Lasers in Medicine: The Changing Role of Therapeutic Laser-Induced Retinal Damage—From de rigeuer to Nevermore
Abstract
1. Introduction
2. Light for Cautery
3. Laser-Induced Retinal Damage (LIRD)
4. Laser Retinal Photocoagulation (RPC)
5. Traditional Theories for the Therapeutic Mechanism of LIRD
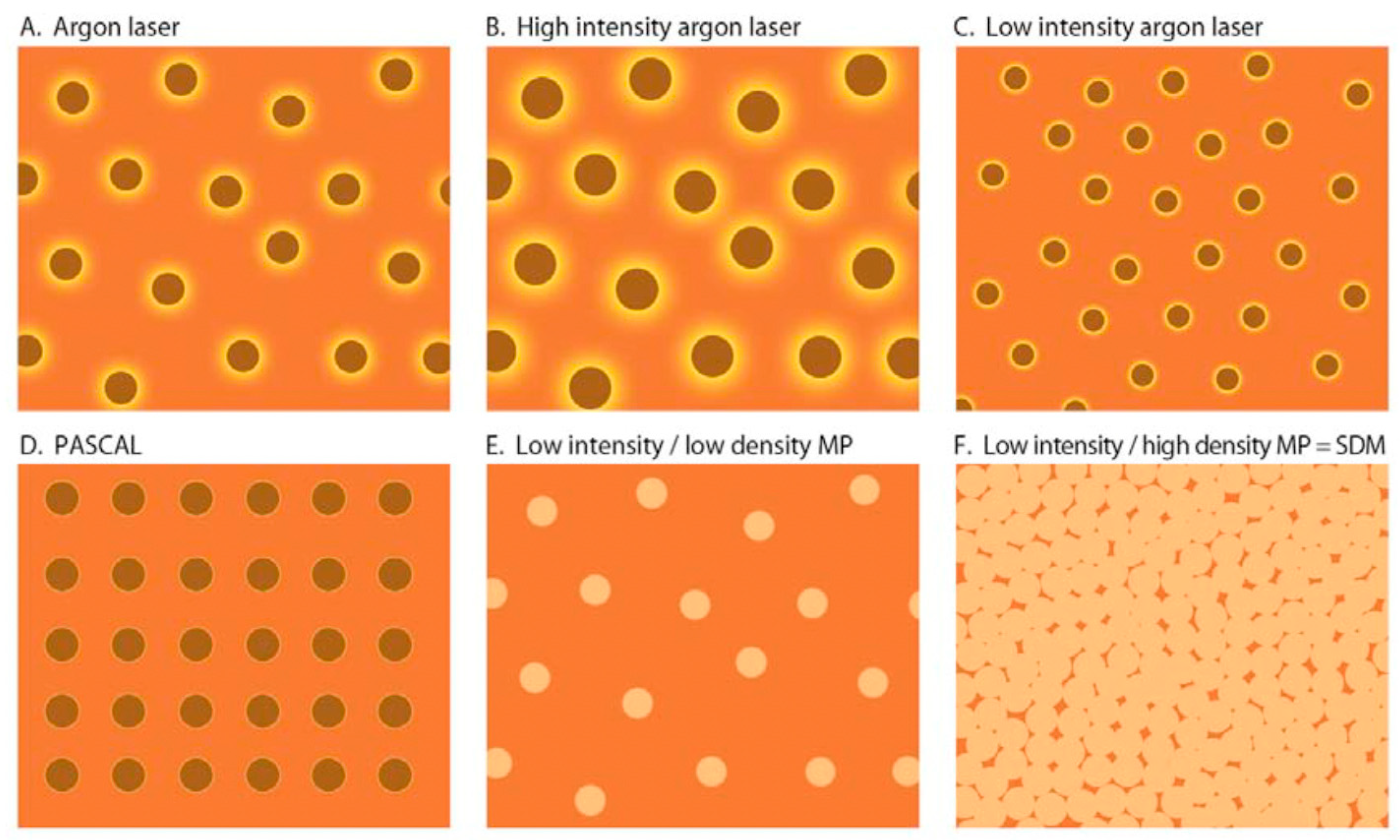
6. Thermal Effects on the Retinal Pigment Epithelium (RPE): The Common Denominator of All Retinal Laser Treatment
7. Current Laser Modes and Platforms for Retinal Laser Treatment and LIRD
7.1. Ultra-Short Pulse Lasers (USPLs)
7.2. Short-Pulse Lasers
7.3. Conventional Retinal Photocoagulation
7.4. Microsecond Pulsed Lasers (MPL)
8. SDM and Modern Retinal Laser Therapy
9. The Clinical Implications of LIRD
10. The Ethical Implications of LIRD
11. Conclusions: The Impact of Elimination of LIRD on Clinical Disease Management
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, V.K.; Saini, C.; Oberoi, M.; Kalra, G.; Nasir, M.I. Semmelweis Reflex: An Age-Old Prejudice. World Neurosurg. 2020, 136, e119–e125. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Feuer, W.J. Warning: Do Not Treat Intermediate AMD with Laser Therapy. Ophthalmology 2019, 126, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.-S. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology 2020, 127, P66–P145, Erratum in Ophthalmology 2020, 127, 1279. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K.; Musch, D.C.; Mainster, M.A. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br. J. Ophthalmol. 2005, 89, 74–80. [Google Scholar] [CrossRef]
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Margolis, B.W.L. Functionally guided retinal protective therapy as prophylaxis for age-related and inherited retinal degenerations. A pilot study. Investig. Ophthalmol. Vis. Sci. 2016, 57, 265–275. [Google Scholar] [CrossRef]
- Sherwood, A.N.; Nikolic, M.; Humphrey, J.W.; Oleson, J.P. Greek and Roman Technology: A Sourcebook of Translated Greek and Roman Texts; Routledge: London, UK, 2019; p. 25. [Google Scholar]
- Grzybowski, A.; Luttrull, J.K.; Kozak, I. (Eds.) History of Lasers in Ophthalmology in Retinal Lasers in Ophthalmology; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Meyer-Schwickerath, G. History and development of photocoagulation. Am. J. Ophthalmol. 1967, 63, 1812–1814. [Google Scholar] [CrossRef]
- Morón, J. Obliteración de los desgarros retinianos por quemadura con luz. Arch. Soc. Oftalmol. Hisp. Am. 1950, 10, 566–578. [Google Scholar]
- Schawlow, A.L.; Townes, C.H. Infrared and optical masers. Phys. Rev. 1958, 112, 1940–1949. [Google Scholar] [CrossRef]
- Campbell, C.J.; Rittler, M.C.; Keoester, C.J. The optical maser as a retinal coagulator: An evaluation. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1963, 67, 58–67. [Google Scholar]
- Maiman, T. Stimulated optical radiation in ruby. Nature 1960, 187, 493–494. [Google Scholar] [CrossRef]
- L’Esperance, F.A., Jr. The treatment of ophthalmic vascular disease by argon laser photocoagulation. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1969, 73, 1077–1096. [Google Scholar]
