A Study of the Effects of Medical Dental Laser and Diamond Drill on Dentin Tissue during Dental Restoration Based on Spectral Imaging and Multivariate Analysis of Synchrotron FTIR Microspectroscopy Data
Abstract
1. Introduction
2. Objects and Methods of Research
3. Results Obtained and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmedt-Aristizabal, D.; Armin, A.; Denman, S.; Fookes, C.; Petersson, L. Graph-Based Deep Learning for Medical Diagnosis and Analysis: Past, Present and Future. Sensors 2021, 21, 4758. [Google Scholar] [CrossRef] [PubMed]
- Besnard, C.; Harper, R.A.; Moxham, T.E.J.; James, J.D.; Storm, M.; Salvati, E.; Landini, G.; Shelton, R.M.; Korsunsky, A.M. 3D Analysis of Enamel Demineralisation in Human Dental Caries Using High-Resolution, Large Field of View Synchrotron X-Ray Micro-Computed Tomography. Mater. Today Commun. 2021, 27, 102418. [Google Scholar] [CrossRef]
- Kenesei, K.; Murali, K.; Czéh, Á.; Piella, J.; Puntes, V.; Madarász, E. Enhanced Detection with Spectral Imaging Fluorescence Microscopy Reveals Tissue- and Cell-Type-Specific Compartmentalization of Surface-Modified Polystyrene Nanoparticles. J. Nanobiotechnol. 2016, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Besnard, C.; Marie, A.; Sasidharan, S.; Harper, R.A.; Shelton, R.M.; Landini, G.; Korsunsky, A.M. Synchrotron X-Ray Studies of the Structural and Functional Hierarchies in Mineralised Human Dental Enamel: A State-of-the-Art Review. Dent. J. 2023, 11, 98. [Google Scholar] [CrossRef]
- Pullano, S.A.; Bianco, M.G.; Greco, M.; Mazzuca, D.; Nisticò, S.P.; Fiorillo, A.S. FT-IR Saliva Analysis for the Diagnosis of Psoriasis: A Pilot Study. Biomed. Signal Process. Control 2022, 74, 103525. [Google Scholar] [CrossRef]
- Calzolari, A.; Pavan, B.; Curtarolo, S.; Buongiorno Nardelli, M.; Fornari, M. Vibrational Spectral Fingerprinting for Chemical Recognition of Biominerals. ChemPhysChem 2020, 21, 770–778. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Emelyanova, A.; Buylov, N.; Ippolitov, Y.; Prutskij, T. Development of a Visualisation Approach for Analysing Incipient and Clinically Unrecorded Enamel Fissure Caries Using Laser-Induced Contrast Imaging, MicroRaman Spectroscopy and Biomimetic Composites: A Pilot Study. J. Imaging 2022, 8, 137. [Google Scholar] [CrossRef]
- Vongsvivut, J.; Pérez-Guaita, D.; Wood, B.R.; Heraud, P.; Khambatta, K.; Hartnell, D.; Hackett, M.J.; Tobin, M.J. Synchrotron Macro ATR-FTIR Microspectroscopy for High-Resolution Chemical Mapping of Single Cells. Analyst 2019, 144, 3226–3238. [Google Scholar] [CrossRef]
- Freitas, R.O.; Cernescu, A.; Engdahl, A.; Paulus, A.; Levandoski, J.E.; Martinsson, I.; Hebisch, E.; Sandt, C.; Gouras, G.K.; Prinz, C.N.; et al. Nano-Infrared Imaging of Primary Neurons. Cells 2021, 10, 2559. [Google Scholar] [CrossRef]
