Tailoring the Thermal Diffusivity of Polyvinylidene Fluoride via Carbon Source Integration: A Photothermal Beam Deflection Study
Abstract
:1. Introduction
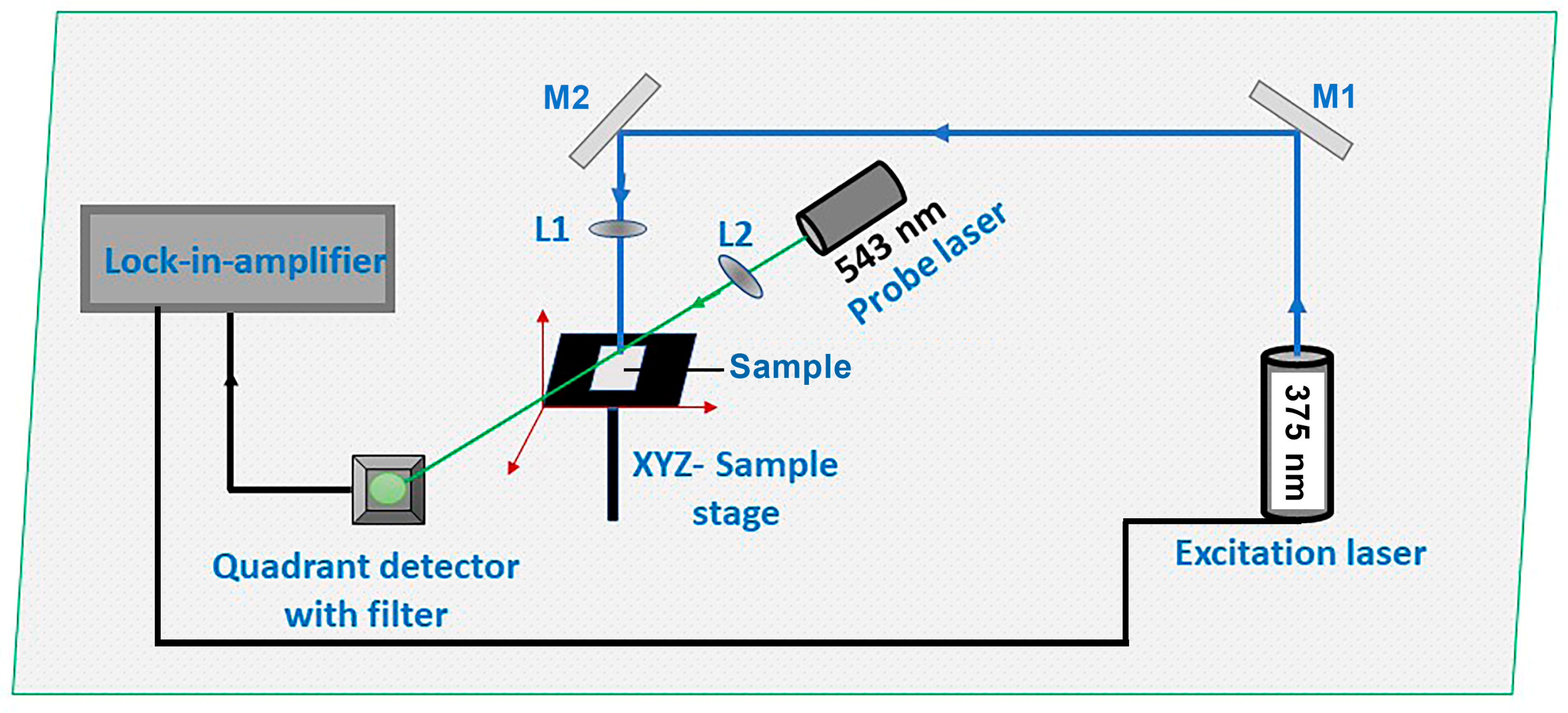
2. Materials and Methods
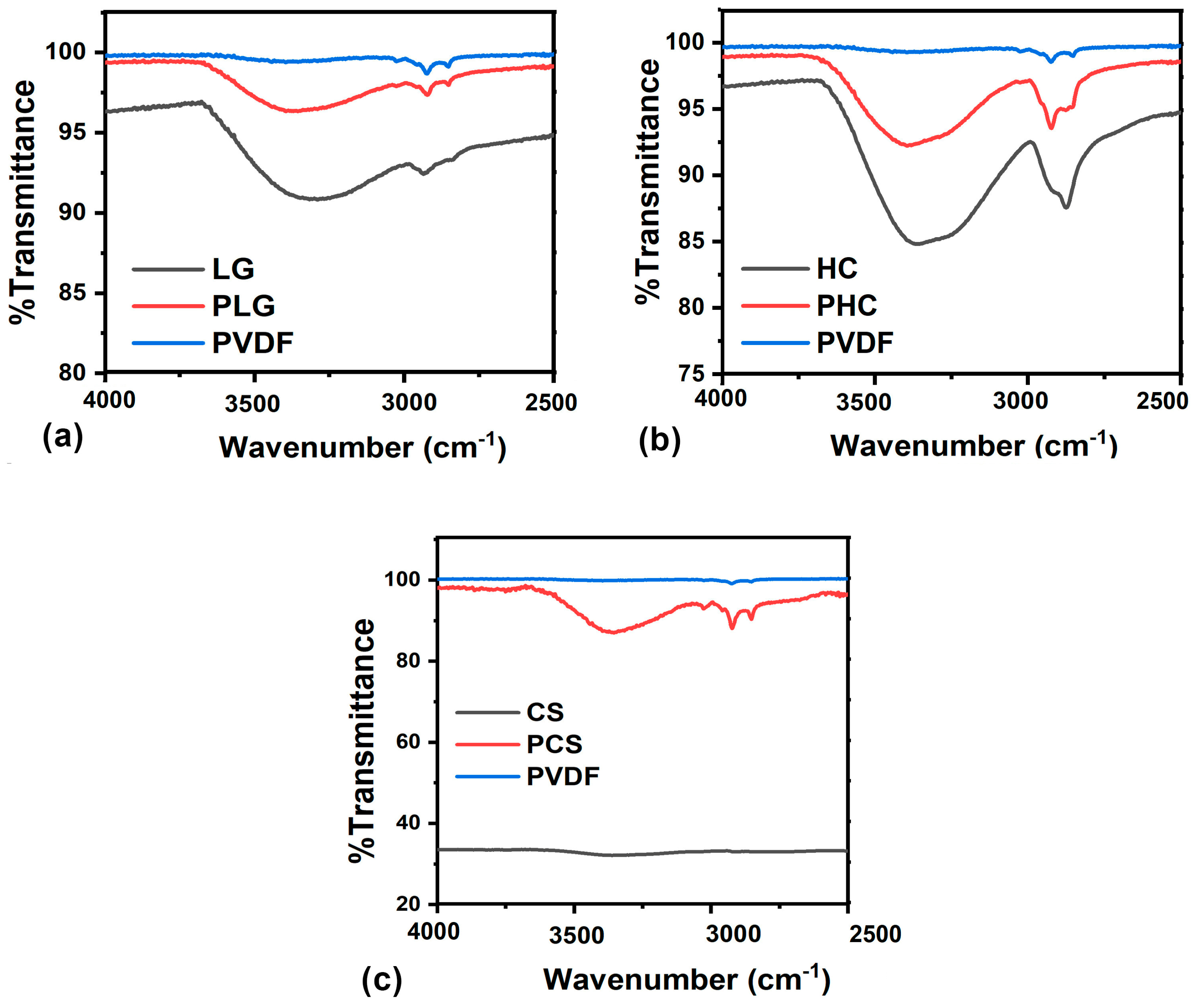
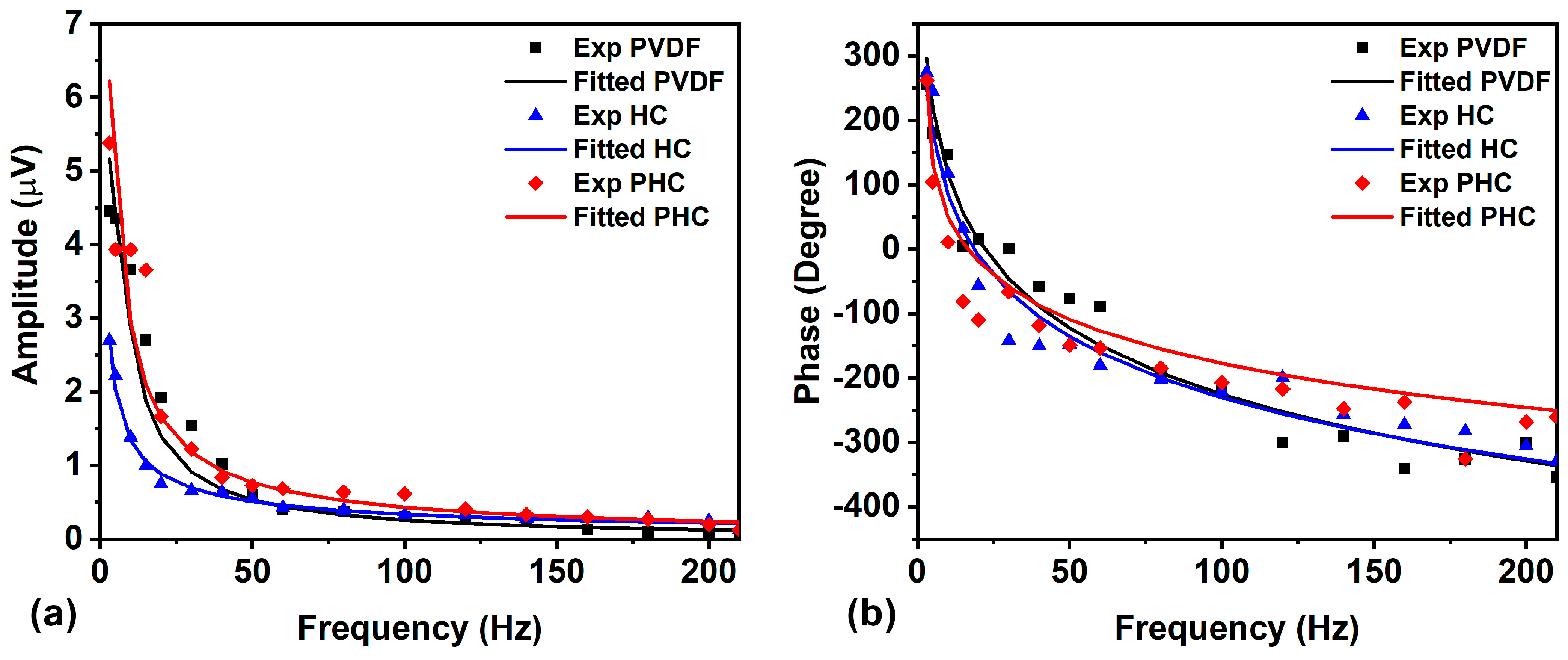
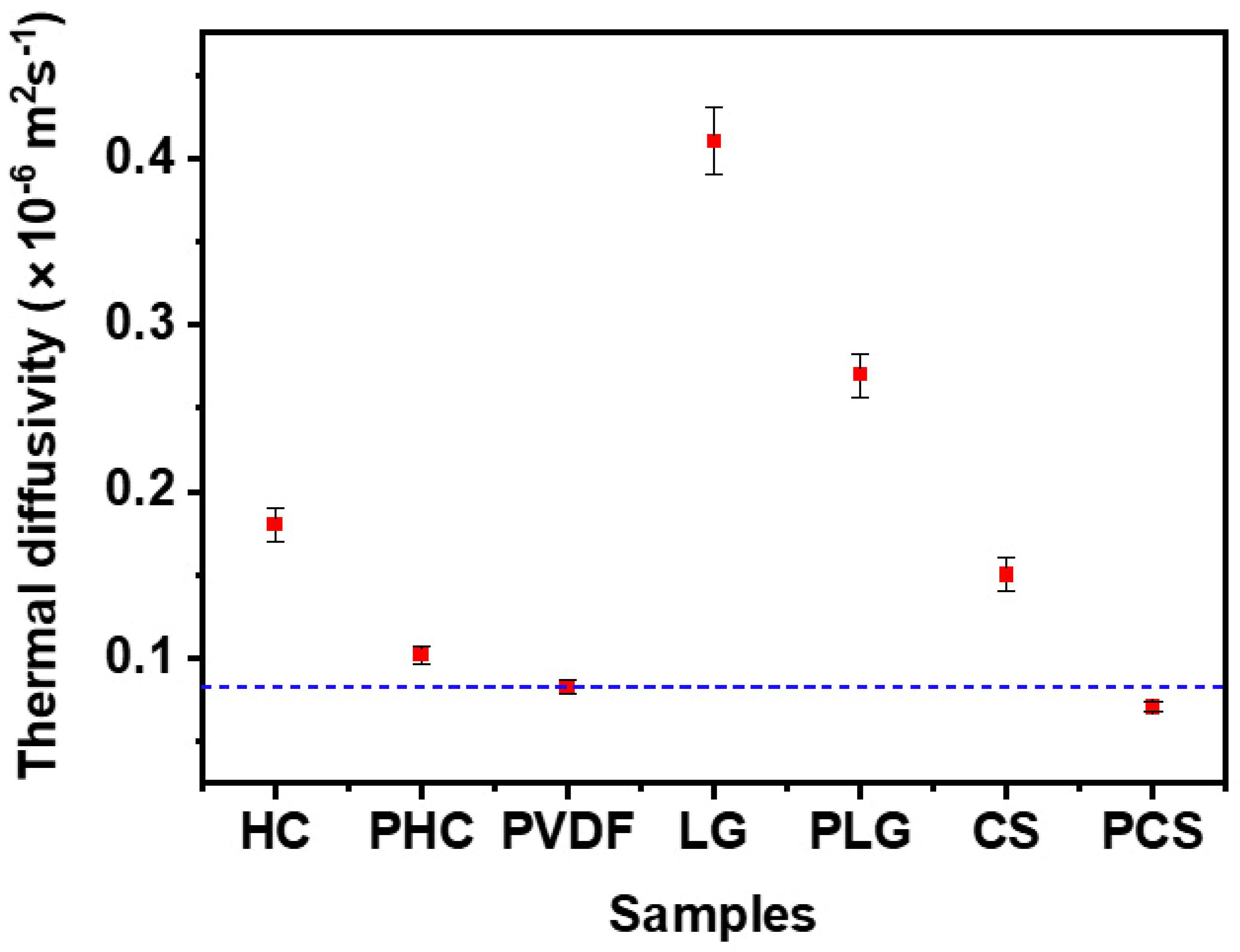
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saxena, P.; Shukla, P. A Comprehensive Review on Fundamental Properties and Applications of Poly(Vinylidene Fluoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Dallaev, R.; Pisarenko, T.; Sobola, D.; Orudzhev, F.; Ramazanov, S.; Trčka, T. Brief Review of PVDF Properties and Applications Potential. Polymers 2022, 14, 4793. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lin, Y.; Ma, W.; Wang, X. A Review on Microporous Polyvinylidene Fluoride Membranes Fabricated via Thermally Induced Phase Separation for MF/UF Application. J. Memb. Sci. 2021, 639, 119759. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, Z. PVDF-based Dielectric Polymers and Their Applications in Electronic Materials. IET Nanodielectrics 2018, 1, 17–31. [Google Scholar] [CrossRef]
- Lavanya Rathi, P.; Ponraj, B.; Deepa, S. Structural, Magnetic and Electrical Properties of Electroactive-Superparamagnetic PVDF-Sn0.2Fe2.8O4 Nanocomposite Films. Ceram. Int. 2021, 47, 9727–9735. [Google Scholar] [CrossRef]
