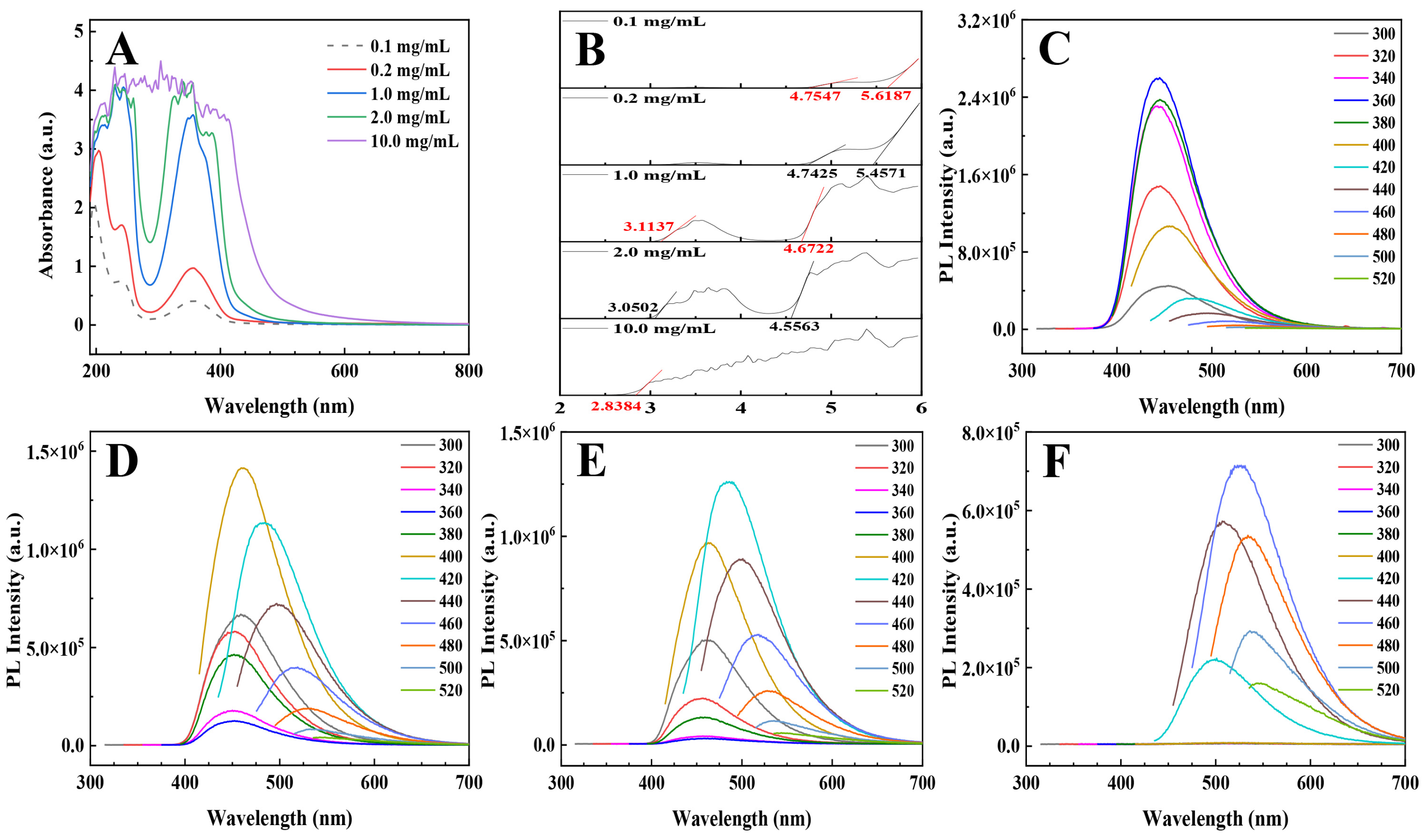
3.4. Photophysical Properties of the PEI@CNDs at High Concentrations
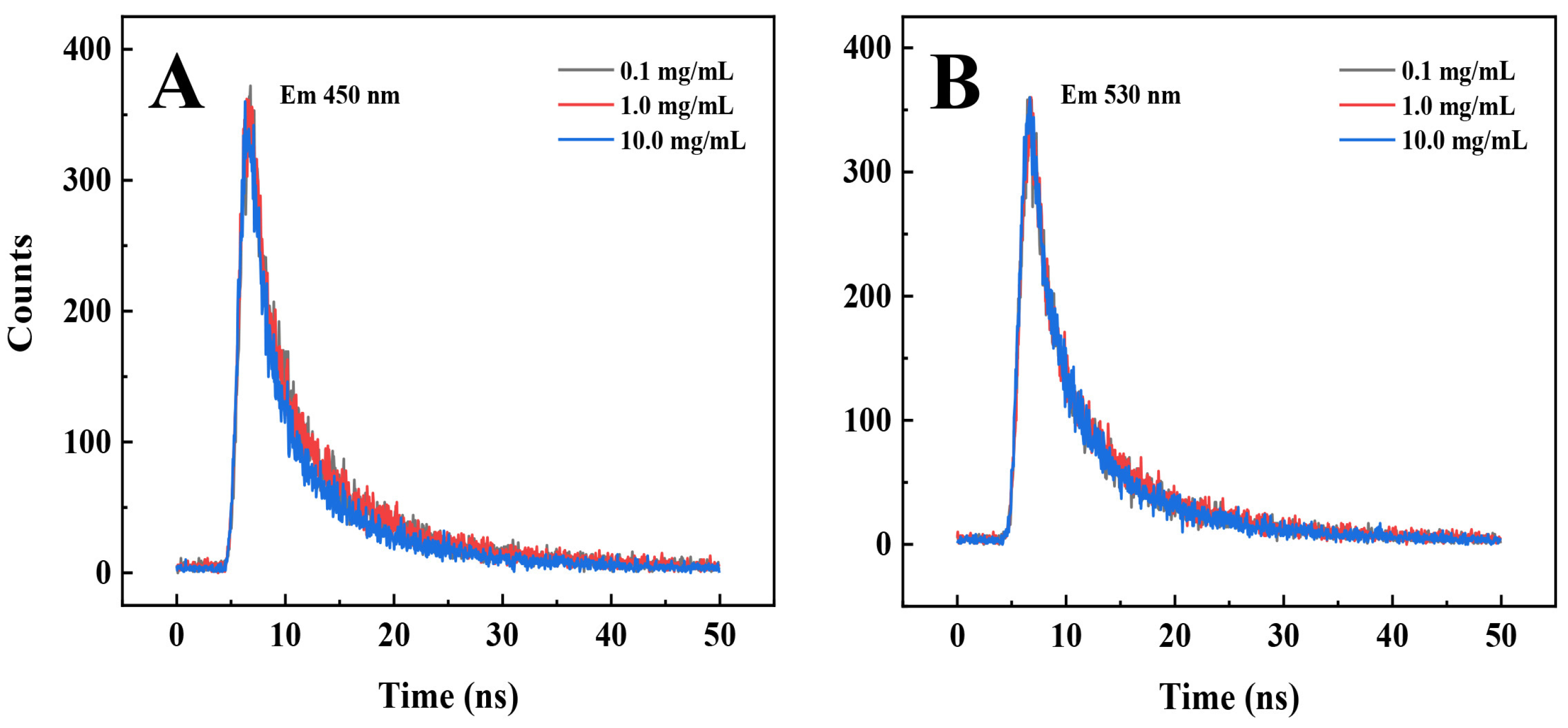
3.5. The PL Mechanism of the PEI@CNDs at High Concentrations
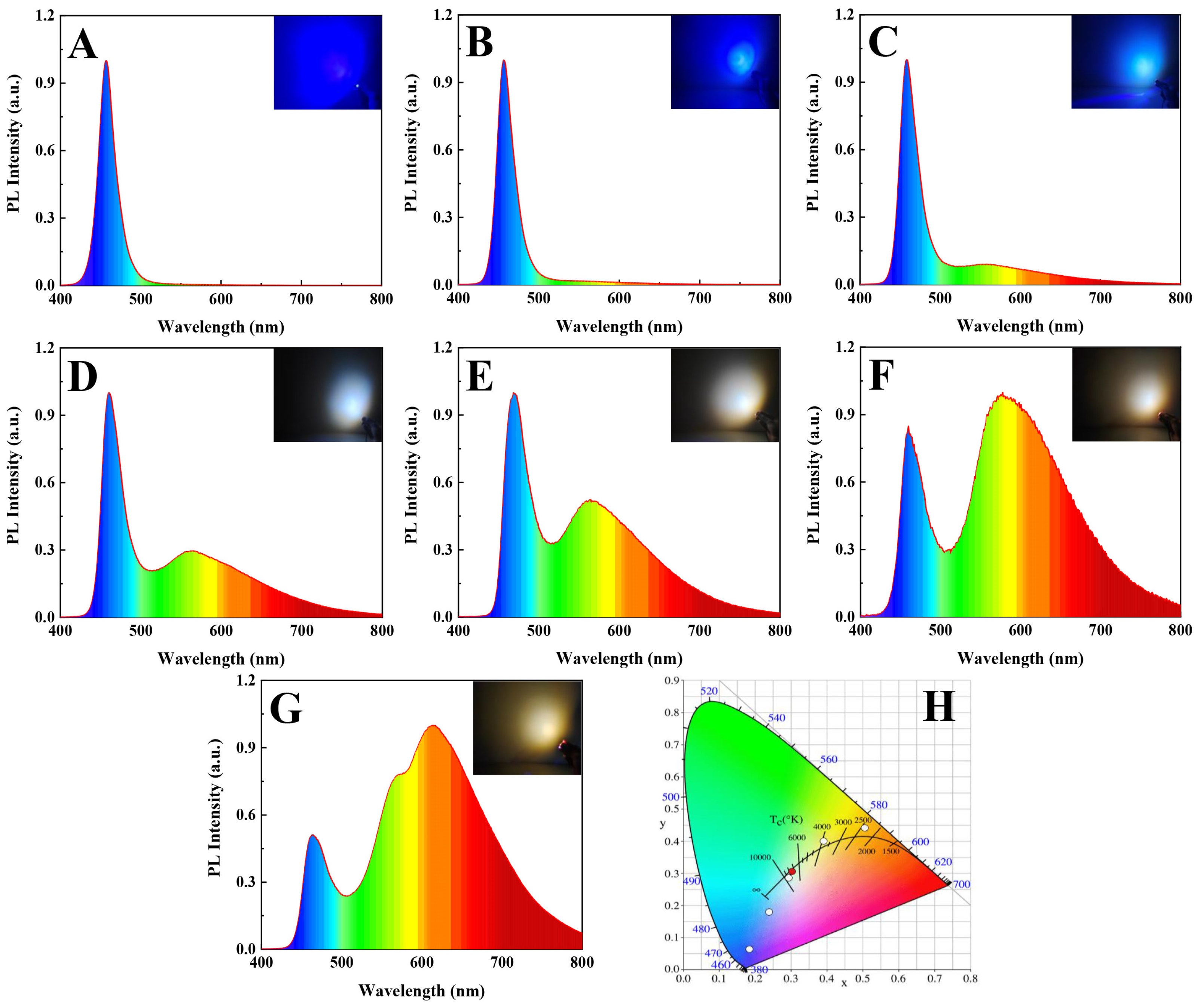
3.6. Performance of WLED
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Won, S.; Park, Y.; Kwon, W. Oxygen-less carbon nanodots with an absolute quantum yield of 80% for display applications. ACS Appl. Nano Mater. 2021, 4, 2462–2469. [Google Scholar] [CrossRef]
- Chinnadurai, S.; Krishnendhu, T.S.; Ashraf, P.M.; Edwin, L. Synthesis and characterization of carbon nanodots from jellyfish. Synth. Met. 2022, 287, 117085. [Google Scholar] [CrossRef]
- Sakthivel, S.; Zhou, X.; Giannelis, E.P.; Kanj, M.Y. Carbon nanodots for enhanced oil recovery in carbonate reservoirs. Energy Rep. 2021, 7, 8943–8959. [Google Scholar] [CrossRef]
- Verma, N.C.; Yadav, A.; Rao, C.; Mishra, P.M.; Nandi, C.K. Emergence of carbon nanodots as a probe for super-resolution microscopy. J. Phys. Chem. C 2021, 125, 1637–1653. [Google Scholar] [CrossRef]
- Liu, S.; Fang, X.; Mi, F.; Sun, K.; Wu, C. Covalent incorporation of metalloporphyrin in luminescent polymer dot transducer for continuous glucose monitoring. J. Lumin. 2022, 251, 119202. [Google Scholar] [CrossRef]
- Han, B.; Yan, Q.; Liu, Q.; Li, D.; Chen, Y.; He, G. Bright green emission non-conjugated polymer dots: pH trigged hydrogel for specific adsorption of anionic dyes and visual detection of tert-butylhydroquinone. Sep. Purif. Technol. 2022, 292, 121023. [Google Scholar] [CrossRef]
- Ghosh, D.; Sarkar, K.; Devi, P.; Kim, K.H.; Kumar, P. Current and future perspectives of carbon and graphene quantum dots: From synthesis to strategy for building optoelectronic and energy devices. Renew. Sust. Energ. Rev. 2021, 135, 110391. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, J.; Shu, L.; Zhu, J.; Fu, B.; Song, C.; Shang, W.; Tao, P.; Deng, T. Self-dispersible graphene quantum dots in ethylene glycol for direct absorption-based medium-temperature solar-thermal harvesting. RSC Adv. 2020, 10, 45028–45036. [Google Scholar] [CrossRef]
- Fu, B.; Liu, Q.; Liu, M.; Chen, X.; Lin, H.; Zheng, Z.; Zhu, J.; Dai, C.; Dong, X.; Yang, D.P. Carbon dots enhanced gelatin/chitosan bio-nanocomposite packaging film for perishable foods. Chin. Chem. Lett. 2022, 33, 4577–4582. [Google Scholar] [CrossRef]
- Mei, S.; Fu, B.; Su, X.; Chen, H.; Lin, H.; Zheng, Z.; Dai, C.; Yang, D.P. Developing silk sericin-based and carbon dots reinforced bio-nanocomposite films and potential application to litchi fruit. LWT—Food Sci.Technol. 2022, 164, 113630. [Google Scholar] [CrossRef]
- Su, X.; Lin, H.; Fu, B.; Mei, S.; Lin, M.; Chen, H.; Zheng, Z.; Bo, H.; Yang, D.P.; Lin, Y. Egg-yolk-derived carbon dots@albumin bio-nanocomposite as multifunctional coating and its application in quality maintenance of fresh litchi fruit during storage. Food Chem. 2023, 405, 134843. [Google Scholar] [CrossRef]