- Peabody, R.R.; Zweng, H.C.; Little, H.L. Treatment of retinal vascular disease with argon laser slit lamp photocoagulation. Trans. Pac. Coast. Otoophthalmol. Soc. Annu. Meet. 1970, 51, 307–326. [Google Scholar] [PubMed]
- Zimmer-Galler, I.E.; Bressler, N.M.; Bressler, S.B. Treatment of choroidal neovascularization: Updated information from recent macular photocoagulation study group reports. Int. Ophthalmol. Clin. 1995, 35, 37–57. [Google Scholar] [CrossRef]
- Kozak, I.; Luttrull, J.K. Modern Retinal Laser Therapy. Saudi. J. Ophthalmol. 2015, 29, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Little, H.L.; Zweng, H.C.; Jack, R.L.; Vassiliadis, A. Techniques of argon laser photocoagulation of diabetic disk new vessels. Am. J. Ophthalmol. 1976, 82, 675–683. [Google Scholar] [CrossRef]
- Alasil, T.; Waheed, N.K. Pan retinal photocoagulation for proliferative diabetic retinopathy: Pattern scan laser versus argon laser. Curr. Opin. Ophthalmol. 2014, 25, 164–170. [Google Scholar] [CrossRef]
- Battaglia Parodi, M.; Arrigo, A.; Iacono, P.; Falcomatà, B.; Bandello, F. Central Serous Chorioretinopathy: Treatment with Laser. Pharmaceuticals 2020, 13, 359. [Google Scholar] [CrossRef]
- Akduman, L.; Olk, R.J. Subthreshold (invisible) modified grid diode laser photocoagulation in diffuse diabetic macular edema (DDME). Ophthalmic Surg. Lasers 1999, 30, 706–714. [Google Scholar] [CrossRef]
- Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch. Ophthalmol. 1985, 103, 1796–1806. [CrossRef]
- Mainster, M.A. Decreasing retinal photocoagulation damage: Principles and techniques. Semin. Ophthalmol. 1999, 14, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Friberg, T.R. Subthreshold (invisible) modified grid diode laser photocoagulation and diffuse diabetic macular edema (DDME). Ophthalmic Surg. Lasers 1999, 30, 705. [Google Scholar] [CrossRef] [PubMed]
- Rodanant, N.; Friberg, T.R.; Cheng, L.; Aurora, A.; Bartsch, D.-U.; Toyoguchi, M.; Corbin, P.S.; El-Bradey, M.H.; Freeman, W.R. Predictors of drusen reduction after subthreshold infrared (810 nm) diode laser macular grid photocoagulation for nonexudative age-related macular degeneration. Am. J. Ophthalmol. 2002, 134, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Guymer, R.H.; Wu, Z.; Hodgson, L.A.B.; Caruso, E.; Brassington, K.H.; Tindill, N.; Aung, K.Z.; McGuinness, M.B.; Fletcher, E.L.; Chen, F.K.; et al. Subthreshold Nanosecond Laser Intervention in Age-Related Macular Degeneration: The LEAD Randomized Controlled Clinical Trial. Ophthalmology 2019, 126, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Muqit, M.M.; Marcellino, G.R.; Henson, D.B.; Young, L.B.; Turner, G.S.; Stanga, P.E. Pascal panretinal laser ablation and regression analysis in proliferative diabetic retinopathy: Manchester Pascal Study Report 4. Eye 2011, 25, 1447–1456. [Google Scholar] [CrossRef]
- Stanga, P.E.; Reck, A.C.; Hamilton, A.M. Micropulse laser in the treatment of diabetic macular edema. Semin. Ophthalmol. 1999, 14, 210–213. [Google Scholar] [CrossRef]