- Babot-Marquillas, C.; Sánchez-Martín, M.-J.; Amigo, J.M.; Yousef, I.H.; Valido, I.; Boada, R.; Valiente, M. Tooth Whitening, Oxidation or Reduction? Study of Physicochemical Alterations in Bovine Enamel Using Synchrotron Based Micro-FTIR. Dent. Mater. 2022, 38, 670–679. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Khydyakov, Y.; Nesterov, D.; Ippolitov, I.; Ippolitov, Y.; Vongsvivut, J. Development of a Hybrid Biomimetic Enamel-Biocomposite Interface and a Study of Its Molecular Features Using Synchrotron Submicron ATR-FTIR Microspectroscopy and Multivariate Analysis Techniques. Int. J. Mol. Sci. 2022, 23, 11699. [Google Scholar] [CrossRef]
- Zepeda-Zepeda, M.A.; Picquart, M.; Irigoyen-Camacho, M.E.; Mejía-Gózalez, A.M. Diagnosis of Dental Fluorosis Using Micro-Raman Spectroscopy Applying a Principal Component-Linear Discriminant Analysis. Int. J. Environ. Res. Public Health 2021, 18, 10572. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Nesterov, D.; Ippolitov, Y.; Ippolitov, I.; Vongsvivut, J. Effect of Exo/Endogenous Prophylaxis Dentifrice/Drug and Cariogenic Conditions of Patient on Molecular Property of Dental Biofilm: Synchrotron FTIR Spectroscopic Study. Pharmaceutics 2022, 14, 1355. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Ippolitov, I.; Vongsvivut, J. To the Question on the Use of Multivariate Analysis and 2D Visualisation of Synchrotron ATR-FTIR Chemical Imaging Spectral Data in the Diagnostics of Biomimetic Sound Dentin/Dental Composite Interface. Diagnostics 2021, 11, 1294. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y. Jitraporn Vongsvivut Spectroscopic Signature of the Pathological Processes of Carious Dentine Based on FTIR Investigations of the Oral Biological Fluids. Biomed. Opt. Express BOE 2019, 10, 4050–4058. [Google Scholar] [CrossRef]
- Querido, W.; Falcon, J.M.; Kandel, S.; Pleshko, N. Vibrational Spectroscopy and Imaging: Applications for Tissue Engineering. Analyst 2017, 142, 4005–4017. [Google Scholar] [CrossRef]
- Ye, Q.; Spencer, P. Analyses of Material-Tissue Interfaces by Fourier Transform Infrared, Raman Spectroscopy, and Chemometrics. In Material-Tissue Interfacial Phenomena; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–251. ISBN 978-0-08-100330-5. [Google Scholar]
- Amarie, S.; Zaslansky, P.; Kajihara, Y.; Griesshaber, E.; Schmahl, W.W.; Keilmann, F. Nano-FTIR Chemical Mapping of Minerals in Biological Materials. Beilstein J. Nanotechnol. 2012, 3, 312–323. [Google Scholar] [CrossRef]
- Delgado, A.H.; Young, A.M. Modelling ATR-FTIR Spectra of Dental Bonding Systems to Investigate Composition and Polymerisation Kinetics. Materials 2021, 14, 760. [Google Scholar] [CrossRef]
- Betancourt, D.E.; Baldion, P.A.; Castellanos, J.E. Resin-Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer. Int. J. Biomater. 2019, 2019, e5268342. [Google Scholar] [CrossRef]