- Singh, S.; Vaishnav, J.K.; Mukherjee, T.K. Quantum dot-based hybrid coacervate nanodroplets for ultrasensitive detection of Hg2+. ACS Appl. Nano Mater. 2020, 3, 3604–3612. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. Bioinspired thiol functionalized carbon dots for rapid detection of lead (II) ions in human serum. Opt. Mater. 2020, 99, 109514. [Google Scholar] [CrossRef]
- Astafiev, A.A.; Shakhov, A.M.; Kritchenkov, A.S.; Khrustalev, V.N.; Shepel, D.V.; Nadtochenko, V.A.; Tskhovrebov, A.G. Femtosecond laser synthesis of nitrogen-doped luminescent carbon dots from acetonitrile. Dyes Pigm. 2021, 188, 109176. [Google Scholar] [CrossRef]
- Bian, H.; Wang, Q.; Yang, S.; Yan, C.; Wang, H.; Liang, L.; Jin, Z.; Wang, G.; Liu, S. Nitrogen-doped graphene quantum dots for 80% photoluminescence quantum yield for inorganic γ-CsPbI3 perovskite solar cells with efficiency beyond 16%. J. Mater. Chem. A 2019, 7, 5740–5747. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhou, Q.; Sheng, X.; Sun, Y.; Zhou, B.; Zhao, J.; Guo, J. A reliable and facile fluorescent sensor from carbon dots for sensing 2,4,6-trinitrophenol based on inner filter effect. Sci. Total Environ. 2020, 720, 137680. [Google Scholar] [CrossRef]
- Gao, T.; Wang, X.; Yang, L.Y.; He, H.; Ba, X.X.; Zhao, J.; Jiang, F.L.; Liu, Y. Red, yellow, and blue luminescence by graphene quantum dots: Syntheses, mechanism, and cellular imaging. ACS Appl. Mater. Interfaces 2017, 9, 24846–24856. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Chen, D.; Gu, B.; Gao, B.; Wang, Z.; Guo, Q.; Wang, G. Multifunctional N-doped graphene quantum dots towards tetracycline detection, temperature sensing and high-performance WLEDs. J. Photochem. Photobiol. A Chem. 2021, 405, 112977. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, G.G.; Li, J.B.; Liu, Z.; Zhao, W.F.; Han, J.C. Towards high-powered remote WLED based on flexible white-luminescent polymer composite films containing S, N co-doped graphene quantum dots. Chem. Eng. J. 2018, 336, 406–415. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Wang, G.G.; Li, J.B.; Liu, Z.; Chen, Y.X.; Liu, L.F.; Han, J.C. Direct white emissive Cl-doped graphene quantum dots-based flexible film as a single luminophore for remote tunable UV-WLEDs. Chem. Eng. J. 2019, 361, 773–782. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, Y.; Yu, C.; Xuan, T. Highly stable carbon nanodot-based phosphor as a color converter for WLED. J. Lumin. 2022, 246, 118836. [Google Scholar] [CrossRef]
- Guo, X.; Xu, L.; Zhang, L.; Wang, H.; Wang, X.; Liu, X.; Yao, J.; Hao, A. One-pot solid phase pyrolysis synthesis of highly fluorescent nitrogen-doped carbon dots and the interaction with human serum albumin. J. Lumin. 2018, 196, 100–110. [Google Scholar] [CrossRef]