- Wolbarsht, M.L.; Landers, M.B., 3rd. The rationale of photocoagulation therapy for proliferative diabetic retinopathy: A review and a model. Ophthalmic Surg. 1980, 11, 235–245. [Google Scholar]
- Stefansson, E.; Landers, M.B., 3rd; Wolbarsht, M.L. Oxygenation and vasodilatation in relation to diabetic and other proliferative retinopathies. Ophthalmic Surg. 1983, 14, 209–226. [Google Scholar]
- Mainster, M.A.; Reichel, E. Transpupillary thermotherapy for age-related macular degeneration: Long-pulse photocoagulation, apoptosis, and heat shock proteins. Ophthalmic Surg. Lasers 2000, 31, 359–373. [Google Scholar]
- Luttrull, J.K.; Chang, D.B.; Margolis, B.W.; Dorin, G.; Luttrull, D.K. Laser Resensitization of Medically Unresponsive Neovascular Age-Related Macular Degeneration: Efficacy and Implications. Retina 2015, 35, 1184–1194. [Google Scholar] [CrossRef]
- Keunen, J.E.E.; Battaglia-Parodi, M.; Vujosevic, S.; Luttrull, J.K. International Retinal Laser Society Guidelines for Subthreshold Laser Treatment. Transl. Vis. Sci. Technol. 2020, 9, 15. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Sinclair, S.D. Safety of transfoveal subthreshold diode micropulse laser for intra-foveal diabetic macular edema in eyes with good visual acuity. Retina 2014, 34, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.B.; Luttrull, J.K. Comparison of subthreshold 577nm and 810nm micropulse laser effects on heat-shock protein activation kinetics: Implications for treatment efficacy and safety. Transl. Vis. Sci. Tecnol. 2020, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Flaxel, C.; Bradle, J.; Acott, T.; Samples, J.R. Retinal pigment epithelium produces matrix metalloproteinases after laser treatment. Retina 2007, 27, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Dorin, G. Subthreshold and micropulse diode laser photocoagulation. Semin. Ophthalmol. 2003, 18, 147–153. [Google Scholar] [CrossRef]
- Chhablani, J.; Roh, Y.J.; Jobling, A.I.; Fletcher, E.L.; Lek, J.J.; Bansal, P.; Guymer, R.; Luttrull, J.K. Restorative retinal laser therapy: Present state and future directions. Surv. Ophthalmol. 2018, 63, 307–328. [Google Scholar] [CrossRef]
- Wood, J.P.; Shibeeb, O.; Plunkett, M.; Casson, R.J.; Chidlow, G. Retinal damage profiles and neuronal effects of laser treatment: Comparison of a conventional photocoagulator and a novel 3-nanosecond pulse laser. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2305–2318. [Google Scholar] [CrossRef]
- Chidlow, G.; Shibeeb, O.; Plunkett, M.; Casson, R.J.; Wood, J.P. Glial cell and inflammatory responses to retinal laser treatment: Comparison of a conventional photocoagulator and a novel, 3-nanosecond pulse laser. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2319–2332. [Google Scholar] [CrossRef]
- Treumer, F.; Klettner, A.; Baltz, J.; Hussain, A.; Miura, Y.; Brinkmann, R.; Roider, J.; Hillenkamp, J. Vectorial release of matrix metalloproteinases (MMPs) from porcine RPE-choroid explants following selective retina therapy (SRT): Towards slowing the macular ageing process. Exp. Eye Res. 2012, 97, 63–72. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Dorin, G. Subthreshold diode micropulse photocoagulation as invisible retinal phototherapy for diabetic macular edema. A review. Curr. Diabetes Rev. 2012, 8, 274–284. [Google Scholar] [CrossRef]