- Sato, T.; Takagaki, T.; Hatayama, T.; Nikaido, T.; Tagami, J. Update on Enamel Bonding Strategies. Front. Dent. Med. 2021, 2, 666379. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Engineering of a Biomimetic Interface between a Native Dental Tissue and Restorative Composite and Its Study Using Synchrotron FTIR Microscopic Mapping. Int. J. Mol. Sci. 2021, 22, 6510. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J. Current Perspectives on Dental Adhesion: (1) Dentin Adhesion—Not There Yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Kaptan, A.; Oznurhan, F. Effects of Er:YAG and Er,Cr:YSGG Laser Irradiation and Adhesive Systems on Microtensile Bond Strength of a Self-Adhering Composite. Lasers Med. Sci. 2023, 38, 41. [Google Scholar] [CrossRef] [PubMed]
- Valinhos Piccioni, M.A.R.; Girotto, A.C.; Saad, J.R.C.; de Campos, E.A. Influence of Different Surface Protocols on Dentin Bond Strength Irradiated with Er,Cr:YSGG Laser. Laser Dent. Sci. 2019, 3, 43–51. [Google Scholar] [CrossRef]
- Chowdhury, A.F.M.A.; Islam, R.; Alam, A.; Matsumoto, M.; Yamauti, M.; Carvalho, R.M.; Sano, H. Variable Smear Layer and Adhesive Application: The Pursuit of Clinical Relevance in Bond Strength Testing. Int. J. Mol. Sci. 2019, 20, 5381. [Google Scholar] [CrossRef]
- Lagunov, V.L.; Rybachuk, M.; Itthagarun, A.; Walsh, L.J.; George, R. Modification of Dental Enamel, Dentin by an Ultra-Fast Femtosecond Laser Irradiation: A Systematic Review. Opt. Laser Technol. 2022, 155, 108439. [Google Scholar] [CrossRef]
- Mahdisiar, F.; Mirzaei, A.; Fallah, A.; Gutknecht, N.; Akhoundan, S. Effect of Duration of Er,Cr:YSGG Laser Etching on Dentin Morphology: An in Vitro Study. Lasers Dent. Sci. 2018, 2, 213–219. [Google Scholar] [CrossRef]
- Petrov, T.; Pecheva, E.; Walmsley, A.D.; Dimov, S. Femtosecond Laser Ablation of Dentin and Enamel for Fast and More Precise Dental Cavity Preparation. Mater. Sci. Eng. C 2018, 90, 433–438. [Google Scholar] [CrossRef]
- Seredin, P.V.; Goloshchapov, D.L.; Prutskij, T.; Ippolitov, Y.A. Fabrication and Characterisation of Composites Materials Similar Optically and in Composition to Native Dental Tissues. Results Phys. 2017, 7, 1086–1094. [Google Scholar] [CrossRef]
- Seredin, P.V.; Goloshchapov, D.L.; Nikitkov, K.A.; Kashkarov, V.M.; Ippolitov, Y.A.; Jitraporn (Pimm), V. Application of Synchrotronic IR-Microspectroscopy for Analysis of Integration of Biomimetic Composites with Native Dental Tissues. Condens. Matter Interphases 2019, 21, 262–277. [Google Scholar] [CrossRef]