- Yang, G.; Wu, C.; Luo, X.; Liu, X.; Gao, Y.; Wu, P.; Cai, C.; Saavedra, S.S. Exploring the emissive states of heteroatom-doped graphene quantum dots. J. Phys. Chem. C 2018, 122, 6483–6492. [Google Scholar] [CrossRef]
- Sadhanala, H.K.; Pagidi, S.; Gedanken, A. High quantum yield boron-doped carbon dots: A ratiometric fluorescent probe for highly selective and sensitive detection of Mg2+ ions. J. Mater. Chem. C 2021, 9, 1632–1640. [Google Scholar] [CrossRef]
- Fan, Z.; Li, Y.; Li, X.; Fan, L.; Zhou, S.; Fang, D.; Yang, S. Surrounding media sensitive photoluminescence of boron-doped graphene quantum dots for highly fluorescent dyed crystals, chemical sensing and bioimaging. Carbon 2014, 70, 149–156. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Shi, H.; Yan, Y.; Liu, E.; Hu, X.; Fan, J. A strong blue fluorescent nanoprobe for highly sensitive and selective detection of mercury (II) based on sulfur doped carbon quantum dots. Mater. Chem. Phys. 2019, 232, 145–151. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Luo, C.; Wang, Y.; Jiang, C.; Qi, R.; Huang, R.; Travas-sejdic, J.; Peng, H. Aggregation induced red shift emission of phosphorus doped carbon dots. RSC Adv. 2017, 7, 32225–32228. [Google Scholar] [CrossRef]
- Omer, K.M.; Hassan, A.Q. Chelation-enhanced fluorescence of phosphorus doped carbon nanodots for multi-ion detection. Microchim. Acta 2017, 184, 2063–2071. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Cheong, W.C.; Zhang, C.; Zheng, L.; Yan, W.; Yu, R.; Chen, C.; Li, Y. Nitrogen-coordinated cobalt nanocrystals for oxidative dehydrogenation and hydrogenation of N-heterocycles. Chem. Sci. 2019, 10, 5345–5352. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhai, Z.; Dong, X.; Zhang, P. Nitrogen doped carbon quantum dots (N-CQDs) with high luminescence for sensitive and selective detection of hypochlorite ions by fluorescence quenching. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 279, 121456. [Google Scholar] [CrossRef]
- Li, W.; Tang, J.; Li, Y.; Bai, H.; Zhang, W.; Zhang, J.; Xiao, Y.; Xu, W. Preparation and fluorescent wavelength control of multi-color nitrogen-doped carbon nano-dots. Nanomaterials 2021, 11, 3190. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Liu, Z.; Qiu, J.; Xu, X. High quality germanate phosphor La(2-x-y)Ge2O7: Tb3+, Eu3+ for WLED applications applications. J. Lumin. 2022, 252, 119295. [Google Scholar] [CrossRef]