- Chehade, L.; Chidlow, G.; Wood, J.; Casson, R.J. Short-pulse duration retinal lasers: A review. Clin. Exp. Ophthalmol. 2016, 44, 714–721. [Google Scholar] [CrossRef]
- Paulus, Y.M.; Kaur, K.; Egbert, P.R.; Blumenkranz, M.S.; Moshfeghi, D.M. Human histopathology of PASCAL laser burns. Eye 2013, 27, 995–996. [Google Scholar] [CrossRef] [PubMed]
- Kandulla, J.; Elsner, H.; Birngruber, R.; Brinkmann, R. Noninvasive optoacoustic online retinal temperature determination during continuous-wave laser irradiation. J. Biomed. Opt. 2006, 11, 041111. [Google Scholar] [CrossRef] [PubMed]
- Lavinsky, D.; Wang, J.; Huie, P.; Dalal, R.; Lee, S.J.; Lee, D.Y.; Palanker, D. Nondamaging Retinal Laser Therapy: Rationale and Applications to the Macula. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2488–2500, Erratum in Investig. Ophthalmol. Vis. Sci. 2016, 57, 3817. [Google Scholar] [CrossRef] [PubMed]
- Sramek, C.; Mackanos, M.; Spitler, R.; Leung, L.-S.; Nomoto, H.; Contag, C.H.; Palanker, D. Non-damaging retinal phototherapy: Dynamic range of heat shock protein expression. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1780–1787. [Google Scholar] [CrossRef]
- Tso, M.O.; Fine, B.S. Repair and late degeneration of the primate foveola after injury by argon laser. Investig. Ophthalmol. Vis. Sci. 1979, 18, 447–461. [Google Scholar]
- Verhoeff, F.H.; Bell, L.; Walker, C.B. The Pathological Effects of Radiant Energy on the Eye: An Experimental Investigation, with a Systematic Review of the Literature. Proc. Am. Acad. Arts Sci. USA 1916, 51, 627, 629–818. [Google Scholar] [CrossRef]
- Gardner, T.W. Complications of retinal laser therapy and their prevention. Semin. Ophthalmol. 1991, 6, 19–26. [Google Scholar] [CrossRef]
- Morgan, C.M.; Schatz, H. Atrophic creep of the retinal pigment epithelium after focal macular photocoagulation. Ophthalmology 1989, 96, 96–103. [Google Scholar] [CrossRef]
- Maeshima, K.; Utsugi-Sutoh, N.; Otani, T.; Kishi, S. Progressive enlargement of scattered photocoagulation scars in diabetic retinopathy. Retina 2004, 24, 507–511. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Sramek, C.; Palanker, D.; Spink, C.J.; Musch, D.C. Long-term safety, high-resolution imaging, and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina 2012, 32, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K.; Kent, D. Modern retinal laser for neuroprotection in open-angle glaucoma. In New Concepts in Glaucoma Surgery; Samples, J.R., Ahmed, I.I.K., Eds.; Kugler Publications: Amsterdam, The Netherlands, 2019; Volume 1, ISBN 978-1461483472/1461483476. [Google Scholar]