- Tekçe, N.; Tuncer, S.; Demirci, M.; Serim, M.E.; Baydemir, C. The Effect of Different Drinks on the Color Stability of Different Restorative Materials after One Month. Restor Dent Endod 2015, 40, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.V.; Uspenskaya, O.A.; Goloshchapov, D.L.; Ippolitov, I.Y.; Vongsvivut, J.; Ippolitov, Y.A. Organic-Mineral Interaction between Biomimetic Materials and Hard Dental Tissues. Sovrem. Tehnol. V Med. 2020, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, X.; Parthasarathy, R. Characterization of Interfacial Chemistry of Adhesive/Dentin Bond Using FTIR Chemical Imaging with Univariate and Multivariate Data Processing. J. Biomed. Mater. Res. A 2009, 91, 251–262. [Google Scholar] [CrossRef]
- Savić, D.; Joković, N.; Topisirović, L. Multivariate Statistical Methods for Discrimination of Lactobacilli Based on Their FTIR Spectra. Dairy Sci. Technol. 2008, 88, 273–290. [Google Scholar] [CrossRef]
- Jegova, G.; Titorenkova, R.; Rashkova, M.; Mihailova, B. Raman and IR Reflection Micro-Spectroscopic Study of Er:YAG Laser Treated Permanent and Deciduous Human Teeth. J. Raman Spectrosc. 2013, 44, 1483–1490. [Google Scholar] [CrossRef]
- Shamsudeen, S.M.; Thavarajah, R.; Joshua, E.; Rao, U.D.K.; Kannan, R. Evaluating and Comparing the Morphological and Histopathological Changes Induced by Erbium:Yttrium-Aluminum-Garnet Laser and Diamond Bur on Enamel, Dentin and Pulp Tissue. J. Investig. Clin. Dent. 2019, 10, e12475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, K.; Xu, W.; Gong, X.; Zhang, F. Mapping the Mechanical Gradient of Human Dentin-Enamel-Junction at Different Intratooth Locations. Dent. Mater. 2018, 34, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Querido, W.; Kandel, S.; Pleshko, N. Applications of Vibrational Spectroscopy for Analysis of Connective Tissues. Molecules 2021, 26, 922. [Google Scholar] [CrossRef]
- Kobrina, Y.; Rieppo, L.; Saarakkala, S.; Pulkkinen, H.J.; Tiitu, V.; Valonen, P.; Kiviranta, I.; Jurvelin, J.S.; Isaksson, H. Cluster Analysis of Infrared Spectra Can Differentiate Intact and Repaired Articular Cartilage. Osteoarthr. Cartil. 2013, 21, 462–469. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Khudyakov, Y.; Ippolitov, I.; Ippolitov, Y.; Vongsvivut, J. Biomimetic Nano-c-HAp Hybrid Layer Engineering and Determination of Mechanisms of Its Integration with Native Hard Dental Tissue. Results Eng. 2021, 11, 100266. [Google Scholar] [CrossRef]
- Ogruc Ildiz, G.; Karadag, A.; Kaygisiz, E.; Fausto, R. PLS-DA Model for the Evaluation of Attention Deficit and Hyperactivity Disorder in Children and Adolescents through Blood Serum FTIR Spectra. Molecules 2021, 26, 3400. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Almança Lopes, C.; Limirio, P.H.J.O.; Novais, V.R.; Dechichi, P. Fourier Transform Infrared Spectroscopy (FTIR) Application Chemical Characterization of Enamel, Dentin and Bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, E.F. XRD and FTIR Crystallinity Indices in Sound Human Tooth Enamel and Synthetic Hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4568–4574. [Google Scholar] [CrossRef] [PubMed]