- Hoerder, G.J.; Seibald, M.; Baumann, D.; Schroder, T.; Peschke, S.; Schmid, P.C.; Tyborski, T.; Pust, P.; Stoll, I.; Bergler, M.; et al. Sr[Li2Al2O2N2]: Eu2+—A high performance red phosphor to brighten the future. Nat. Commun. 2019, 10, 1824. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Wang, N.; Sun, Z.; Wang, J.; Li, J.; Yu, J. Carbogenic nanodots derived from organo-templated zeolites with modulated full-color luminescence. Chem. Sci. 2016, 7, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Singh, S.; Sharma, S.; Batra, G.; Kaushik, K.; Rao, C.; Verma, N.C.; Mondal, B.; Yadav, A.; Nandi, C.K. Absorption and emission of light in red emissive carbon nanodots. Chem. Sci. 2021, 12, 3615–3626. [Google Scholar] [CrossRef]
- Jang, H.S.; Yang, H.; Kim, S.W.; Han, J.Y.; Lee, S.G.; Jeon, D.Y. White light-emitting diodes with excellent color rendering based on organically capped CdSe quantum dots and Sr3SiO5: Ce3+, Li+ phosphors. Adv. Mater. 2008, 20, 2696–2702. [Google Scholar] [CrossRef]
- Dutzler, D.; Seibald, M.; Baumann, D.; Huppertz, H. Alkali Lithosilicates: Renaissance of a reputable substance class with surprising luminescence properties. Angew. Chem. Int. Ed. Engl. 2018, 57, 13676–13680. [Google Scholar] [CrossRef]
- Hudson, M.H.; Chen, M.; Kamysbayev, V.; Janke, E.M.; Lan, X.; Allan, G.; Delerue, C.; Lee, B.; Guyot-Sionnest, P.; Talapin, D.V. Conduction band fine structure in colloidal HgTe quantum dots. ACS Nano 2018, 12, 9397–9404. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% efficient red emissive carbon quantum dots for high color rendering and stable warm white-light-emitting diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Zhou, D.; Li, D.; Ji, W.; Jing, P.; Han, D.; Liu, L.; Zeng, H.; Shen, D. Toward efficient orange emissive carbon nanodots through conjugated sp2-domain controlling and surface charges engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, H.; Tang, Z.; Yang, B.; Lu, S. Ethanol-derived white emissive carbon dots: The formation process investigation and multi-color/white LEDs preparation. Nano Res. 2021, 15, 942–949. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Yin, L.; Liu, Y.; Guo, H.; Lai, J.; Han, Y.; Li, G.; Li, M.; Zhang, J.; et al. Full-color fluorescent carbon quantum dots. Sci. Adv. 2020, 6, eabb6772. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, L.; Sun, T.; Xue, X.; Wei, B. Facilely synthesized carbon dots and configurated light-emitting diodes with efficient electroluminescence. Phys. Chem. Chem. Phys. 2022, 24, 26511–26518. [Google Scholar] [CrossRef]
- Li, W.; Wu, M.; Jiang, H.; Yang, L.; Liu, C.; Gong, X. Carbon dots/ZnO quantum dots composite-based white phosphors for white light-emitting diodes. Chem. Commun. 2022, 58, 1910–1913. [Google Scholar] [CrossRef]
- Yan, X.; Wei, P.; Liu, Y.; Wang, M.; Chen, C.; Zhao, J.; Li, G.; Saha, M.L.; Zhou, Z.; An, Z.; et al. Endo- and exo-functionalized tetraphenylethylene M12L24 nanospheres: Fluorescence emission inside a confined space. J. Am. Chem. Soc. 2019, 141, 9673–9679. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, A.; Mishra, N.K.; Sahu, S.K. Polymer-induced emission-active fluorine-embedded carbon dots for the preparation of warm WLEDs with a high color rendering index. Langmuir 2022, 38, 9389–9399. [Google Scholar] [CrossRef]