- Vodišek, N.; Šuligoj, A.; Korte, D.; Štangar, U.L. Transparent Photocatalytic Thin Films on Flexible Polymer Substrates. Materials 2018, 11, 1945. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, C.Y.; dos Santos, W.N.; Gregorio, R. Determination of Thermal Properties of Pyroelectric Polymers, Copolymers and Blends by the Laser Flash Technique. Polym. Test. 2007, 26, 788–792. [Google Scholar] [CrossRef]
- Tsutsumi, N.; Terao, M.; Kiyotsukuri, T. Thermal Diffusivity and Heat Capacity of Poly(Vinylidene Fluoride)/Poly(Methyl Methacrylate) Blends by Flash Radiometry. Polymer 1993, 34, 90–94. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, F.; Yuan, M.; Li, B.; Peng, J.; Lv, Y.; Cai, H.; Liu, X.; Chen, Q.; Dang, Z.-M. Improved Dielectric Properties and Thermal Conductivity of PVDF Composites Filled with Core–Shell Structured Cu@CuO Particles. J. Mater. Sci. Mater. Electron. 2019, 30, 18350–18361. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, X.; Yang, J.; Huang, T.; Zhang, N.; Wang, Y.; Zhou, Z. High Thermal Conductivity of Poly(Vinylidene Fluoride)/Carbon Nanotubes Nanocomposites Achieved by Adding Polyvinylpyrrolidone. Compos. Sci. Technol. 2015, 106, 1–8. [Google Scholar] [CrossRef]
- Korte, D.; Franko, M. Application of Complex Geometrical Optics to Determination of Thermal, Transport, and Optical Parameters of Thin Films by the Photothermal Beam Deflection Technique. J. Opt. Soc. Am. A 2015, 32, 61. [Google Scholar] [CrossRef]
- Sell, J. Photothermal Investigations of Solids and Fluids, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0323154220. [Google Scholar]
- Liu, J.; Han, M.; Wang, R.; Xu, S.; Wang, X. Photothermal Phenomenon: Extended Ideas for Thermophysical Properties Characterization. J. Appl. Phys. 2022, 131, 065107. [Google Scholar] [CrossRef]