- Jeon, R.J.; Hellen, A.; Matvienko, A.; Mandelis, A.; Abrams, S.H.; Amaechi, B.T. In Vitro Detection and Quantification of Enamel and Root Caries Using Infrared Photothermal Radiometry and Modulated Luminescence. J Biomed. Opt. 2008, 13, 034025. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Marsan, O.; Combes, C.; Drouet, C.; Grossin, D.; Sarda, S. Characterization of Calcium Phosphates Using Vibrational Spectroscopies. In Advances in Calcium Phosphate Biomaterials; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2014; pp. 229–266. ISBN 978-3-642-53979-4. [Google Scholar]
- Bakry, A.S.; Sadr, A.; Takahashi, H.; Otsuki, M.; Tagami, J. Analysis of Er:YAG Lased Dentin Using Attenuated Total Reflectance Fourier Transform Infrared and X-Ray Diffraction Techniques. Dent. Mater. J. 2007, 26, 422–428. [Google Scholar] [CrossRef]
- Zezell, D.M.; Benetti, C.; Veloso, M.N.; Castro, P.A.A.; Ana, P.A. FTIR Spectroscopy Revealing the Effects of Laser and Ionizing Radiation on Biological Hard Tissues. J. Braz. Chem. Soc. 2015, 26, 2571–2582. [Google Scholar] [CrossRef]
- Le, Q.-T.; Bertrand, C.; Vilar, R. Structural Modifications Induced in Dentin by Femtosecond Laser. J. Biomed. Opt. 2016, 21, 125007. [Google Scholar] [CrossRef]
- Le, Q.T.; Vilar, R.; Bertrand, C. Influence of External Cooling on the Femtosecond Laser Ablation of Dentin. Lasers Med. Sci. 2017, 32, 1943–1951. [Google Scholar] [CrossRef]
- Sasaki, K.M.; Aoki, A.; Masuno, H.; Ichinose, S.; Yamada, S.; Ishikawa, I. Compositional Analysis of Root Cementum and Dentin after Er:YAG Laser Irradiation Compared with CO2 Lased and Intact Roots Using Fourier Transformed Infrared Spectroscopy. J. Periodontal Res. 2002, 37, 50–59. [Google Scholar] [CrossRef]
- Orsini, G.; Orilisi, G.; Notarstefano, V.; Monterubbianesi, R.; Vitiello, F.; Tosco, V.; Belloni, A.; Putignano, A.; Giorgini, E. Vibrational Imaging Techniques for the Characterization of Hard Dental Tissues: From Bench-Top to Chair-Side. Appl. Sci. 2021, 11, 11953. [Google Scholar] [CrossRef]
- Almhöjd, U.S.; Norén, J.G.; Arvidsson, A.; Nilsson, Å.; Lingström, P. Analysis of Carious Dentine Using FTIR and ToF-SIMS. Oral Health Dent Manag 2014, 13, 735–744. [Google Scholar] [PubMed]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting Carbonate Quantification in Apatite (Bio)Minerals: A Validated FTIR Methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.; Liu, Y.W.; Wang, Y. A Fourier Transform Infrared Spectroscopy Analysis of Carious Dentin from Transparent Zone to Normal Zone. Caries Res. 2014, 48, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, L.; Diebolder, R.; Hibst, R.; Zezell, D. Infrared Spectroscopy of Dentin Irradiated by Erbium Laser. Int. Congr. Ser. 2003, 1248, 153–156. [Google Scholar] [CrossRef]