- Aghamali, A.; Khosravi, M.; Hamishehkar, H.; Modirshahla, N.; Behnajady, M.A. Preparation of novel high performance recoverable and natural sunlight-driven nanocomposite photocatalyst of Fe3O4/C/TiO2/N-CQDs. Mater. Sci. Semicond. Process. 2018, 87, 142–154. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.T.; Ashraf Gandomi, Y.; Li, J.; Yue, X.X.; Chang, J.K. Tailoring fluorescence emissions, quantum yields, and white light emitting from nitrogen-doped graphene and carbon nitride quantum dots. Nanoscale 2019, 11, 16553–16561. [Google Scholar] [CrossRef]
- Tarakeshwar, P.; Manogaran, S. Ground state vibrations of citric acid and the citrate trianion—An ab initio study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1994, 50, 2327–2343. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Li, Y.; Li, S. A sensitive enzyme-free hydrogen peroxide sensor based on a chitosan–graphene quantum dot/silver nanocube nanocomposite modified electrode. Anal. Methods 2016, 8, 2448–2455. [Google Scholar] [CrossRef]
- Strauss, V.; Wang, H.; Delacroix, S.; Ledendecker, M.; Wessig, P. Carbon nanodots revised: The thermal citric acid/urea reaction. Chem. Sci. 2020, 11, 8256–8266. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.Y.; Bae, Y.H. Self-assembled polyethylenimine-graft-poly(epsilon-caprolactone) micelles as potential dual carriers of genes and anticancer drugs. Biomaterials 2007, 28, 4132–4142. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, N.; Salehi, Z.; Khodadadi, A.A.; Shokrgozar, M.A.; Saboury, A.A. Glucosamine-conjugated graphene quantum dots as versatile and pH-sensitive nanocarriers for enhanced delivery of curcumin targeting to breast cancer. Mater. Sci. Eng. C 2021, 121, 111809. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of seaweed extracts from laminaria and ascophyllum nodosum spp. as biostimulants in Zea mays L. Using a combination of chemical, biochemical and morphological approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, N.; Cui, Z.; Du, H.; Fu, L.; Huang, C.; Yang, Z.; Shen, X.; Yi, Y.; Tu, Z.; et al. Hydrogen substituted graphdiyne as carbon-rich flexible electrode for lithium and sodium ion batteries. Nat. Commun. 2017, 8, 1172. [Google Scholar] [CrossRef]
- Wen, D.; Liu, W.; Haubold, D.; Zhu, C.; Oschatz, M.; Holzschuh, M.; Wolf, A.; Simon, F.; Kaskel, S.; Eychmuller, A. Gold aerogels: Three-dimensional assembly of nanoparticles and their use as electrocatalytic interfaces. ACS Nano 2016, 10, 2559–2567. [Google Scholar] [CrossRef]