- Usoltseva, L.O.; Korobov, M.V.; Proskurnin, M.A. Photothermal Spectroscopy: A Promising Tool for Nanofluids. J. Appl. Phys. 2020, 128, 190901. [Google Scholar] [CrossRef]
- Soumya, S.; Raj, V.; Swapna, M.S.; Sankararaman, S. Thermal Diffusivity Downscaling of Molybdenum Oxide Thin Film through Annealing Temperature-Induced Nano-Lamelle Formation: A Photothermal Beam Deflection Study. Eur. Phys. J. Plus 2021, 136, 187. [Google Scholar] [CrossRef]
- Warrier, A.R.; Vijayakumar, K.P.; Sudha Kartha, C.; Manoharan, N.; Venkatraman, B. Photothermal Beam Deflection Technique for Nondestructive Evaluation of Thin Film Photovoltaic Cells. In Proceedings of the 2015 Asia International Conference on Quantitative InfraRed Thermography, Mahabalipuram, India, 6–10 July 2015; QIRT Council: Mahabalipuram, India, 2015. [Google Scholar]
- Leahu, G.; Petronijevic, E.; Li Voti, R.; Belardini, A.; Cesca, T.; Mattei, G.; Sibilia, C. Diffracted Beams from Metasurfaces: High Chiral Detectivity by Photothermal Deflection Technique. Adv. Opt. Mater. 2021, 9, 2100670. [Google Scholar] [CrossRef]
- Swapna, M.S.; Saritha Devi, H.V.; Sankararaman, S. Camphor Soot: A Tunable Light Emitter. Appl. Phys. A 2018, 124, 50. [Google Scholar] [CrossRef]
- Swapna, M.N.S.; Korte, D.; Sankararaman, S.I. Unveiling the Role of the Beam Shape in Photothermal Beam Deflection Measurements: A 1D and 2D Complex Geometrical Optics Model Approach. Photonics 2022, 9, 991. [Google Scholar] [CrossRef]