- Spevak, L.; Flach, C.R.; Hunter, T.; Mendelsohn, R.; Boskey, A. Fourier Transform Infrared Spectroscopic Imaging Parameters Describing Acid Phosphate Substitution in Biologic Hydroxyapatite. Calcif. Tissue Int. 2013, 92, 418–428. [Google Scholar] [CrossRef]
- Spencer, P.; Wang, Y.; Katz, J.L.; Misra, A. Physicochemical Interactions at the Dentin/Adhesive Interface Using FTIR Chemical Imaging. J. Biomed. Opt. 2005, 10, 031104. [Google Scholar] [CrossRef]
- Villegas, M.F.; Garcia-Uriostegui, L.; Rodríguez, O.; Izquierdo-Barba, I.; Salinas, A.J.; Toriz, G.; Vallet-Regí, M.; Delgado, E. Lysine-Grafted MCM-41 Silica as an Antibacterial Biomaterial. Bioengineering 2017, 4, 80. [Google Scholar] [CrossRef]
- Goloshchapov, D.L.; Ippolitov, Y.A.; Seredin, P.V. Mechanism of Interaction among Nanocrystalline Carbonate-Substituted Hydroxyapatite and Polar Amino-Acids for the Biomimetic Composite Technology: Spectroscopic and Structural Study. Results Phys. 2020, 18, 103277. [Google Scholar] [CrossRef]
- Hernández, B.; Pflüger, F.; Derbel, N.; De Coninck, J.; Ghomi, M. Vibrational Analysis of Amino Acids and Short Peptides in Hydrated Media. VI. Amino Acids with Positively Charged Side Chains: l-Lysine and l-Arginine. J. Phys. Chem. B 2010, 114, 1077–1088. [Google Scholar] [CrossRef]
- Guo, H.; Xun, Q.; Liu, S.; Wang, X. Investigation of Ethylene Glycol Monomethyl Ether Soyate as a Biofuel; SAE International: Warrendale, PA, USA, 2015; p. 2015–01–0955. [Google Scholar]
- Gupta, B.S.; Jelle, B.P.; Gao, T. In Vitro Cell Composition Identification of Wood Decay Fungi by Fourier Transform Infrared Spectroscopy. R. Soc. Open Sci. 2022, 9, 201935. [Google Scholar] [CrossRef]
- Barth, A. The Infrared Absorption of Amino Acid Side Chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Swee Heng, C.; Neo Chiew Lian, J.; Fawzy, A.S. In Vitro Analysis of Riboflavin-Modified, Experimental, Two-Step Etch-and-Rinse Dentin Adhesive: Fourier Transform Infrared Spectroscopy and Micro-Raman Studies. Int. J. Oral Sci. 2015, 7, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Parthasarathy, R.; Abedin, F.; Laurence, J.S.; Misra, A.; Spencer, P. Multivariate Analysis of Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopic Data to Confirm Phase Partitioning in Methacrylate-Based Dentin Adhesive. Appl. Spectrosc. 2013, 67, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational Spectroscopy of Selective Dental Restorative Materials. Appl. Spectrosc. Rev. 2017, 52, 507–540. [Google Scholar] [CrossRef]
- Nayyer, M.; Zahid, S.; Hassan, S.H.; Mian, S.A.; Mehmood, S.; Khan, H.A.; Kaleem, M.; Zafar, M.S.; Khan, A.S. Comparative Abrasive Wear Resistance and Surface Analysis of Dental Resin-Based Materials. Eur. J. Dent. 2018, 12, 057–066. [Google Scholar] [CrossRef]
- Hędzelek, W.; Marcinkowska, A.; Domka, L.; Wachowiak, R. Infrared Spectroscopic Identification of Chosen Dental Materials and Natural Teeth. Acta Phys. Pol. A 2008, 114, 471–484. [Google Scholar] [CrossRef]