- Liang, K.; Spiesz, E.M.; Schmieden, D.T.; Xu, A.W.; Meyer, A.S.; Aubin-Tam, M.E. Bioproduced polymers self-assemble with graphene oxide into nanocomposite films with enhanced mechanical performance. ACS Nano 2020, 14, 14731–14739. [Google Scholar] [CrossRef]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yuksekkaya, M.; Wan, K.T.; et al. Reduced graphene Oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef]
- Dostert, K.H.; O’Brien, C.P.; Mirabella, F.; Ivars-Barcelo, F.; Schauermann, S. Adsorption of acrolein, propanal, and allyl alcohol on Pd(111): A combined infrared reflection-absorption spectroscopy and temperature programmed desorption study. Phys. Chem. Chem. Phys. 2016, 18, 13960–13973. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Nicolas, C.; Bentzer, J.; Ellstrom, M.; Smits, M.; Rineau, F.; Canback, B.; Floudas, D.; Carleer, R.; Lackner, G.; et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol. 2016, 209, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Schroer, Z.S.; Wu, Y.; Xing, Y.; Wu, X.; Liu, X.; Wang, X.; Pino, O.G.; Zhou, C.; Combs, C.; Pu, Q.; et al. Nitrogen–sulfur-doped graphene quantum dots with metal ion-resistance for bioimaging. ACS Appl. Nano Mater. 2019, 2, 6858–6865. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, F.; Li, L.; Qi, B.; Zhu, D.; Lu, J.; Lu, C. Tricolor white-light-emitting carbon dots with multiple-cores@shell structure for WLED application. ACS Appl. Mater. Interfaces 2018, 10, 19796–19805. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, W.; Zhou, T.; Wang, B.; Li, H.; Ding, L. Synthesis and formation mechanistic investigation of nitrogen-doped carbon dots with high quantum yields and yellowish-green fluorescence. Nanoscale 2016, 8, 11185–11193. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Song, X.; Zhao, Z.; Zhang, N.; Hao, C. Pitch-derived carbon quantum dots as fluorescent probe for selective and sensitive detection of ferric ions and bioimaging. J. Photochem. Photobiol. A Chem. 2021, 412, 113253. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wang, J.; Liu, H.; Li, S.; Hou, Y.; Wan, W.; Xue, W.; Ma, N.; Zhang, J.Z. Chemical nature of redox-controlled photoluminescence of graphene quantum dots by post-synthesis treatment. J. Phys. Chem. C 2016, 120, 26004–26011. [Google Scholar] [CrossRef]
- Hola, K.; Sudolska, M.; Kalytchuk, S.; Nachtigallova, D.; Rogach, A.L.; Otyepka, M.; Zboril, R. Graphitic nitrogen triggers red fluorescence in carbon dots. ACS Nano 2017, 11, 12402–12410. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Mernaugh, R.L.; Zeng, X. Single chain fragment variable recombinant antibody functionalized gold nanoparticles for a highly sensitive colorimetric immunoassay. Biosens. Bioelectron. 2009, 24, 2853–2857. [Google Scholar] [CrossRef]
