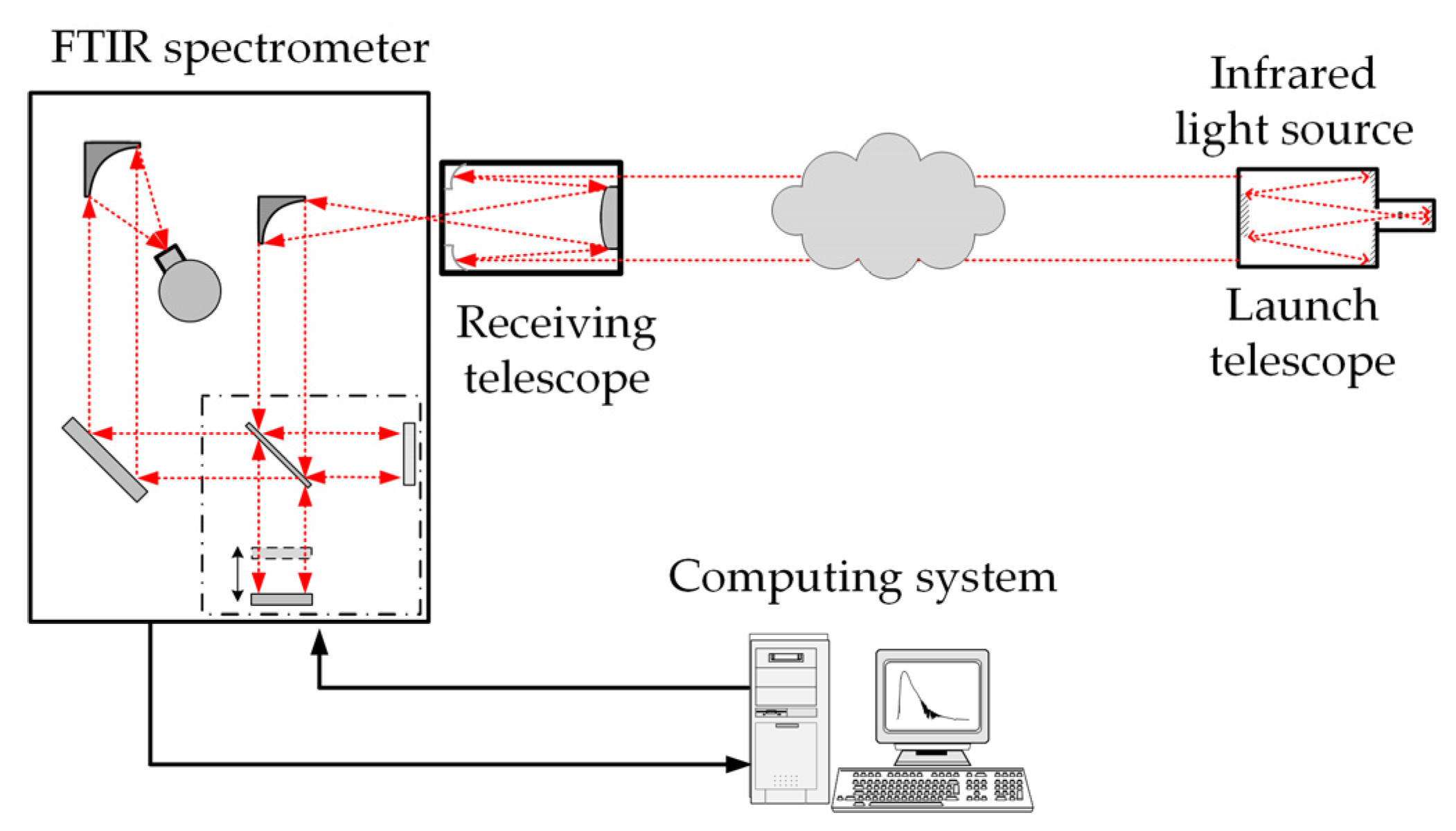
2.1. Synthetic Background Spectrum
The background spectrum used to calculate absorbance is an essential component affecting OP-FTIR quantitative analysis. Under laboratory conditions, it is not difficult to measure the background spectra for an FTIR spectrometer in a closed absorption cell; it is only necessary to fill the closed sample cell with N
2 to obtain an ideal background spectrum. However, in OP-FTIR measurements, it is difficult to directly remove the target gas from the open path due to the presence of a large number of interfering gases and environmental background noise. Therefore, alternative methods are required to reduce this interference. One commonly used method is the synthetic background spectrum method [
4,
17]. The synthetic background spectrum method used in this paper is a filtering method based on moving average filtering.
The basic principle of obtaining the background spectrum is to treat the background spectrum as a slowly changing baseline superimposed on the absorption spectrum structure. Then, extract the background spectrum from the measurement spectrum through filtering and smoothening. Moving average filtering is a classic filtering method, which smoothens spectral data to remove noise and obtain a background spectrum. The specific operation process is as follows:
- (1)
Perform moving average filtering on the spectral data S, as shown in Equation (1):
where
S(
i) is the spectral data and
N = 2
m + 1 is the width of the filter,
m represents the number of smoothening points.
- (2)
After filtering and smoothening, the spectral data S1 is obtained.