- Uskoković, V. Visualizing Different Crystalline States during the Infrared Imaging of Calcium Phosphates. Vib. Spectrosc. 2020, 108, 103045. [Google Scholar] [CrossRef]
- Vieira, A.A.; Silva, A.C.N. Effects of Erbium Laser Radiation on the Dentin Organic Matrix. Laser Dent. Sci. 2021, 5, 69–78. [Google Scholar] [CrossRef]
- de Oliveira, M.T.; de Freitas, P.M.; de Paula Eduardo, C.; Ambrosano, G.M.B.; Giannini, M. Influence of Diamond Sono-Abrasion, Air-Abrasion and Er:YAG Laser Irradiation on Bonding of Different Adhesive Systems to Dentin. Eur. J. Dent. 2007, 1, 158–166. [Google Scholar] [CrossRef]
- Torres, C.R.G.; Huhtala, M.F.R.L.; Teixeira, S.C.; Araujo, M.A.M. de Morphological Evaluation of the Bovine Dentin Prepared with High-Speed Turbine or Er:YAG Laser and Submitted to Different Adhesive Systems after Laser Etching. World J. Dent. 2011, 2, 117–123. [Google Scholar] [CrossRef]
- Wang, K.; Yuan, Y.; Han, S.; Yang, H. Application of Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) and Principal Component Analysis (PCA) for Quick Identifying of the Bitumen Produced by Different Manufacturers. Road Mater. Pavement Des. 2018, 19, 1940–1949. [Google Scholar] [CrossRef]
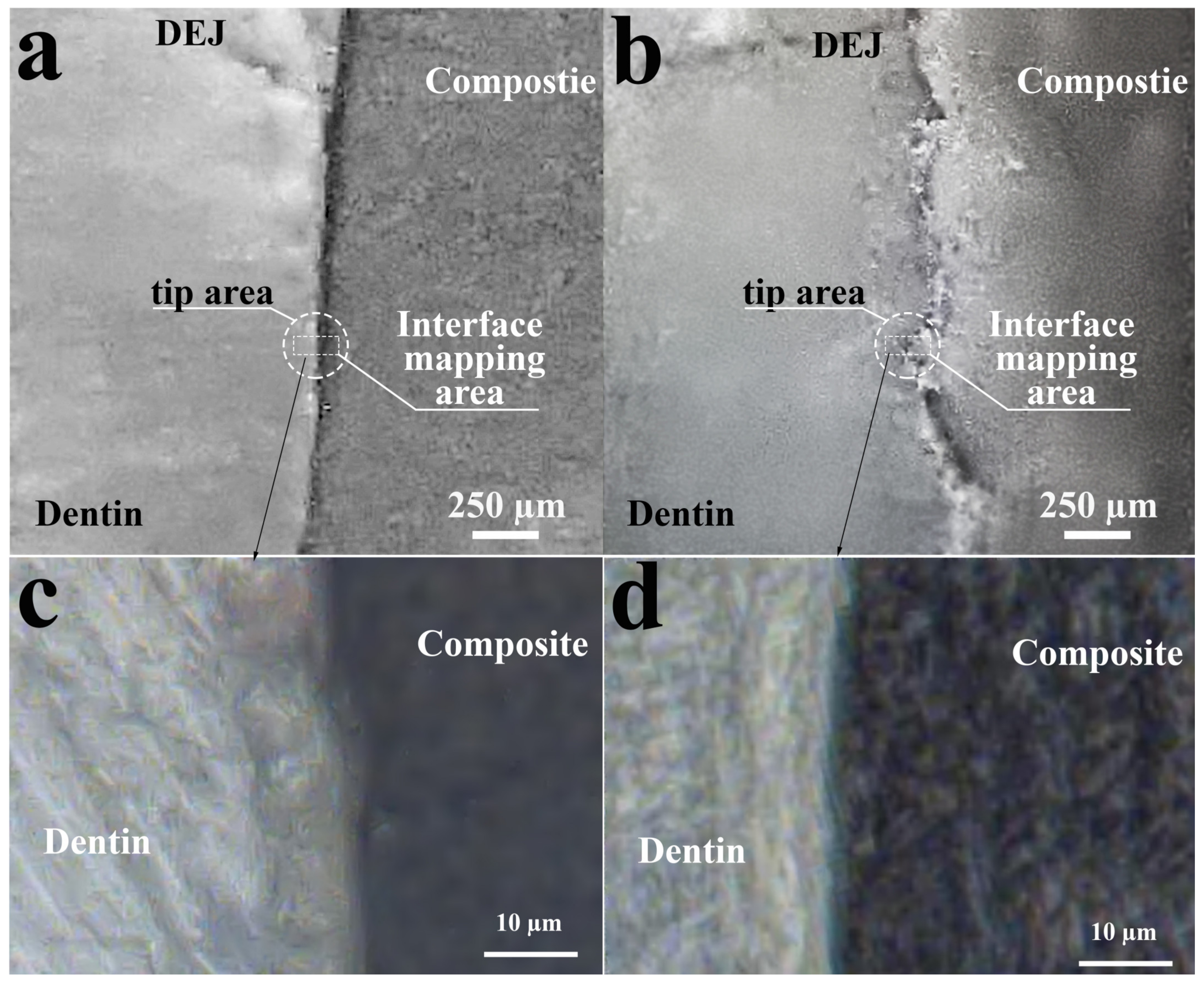

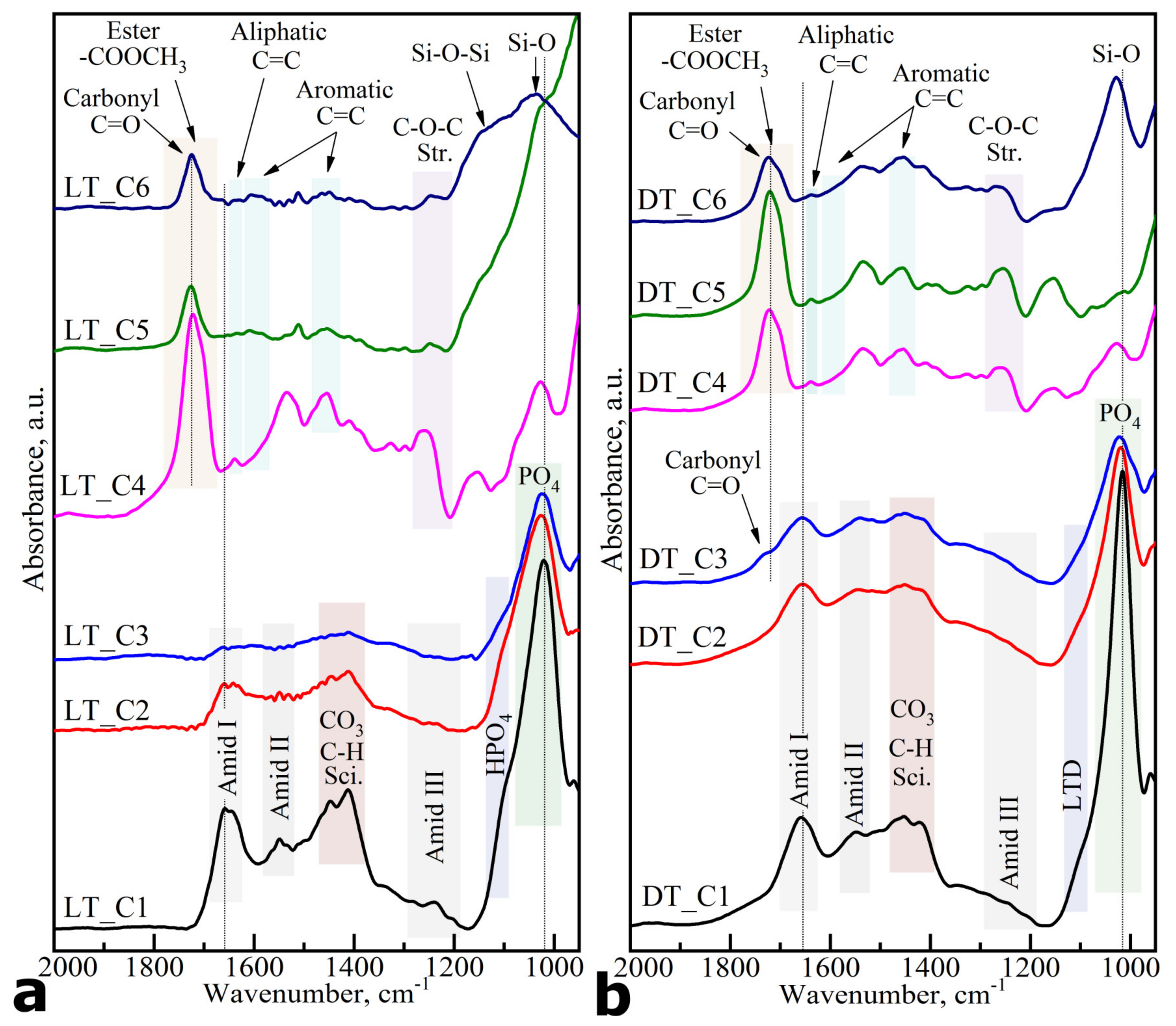

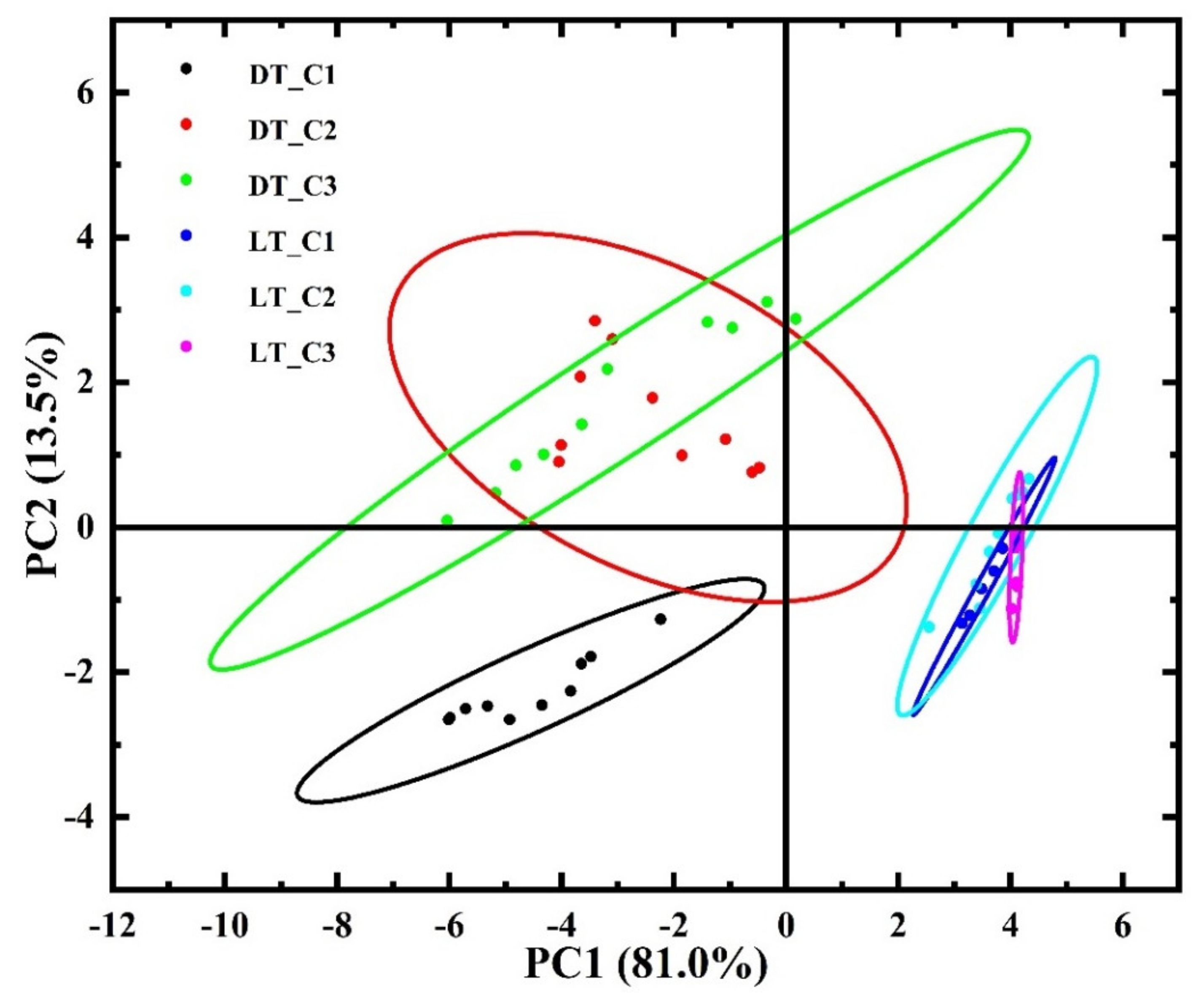

| Substance/ Material | Spectral Area, cm−1 | Functional (Molecular) Group | References |
|---|---|---|---|
| Dentin | 1687–1662 | LTD, –CO–NH2- | [47,48,49,50] |
| 1644–1658 | Amide I C=O stretching | [43,51,52,53] | |
| 1636 | LTD | [47,48,49,50] | |
| 1605 | LTD | [47,48,49,50] | |
| 1549–1563 | Amide II C–N stretching and N–H deformation modes, CNH | [43,53] | |
| 1445–1456 | Collagen C–H Scissoring, C-H bending | [43,52,53,54,55] | |
| 1450 cm−1 1415 and | ν3 CO32− substituted in B-type PO3− and A-type OH and C–H Scissoring | [43,50,53,54,55] | |
| 1330 | LTD | [47,48,49,50] | |
| 1320 | C-H bending | [56] | |
| 1246 1234 | amide III, N–H deformation C–N stretching | [43,52] | |
| 1165 | LTD | [47,48,49,50] | |
| 1127–1145 | HPO4 2− | [57,58] | |
| 1122 | LTD | ||
| 1100–1110 | υ3 PO4 | [57] | |
| 1090 | Phosphate in dentin | [43] | |
| 1061–1075 | LTD Apatite υ3 PO4, after laser ablation, pure HAP | [47,48,49,50,58] | |
| 1030–1047 | υ3 PO4 apatite in dentin | [43,52,53] | |
| 1027–1034 | υ3 PO4 Apatite PO4, after laser ablation, pure HAP | [52,55,58] | |
| 1010–1020 | υ3 PO4 in poorly crystalline apatites | [58] | |
| 964 | υ1 PO4 | [58] | |
| Conditioner + Amino acids booster | 1718 | C=O carbonyl group of AA | [59] |
| 1635 | v as, COO- vas(CN3H+5) | [60] | |
| 1460–1445 | CH2/CH3 | [60] | |
| 1362 | N-Cα-Hα, Cβ-Cα-Hα | [60] | |
| 1226 | NH3+ | [60,61] | |
| 1185 | ρ, NH3+ | [60] | |
| Bioprimer | 1703 | C=O stretching | [62] |