- Luttrull, J.K.; Kent, D. Laser therapy to prevent choroidal neovascularization. In Choroidal Neovascularization; Chhablanni, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Sinclair, S.H.; Elmann, S.; Chang, D.B.; Kent, D. Slowed progression of age-related geographic atrophy following subthreshold laser. Clin. Ophthalmol. 2020, 14, 2983–2993. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K. Low-intensity/high-density subthreshold diode micropulse laser (SDM) for central serous chorioretinopathy. Retina 2016, 36, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K. Improved retinal and visual function following subthreshold diode micropulse laser (SDM) for retinitis pigmentosa. Eye 2018, 32, 1099–1110. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Sinclair, S.H.; Elmann, S.; Glaser, B.M. Low incidence of choroidal neovascularization following subthreshold diode micropulse laser (SDM) for high-risk AMD. PLoS ONE 2018, 13, e0202097. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Gray, G. Real World Data Comparison of Standard Care vs SDM Laser Vision Protection Therapy for Prevention of Neovascular AMD. Clin. Ophthalmol. 2022, 16, 1555–1568. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Spink, C.J. Serial optical coherence tomography of subthreshold diode laser micropulse photocoagulation for diabetic macular edema. Ophthalmic Surg. Lasers Imaging 2006, 37, 370–377. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Spink, C.J.; Musch, D.A. Subthreshold diode micropulse panretinal photocoagulation for proliferative diabetic retinopathy. Eye 2008, 22, 607–612. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Samples, J.R.; Kent, D.; Lum, B.J. Panmacular subthreshold diode micropulse laser (SDM) as neuroprotective therapy in primary open-angle glaucoma. In Glaucoma Research 2018–2020; Samples, J.R., Knepper, P.A., Eds.; Kugler Publications: Amsterdam, The Netherlands; pp. 281–294.
- Luttrull, J.K. Modern Retinal Laser Therapy; Principles and Application; Kugler Publications: Amsterdam, The Netherlands, 2023; ISBN 978-90-6299-298-0. [Google Scholar]
- Caballero, S.; Kent, D.L.; Sengupta, N.; Calzi, S.L.; Shaw, L.; Beli, E.; Moldovan, L.; Dominguez, J.M.; Moorthy, R.S.; Grant, M.B. Bone Marrow-Derived Cell Recruitment to the Neurosensory Retina and Retinal Pigment Epithelial Cell Layer Following Subthreshold Retinal Phototherapy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5164–5176. [Google Scholar] [CrossRef]
- Eells, J.T.; Wong-Riley, M.T.; VerHoeve, J.; Henry, M.; Buchman, E.V.; Kane, M.P.; Gould, L.J.; Das, R.; Jett, M.; Hodgson, B.D.; et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion 2004, 4, 559–567. [Google Scholar] [CrossRef]
- Frizziero, L.; Calciati, A.; Midena, G.; Torresin, T.; Parrozzani, R.; Pilotto, E.; Midena, E. Subthreshold Micropulse Laser Modulates Retinal Neuroinflammatory Biomarkers in Diabetic Macular Edema. J. Clin. Med. 2021, 10, 3134. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Yu, M.; Lu, L.; Jin, C.; Luo, G. Electroretinogram evaluation for the treatment of proliferative diabetic retinopathy by short-pulse pattern scanning laser panretinal photocoagulation. Lasers Med. Sci. 2018, 33, 1095–1102, Erratum in Lasers Med. Sci. 2018, 33, 1103. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.M.; Chao, D.L. Application of subthreshold laser therapy in retinal diseases: A review. Expert. Rev. Ophthalmol. 2018, 13, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L.; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 1138–1148, Erratum in JAMA Ophthalmol. 2019, 137, 467. [Google Scholar] [CrossRef] [PubMed]