- Soumya, S.; Arun Kumar, R.; Raj, V.; Swapna, M.S.; Sankararaman, S. Thermal Diffusivity of Molybdenum Oxide Nanowire Film: A Photothermal Beam Deflection Study. Opt. Laser Technol. 2021, 139, 106993. [Google Scholar] [CrossRef]
- Cabrera, H.; Korte, D.; Budasheva, H.; Abbasgholi, N.; Asbaghi, B.; Bellucci, S. Through-Plane and In-Plane Thermal Diffusivity Determination of Graphene Nanoplatelets by Photothermal Beam Deflection Spectrometry. Materials 2021, 14, 7273. [Google Scholar] [CrossRef]
- Devi, P.I.; Ramachandran, K. Dielectric Studies on Hybridised PVDF–ZnO Nanocomposites. J. Exp. Nanosci. 2011, 6, 281–293. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker—A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Siesler, H.W. Fourier Transform Infrared (Ftir) Spectroscopy in Polymer Research. J. Mol. Struct. 1980, 59, 15–37. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β and γ Phases in Poly(Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Demina, T.S.; Birdibekova, A.V.; Svidchenko, E.A.; Ivanov, P.L.; Kuryanova, A.S.; Kurkin, T.S.; Khaibullin, Z.I.; Goncharuk, G.P.; Zharikova, T.M.; Bhuniya, S.; et al. Solid-State Synthesis of Water-Soluble Chitosan-g-Hydroxyethyl Cellulose Copolymers. Polymers 2020, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- Lanceros-Méndez, S.; Mano, J.F.; Costa, A.M.; Schmidt, V.H. FTIR and DSC Studies of Mechanically Deformed β-PVDF Films. J. Macromol. Sci. Part B 2001, 40, 517–527. [Google Scholar] [CrossRef]
- Alharbi, N.D.; Guirguis, O.W. Macrostructure and Optical Studies of Hydroxypropyl Cellulose in Pure and Nano-Composites Forms. Results Phys. 2019, 15, 102637. [Google Scholar] [CrossRef]