- (3)
Replace the values in S that are less than S1 with S1 to construct a new relative intensity vector S0, that is,
where
S1 is the synthesized background spectrum,
S is the measured spectrum, and
S0 is the target iteration spectrum.
- (4)
Repeat the above process for S0 until the filtering loop stops (after satisfying the pre-set number of iterations). The synthetic background spectrum S1 is thus obtained.
The accuracy of the synthetic background spectrum depends on the absorption peak width and height of the gas spectrum and filter type [
17]. For certain gases, the absorption peak width and height of the gas spectrum depend on the spectral resolution. Therefore, when using the same filtering method, spectral resolution is an important factor that affects the synthetic background spectrum.
The value function E1 is used to represent the error of the synthesized background spectrum in this paper, as shown in Equation (3):
where
Sa is the actual background spectrum,
S1 is the synthesized background spectrum,
S is the measured spectrum,
S″ is the second derivative of the measured spectrum, ϖ(
i) is the noise signal, and
λ1 is the impact parameter of spectral resolution.
In Equation (3), the second derivative of the measured spectrum is used to represent the spectral resolution, the impact of which can be quantified on the synthesized background spectrum. The gas spectrum characteristics are dependent on the spectral FWHM (both narrow and broad), which is mainly affected by the characteristics of the gas. Gas spectra with different FWHM are also affected differently by the resolution and have different impacts on the synthesized background.
For gas spectra with a narrow FWHM, the signal-to-noise ratio (SNR) is improved at low resolutions, which means that the value of ϖ(i) decreases. However, gas spectra with a narrow FWHM may be drowned out by the background spectrum and thus the background spectrum cannot be effectively extracted (this makes the value of λ1 increase). Therefore, the influence of spectral resolution on the value function is significantly increased in the synthesized background spectrum, which in turn makes the value of E1 increase.
For gas spectra with a broad FWHM, not only is the spectral SNR improved at low resolutions, but the spectral information can also be effectively preserved. In other words, the value of λ1 and ϖ(i) make E1 decrease. Therefore, even with a lower spectral resolution, a thorough filtering and smoothening effect can be obtained, which is favorable for improving the accuracy of the synthesized background spectrum.
2.2. NLLS
Considering the nonlinear relationship between spectral response and concentration, the NLLS method [
18,
19,
20,
21] was introduced to correct the nonlinear behavior of the spectrum and improve its quantitative accuracy. This section will analyze the impact of spectral resolution on NLLS.
The absorption coefficient σ(
ν) is expressed as
where σ
0(
ν) is the absorption coefficient from the standard database,
fL is pressure broadening,
fG is temperature broadening, and ILS(
ν) is Instrumental Line Shape effects (including resolution, window function, Field of View, etc.).
Gas concentration inversion can be represented as a multi-parameter NLLS fitting process, which can be represented by the best fitting function as follows:
where
Im represents the measured spectral intensity,
I0 represents the intensity of the synthesized background spectrum,
L is the optical path length, σ
i is the absorption coefficient of component
i, C
i is the concentration of component
i, and ν is the wavenumber.
For simplicity,
is used to represent the coefficient vector, which is comprised of the concentration, environmental parameters, and instrument parameters. Then Equation (5) can be rewritten as follows:
The purpose of NLLS fitting is to minimize the difference between the fitting data and the actual data. Typically, this difference can be represented by the value function E2, that is:
where
SF is the fitting spectrum represented, and
SM is the measured spectrum.
The size of this difference is mainly affected by the SNR. For an FTIR spectrometer, the SNR is proportional to the square root of observation time t and spectral resolution , where Δν represents the spectral resolution. When the resolution causes a change in the SNR, the SNR becomes the main impact on the NLLS fitting. The noise in the spectral data can interfere with the recognition of features, thus reducing the fitting accuracy. However, for some gas spectra, low resolution will also interfere with feature recognition. Therefore, an analysis of the impact of spectral resolution must also take into account the specific characteristics of the gas spectrum.
Therefore, Equation (7) is modified to the form of Equation (8), as follows:
where
SF is the fitting spectrum represented,
SM is the measured spectrum,
is the second derivative of the measured spectrum. ϖ(
ν) is the noise signal, and λ
2 is the impact parameter of spectral resolution.
In Equation (8), the second derivative of the measured spectrum is used to represent the spectral resolution, thus quantifying its impact on the synthetic background spectrum. In nonlinear least squares (NLLS) quantitative analyses, resolution and SNR represent a pair of opposite parameters. In SNR-centric analyses, higher resolution may provide more spectral information, thereby reducing the impact of SNR. Therefore, a negative relationship is expressed using a minus sign to denote the contradictory nature of these parameters.
The fitting difference is analyzed with the SNR as the main influence, where a higher SNR at low resolution indicates a more accurate fitting. However, for gas spectra with different FWHM, the influence of resolution is different, which requires separate discussion.
For gas spectra with a narrow FWHM, high spectral resolution leads to a larger second derivative of the spectrum, making the spectral peaks sharper and allowing for more accurate identification of the spectral features. Thus, the impact of the SNR on the NLLS fitting accuracy is reduced to some extent.
For gas spectra with a broad FWHM, high spectral resolution results in smoother spectral peaks and a smaller second derivative of the spectrum. The improvement of the spectral features at high resolution is limited, and the fitting accuracy is primarily affected by the changes in the SNR, which are themselves caused by changes in the resolution.