- Jhingan, M.; Goud, A.; Peguda, H.K.; Tyagi, M.; Luttrull, J.K.; Chhablani, J. Subthreshold microsecond laser for proliferative diabetic retinopathy: A randomized pilot study. Clin. Ophthalmol. 2018, 12, 141–145. [Google Scholar] [CrossRef]
- Frizziero, L.; Calciati, A.; Torresin, T.; Midena, G.; Parrozzani, R.; Pilotto, E.; Midena, E. Diabetic Macular Edema Treated with 577-nm Subthreshold Micropulse Laser: A Real-Life, Long-Term Study. J. Pers. Med. 2021, 11, 405. [Google Scholar] [CrossRef]
- Lavinsky, D.; Cardillo, J.A.; Melo, L.A., Jr.; Dare, A.; Farah, M.E.; Belfort, R., Jr. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4314–4323. [Google Scholar] [CrossRef]
- Lois, N.; Campbell, C.; Waugh, N.; Azuara-Blanco, A.; Maredza, M.; Mistry, H.; McAuley, D.; Acharya, N.; Aslam, T.M.; Bailey, C.; et al. Diabetic Macular Edema and Diode Subthreshold Micropulse Laser: A Randomized Double-Masked Noninferiority Clinical Trial. Ophthalmology 2023, 130, 14–27. [Google Scholar] [CrossRef]
- Sabal, B.; Teper, S.; Wylęgała, E. Subthreshold Micropulse Laser for Diabetic Macular Edema: A Review. J. Clin. Med. 2022, 12, 274. [Google Scholar] [CrossRef]
- Sober, E. Ockam’s Razor: A User’s Manual; Cambridge University Press: Cambridge, UK, 2015; ISBN 978-1107692534. [Google Scholar]
- Brown, D.M.; Nguyen, Q.D.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Schlottmann, P.G.; Rundle, A.C.; Zhang, J.; Rubio, R.G.; et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: The 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013, 120, 2013–2022. [Google Scholar] [CrossRef]









| Laser Type | LIRD? | LIRD Mechanism | Primary Therapeutic Mechanism | Foveal Treatment? | High Density? | Repeatable? | SAEs? |
|---|---|---|---|---|---|---|---|
| 2RT | + | PD | W | - | - | L | + |
| SRT | + | PA | W | - | - | L | + |
| PASCAL | + | PA | W | - | - | L | + |
| CW RPC | + | PC | R | - | - | L | + |
| SDM | - | - | R | + | + | U | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luttrull, J.K. Lasers in Medicine: The Changing Role of Therapeutic Laser-Induced Retinal Damage—From de rigeuer to Nevermore. Photonics 2023, 10, 999. https://doi.org/10.3390/photonics10090999
Luttrull JK. Lasers in Medicine: The Changing Role of Therapeutic Laser-Induced Retinal Damage—From de rigeuer to Nevermore. Photonics. 2023; 10(9):999. https://doi.org/10.3390/photonics10090999
Chicago/Turabian StyleLuttrull, Jeffrey K. 2023. "Lasers in Medicine: The Changing Role of Therapeutic Laser-Induced Retinal Damage—From de rigeuer to Nevermore" Photonics 10, no. 9: 999. https://doi.org/10.3390/photonics10090999
APA StyleLuttrull, J. K. (2023). Lasers in Medicine: The Changing Role of Therapeutic Laser-Induced Retinal Damage—From de rigeuer to Nevermore. Photonics, 10(9), 999. https://doi.org/10.3390/photonics10090999