- Jacob, M.M.; Arof, A. FTIR Studies of DMF Plasticized Polyvinyledene Fluoride Based Polymer Electrolytes. Electrochim. Acta 2000, 45, 1701–1706. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, K.A.R.; Rangel, E.Q.; Sant’Anna, A.R.; Louzada, D.R.; Barbosa, C.R.H.; D’Almeida, J.R.M. Evaluation of the Electromechanical Behavior of Polyvinylidene Fluoride Used as a Component of Risers in the Offshore Oil Industry. Oil Gas Sci. Technol.—Rev. d’IFP Energies Nouv. 2018, 73, 48. [Google Scholar] [CrossRef]
- Kaspar, P.; Sobola, D.; Částková, K.; Knápek, A.; Burda, D.; Orudzhev, F.; Dallaev, R.; Tofel, P.; Trčka, T.; Grmela, L.; et al. Characterisation of Polyvinylidene Fluoride (PVDF) Electrospun Fibers Doped by Carbon Flakes. Polymers 2020, 12, 2766. [Google Scholar] [CrossRef]
- Swapna, M.S.; Sankararaman, S. Thermal Induced Order Fluctuations in Carbon Nanosystem with Carbon Nanotubes. Nano-Struct. Nano-Objects 2019, 19, 100375. [Google Scholar] [CrossRef]
- Bai, H.; Wang, X.; Zhou, Y.; Zhang, L. Preparation and Characterization of Poly(Vinylidene Fluoride) Composite Membranes Blended with Nano-Crystalline Cellulose. Prog. Nat. Sci. Mater. Int. 2012, 22, 250–257. [Google Scholar] [CrossRef]
- Chai, M.N.; Isa, M.I.N. The Oleic Acid Composition Effect on the Carboxymethyl Cellulose Based Biopolymer Electrolyte. J. Cryst. Process Technol. 2013, 3, 27263. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Hulteberg, C.P. Physicochemical Characterisation of Technical Lignins for Their Potential Valorisation. Waste Biomass Valorization 2017, 8, 859–869. [Google Scholar] [CrossRef]