| 1635 | vas(CN3H+5) protein amino acid, arginine | [63,64] | |
| 1454 | –CH2 | [65] | |
| 1320–1298 | [v(C-O)] stretch doublet δ(CH) | [62] | |
| 1164 | C–O–C, δ(CH) | [62] | |
| 1078 | ν(CO) | [65] | |
| 1026 | ν(CC) | [65] | |
| BisGMA Adhesive | 1721 | C=O carbonyl | [19,66] |
| 1636 | C=C Aliphatic C=C methacrylate groups | [66] | |
| 1609 | phenyl C=C | [66,67] | |
| 1513 | Aromatic C=C | [66] | |
| 1452 | CH2 CH3 | [66,67] | |
| 1402 | =CH2 deformation | [19] | |
| 1320–1290 | [v(C-O)] stretch dublet | [19] | |
| 1242 | Aromatic C–O | [19] | |
| DyractXP commercial material | 1700–1740 | Ester groups –COOCH3 attached to the methacrylate | [19,68]. |
| 1636 | C=C stretching vibration of the methacrylate group | [68,69] | |
| 1608 | C=C in an aromatic ring | [68,69] | |
| 1511 | N–H deformation stretching of urethane dimethacrylate (UDMA) | [68,69] | |
| 1454 | C–H in constituent monomers | [68] | |
| 1297 | symmetric stretching of -O in monomers, Si–O stretching | [68] | |
| 1233 | C–O–C stretching | [19,69] | |
| 1150 | C–O–C stretching | [19,69] | |
| 1040–1060 | Si–O from SiO2 -containing fillers | [68,70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seredin, P.; Goloshchapov, D.; Buylov, N.; Nesterov, D.; Kashkarov, V.; Ippolitov, Y.; Ippolitov, I.; Kuyumchyan, S.; Vongsvivut, J. A Study of the Effects of Medical Dental Laser and Diamond Drill on Dentin Tissue during Dental Restoration Based on Spectral Imaging and Multivariate Analysis of Synchrotron FTIR Microspectroscopy Data. Photonics 2023, 10, 881. https://doi.org/10.3390/photonics10080881
Seredin P, Goloshchapov D, Buylov N, Nesterov D, Kashkarov V, Ippolitov Y, Ippolitov I, Kuyumchyan S, Vongsvivut J. A Study of the Effects of Medical Dental Laser and Diamond Drill on Dentin Tissue during Dental Restoration Based on Spectral Imaging and Multivariate Analysis of Synchrotron FTIR Microspectroscopy Data. Photonics. 2023; 10(8):881. https://doi.org/10.3390/photonics10080881
Chicago/Turabian StyleSeredin, Pavel, Dmitry Goloshchapov, Nikita Buylov, Dmitry Nesterov, Vladimir Kashkarov, Yuri Ippolitov, Ivan Ippolitov, Sergey Kuyumchyan, and Jitraporn Vongsvivut. 2023. "A Study of the Effects of Medical Dental Laser and Diamond Drill on Dentin Tissue during Dental Restoration Based on Spectral Imaging and Multivariate Analysis of Synchrotron FTIR Microspectroscopy Data" Photonics 10, no. 8: 881. https://doi.org/10.3390/photonics10080881
APA StyleSeredin, P., Goloshchapov, D., Buylov, N., Nesterov, D., Kashkarov, V., Ippolitov, Y., Ippolitov, I., Kuyumchyan, S., & Vongsvivut, J. (2023). A Study of the Effects of Medical Dental Laser and Diamond Drill on Dentin Tissue during Dental Restoration Based on Spectral Imaging and Multivariate Analysis of Synchrotron FTIR Microspectroscopy Data. Photonics, 10(8), 881. https://doi.org/10.3390/photonics10080881