- Rao, L.; Tang, Y.; Lu, H.; Yu, S.; Ding, X.; Xu, K.; Li, Z.; Zhang, J.Z. Highly photoluminescent and stable N-doped carbon dots as nanoprobes for Hg2+ detection. Nanomaterials 2018, 8, 900. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Wang, H.; Yang, A.; Wang, X.; Xu, J. Facile fabrication of highly fluorescent N-doped carbon quantum dots using an ultrasonic-assisted hydrothermal method: Optical properties and cell imaging. ACS Omega 2021, 6, 32904–32916. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, C.; Yang, A.; Wang, X.; Xu, J. One-pot synthesis of bright blue luminescent N-doped GQDs: Optical properties and cell imaging. Nanomaterials 2021, 11, 2798. [Google Scholar] [CrossRef]
- Page, R.C.; Espinobarro-Velazquez, D.; Leontiadou, M.A.; Smith, C.; Lewis, E.A.; Haigh, S.J.; Li, C.; Radtke, H.; Pengpad, A.; Bondino, F.; et al. Near-unity quantum yields from chloride treated CdTe colloidal quantum dots. Small 2015, 11, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Slimani, Y.; Almessiere, M.A.; Guner, S.; Kurtan, U.; Baykal, A. Impacts of sol-gel auto-combustion and ultrasonication approaches on structural, magnetic, and optical properties of Sm-Tm co-substituted Sr0.5Ba0.5Fe12O19 nanohexaferrites: Comparative study. Nanomaterials 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, A.S.; Hussain, S.; Malik, A.H.; Afroz, M.A.; Iyer, P.K. Inner filter effect based selective detection of nitroexplosive-picric acid in aqueous solution and solid support using conjugated polymer. ACS Sens. 2016, 1, 1070–1077. [Google Scholar] [CrossRef]
- Jiang, K.; Luo, S.H.; Pang, C.M.; Wang, B.W.; Wu, H.Q.; Wang, Z.Y. A functionalized fluorochrome based on quinoline-benzimidazole conjugate: From facile design to highly sensitive and selective sensing for picric acid. Dyes Pigm. 2019, 162, 367–376. [Google Scholar] [CrossRef]
- Gu, B.; Liu, Z.; Chen, D.; Gao, B.; Yang, Y.; Guo, Q.; Wang, G. Solid-state fluorescent nitrogen doped graphene quantum dots with yellow emission for white light-emitting diodes. Synth. Met. 2021, 277, 116787. [Google Scholar] [CrossRef]
- Kumar Panigrahi, S.; Kumar Mishra, A. Inner filter effect in fluorescence spectroscopy: As a problem and as a solution. J. Photochem. Photobiol. C 2019, 41, 100318. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Liang, M.; Xu, L.; Qi, S.; Chen, H.; Chen, X. Solid-phase synthesis of highly fluorescent nitrogen-doped carbon dots for sensitive and selective probing ferric ions in living cells. Anal. Chem. 2014, 86, 9846–9852. [Google Scholar] [CrossRef]
- Meng, X.; Chang, Q.; Xue, C.; Yang, J.; Hu, S. Full-colour carbon dots: From energy-efficient synthesis to concentration-dependent photoluminescence properties. Chem. Commun. 2017, 53, 3074–3077. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, H.; Wei, Y.; He, X.; Chen, Y.; Wang, B.; Zeng, Q.; Lin, J. Concentration-induced multi-colored emissions in carbon dots: Origination from triple fluorescent centers. Nanoscale 2018, 10, 6734–6743. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Phatake, R.S.; Nabha Barnea, S.; Zerby, N.; Zhu, J.J.; Shikler, R.; Lemcoff, N.G.; Jelinek, R. Fluorescent self-healing carbon dot/polymer gels. ACS Nano 2019, 13, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Yuan, Y.; Zhu, Z.L.; Jiang, Z.Q.; Su, S.J.; Liao, L.S.; Lee, C.S. De novo design of D-sigma-A molecules as universal hosts for monochrome and white phosphorescent organic light-emitting diodes. Chem. Sci. 2018, 9, 4062–4070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuo, P.; Yin, H.; Fan, Y.; Zhang, J.; Liu, X.; Chen, Z. Solid-state fluorescent carbon dots with aggregation-induced yellow emission for white light-emitting diodes with high luminous efficiencies. ACS Appl. Mater. Interfaces 2019, 11, 24395–24403. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, Z.; Lu, S.; Wang, L.; Feng, X.; Yang, D.; Wang, K.; Xiao, G.; Zhang, L.; Redfern, S.A.T.; et al. Pressure-induced emission of cesium lead halide perovskite nanocrystals. Nat. Commun. 2018, 9, 4506. [Google Scholar] [CrossRef]
- Ha, J.; Seo, Y.; Kim, Y.; Lee, J.; Lee, H.; Kim, S.; Choi, Y.; Oh, H.; Lee, Y.; Park, E.; et al. Synthesis of nitrogen-doped carbon nanodots to destroy bacteria competing with Campylobacter jejuni in enrichment medium, and development of a monoclonal antibody to detect C. jejuni after enrichment. Int. J. Food Microbiol. 2021, 339, 109014. [Google Scholar] [CrossRef]


| Ex/Em (nm) | Concentration (mg/mL) | τ1 (ns) | α1 (%) | τ2 (ns) | α2 (%) | τav (ns) |
|---|---|---|---|---|---|---|
| 375/450 | 0.1 | 1.89 | 21.66 | 8.97 | 78.34 | 7.44 |
| 1.0 | 2.01 | 25.09 | 8.72 | 74.91 | 7.03 | |
| 10.0 | 1.66 | 25.26 | 7.63 | 74.74 | 6.13 | |
| 420/530 | 0.1 | 1.89 | 24.92 | 8.44 | 75.08 | 6.81 |
| 1.0 | 1.59 | 18.65 | 7.84 | 81.35 | 6.67 | |
| 10.0 | 1.92 | 26.55 | 7.86 | 73.45 | 6.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, A.; Zhang, K. Photophysical Properties of Bright Luminescent Polyethyleneimine@Carbon Nanodots and Their Application in White Light-Emitting Diodes. Photonics 2023, 10, 262. https://doi.org/10.3390/photonics10030262
Zhang J, Yang A, Zhang K. Photophysical Properties of Bright Luminescent Polyethyleneimine@Carbon Nanodots and Their Application in White Light-Emitting Diodes. Photonics. 2023; 10(3):262. https://doi.org/10.3390/photonics10030262
Chicago/Turabian StyleZhang, Junming, Ailing Yang, and Kang Zhang. 2023. "Photophysical Properties of Bright Luminescent Polyethyleneimine@Carbon Nanodots and Their Application in White Light-Emitting Diodes" Photonics 10, no. 3: 262. https://doi.org/10.3390/photonics10030262
APA StyleZhang, J., Yang, A., & Zhang, K. (2023). Photophysical Properties of Bright Luminescent Polyethyleneimine@Carbon Nanodots and Their Application in White Light-Emitting Diodes. Photonics, 10(3), 262. https://doi.org/10.3390/photonics10030262