- Alsubaie, A.S.A. Characterization and Optical Studies of Hydroxyethyl Cellulose-Copper Oxide Nanocomposites. J. Spectrosc. 2022, 2022, 8422803. [Google Scholar] [CrossRef]
- Touloukian, Y.S.; Powell, R.W.; Ho, C.Y.; Klemens, P.G. Thermophysical Properties of Matter-the TPRC Data Series. Volume 1. Thermal Conductivity-Metallic Elements and Alloys. (Reannouncement). Data Book; Thermophysical Properties Research Center, Purdue University: Lafayette, IN, USA, 1970. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swapna, M.N.S.; Korte, D.; Sankararaman, S.I. Tailoring the Thermal Diffusivity of Polyvinylidene Fluoride via Carbon Source Integration: A Photothermal Beam Deflection Study. Photonics 2023, 10, 942. https://doi.org/10.3390/photonics10080942
Swapna MNS, Korte D, Sankararaman SI. Tailoring the Thermal Diffusivity of Polyvinylidene Fluoride via Carbon Source Integration: A Photothermal Beam Deflection Study. Photonics. 2023; 10(8):942. https://doi.org/10.3390/photonics10080942
Chicago/Turabian StyleSwapna, Mohanachandran Nair Sindhu, Dorota Korte, and Sankaranarayana Iyer Sankararaman. 2023. "Tailoring the Thermal Diffusivity of Polyvinylidene Fluoride via Carbon Source Integration: A Photothermal Beam Deflection Study" Photonics 10, no. 8: 942. https://doi.org/10.3390/photonics10080942
APA StyleSwapna, M. N. S., Korte, D., & Sankararaman, S. I. (2023). Tailoring the Thermal Diffusivity of Polyvinylidene Fluoride via Carbon Source Integration: A Photothermal Beam Deflection Study. Photonics, 10(8), 942. https://doi.org/10.3390/photonics10080942






