In Vivo Insights: Near-Infrared Photon Sampling of Reflectance Spectra from Cranial and Extracranial Sites in Healthy Individuals and Patients with Essential Tremor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Spectra Collection and Analysis
- ET biceps (n = 800) vs. Normal biceps (n = 800);
- ET triceps (n = 800) vs. Normal triceps (n = 800);
- ET brainstem (n = 320) vs. Normal brainstem (n = 320);
- ET cortical (n = 320) vs. Normal cortical (n = 320).
- ET biceps (n = 800) vs. Normal biceps (n = 800);
- ET triceps (n = 800) vs. Normal triceps (n = 800);
- ET brainstem (n = 320) vs. Normal brainstem (n = 320);
- ET cortical (n = 320) vs. Normal cortical (n = 320).
- ET biceps (n = 800) vs. Normal biceps (n = 800) for age;
- ET biceps (n = 800) vs. Normal biceps (n = 800) for BMI;
- ET triceps (n = 800) vs. Normal triceps (n = 800) for age;
- ET triceps (n = 800) vs. Normal triceps (n = 800) for BMI;
- ET brainstem (n = 320) vs. Normal brainstem (n = 320) for age;
- ET brainstem (n = 320) vs. Normal brainstem (n = 320) for BMI;
- ET cortical (n = 320) vs. Normal cortical (n = 320) for age;
- ET cortical (n = 320) vs. Normal cortical (n = 320) for BMI.
3. Results
4. Discussion
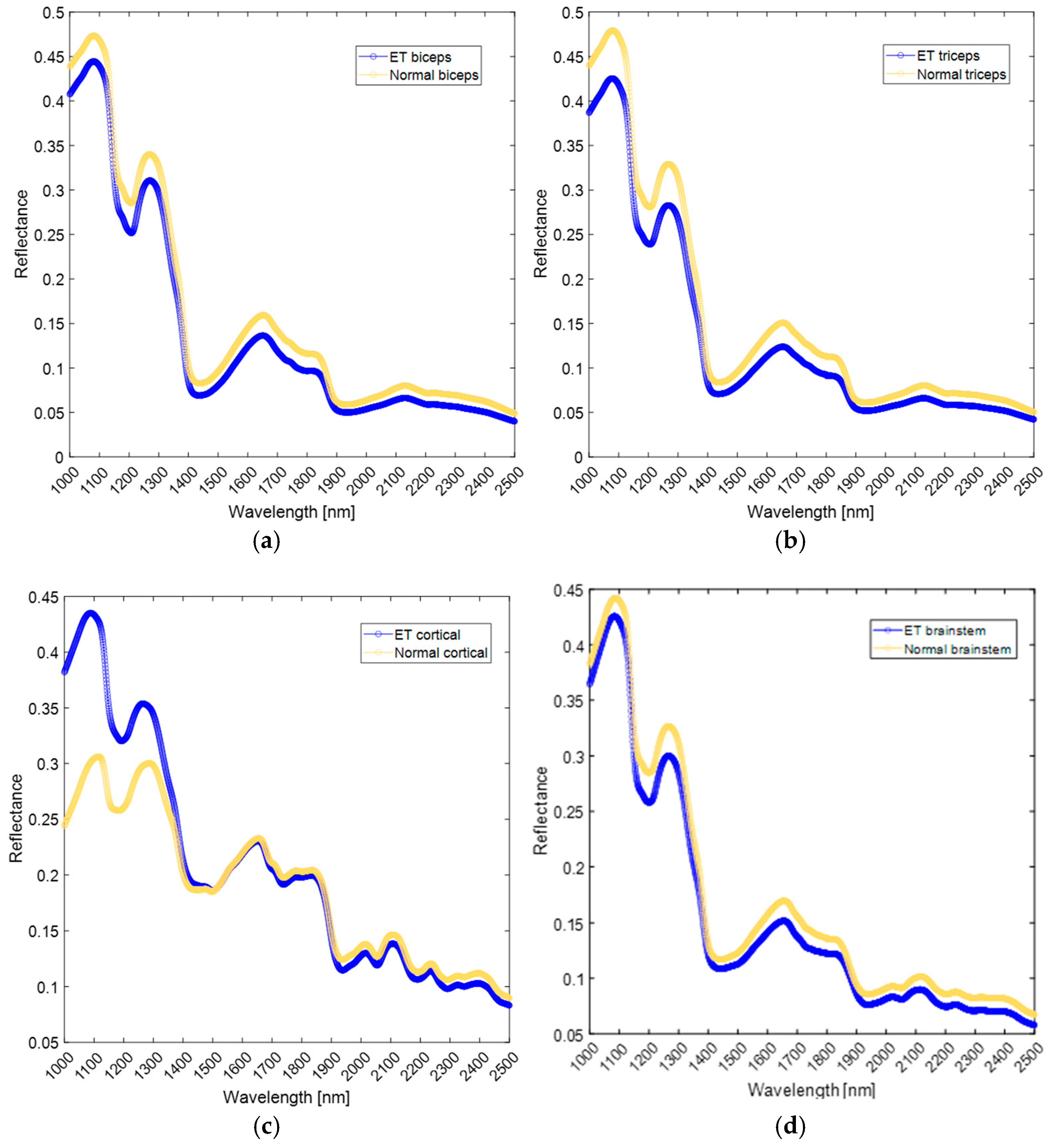
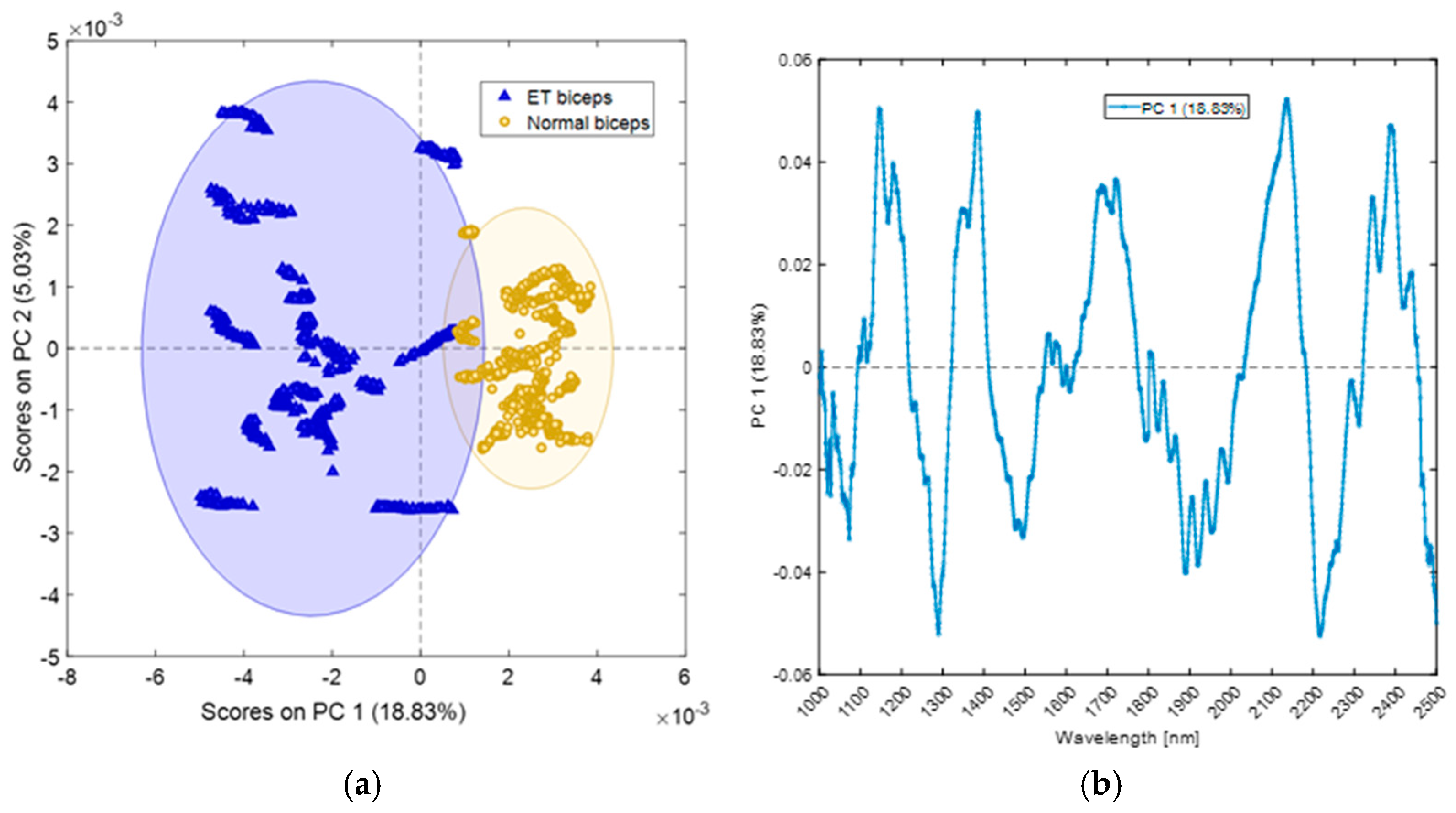
4.1. Extracranial Sites
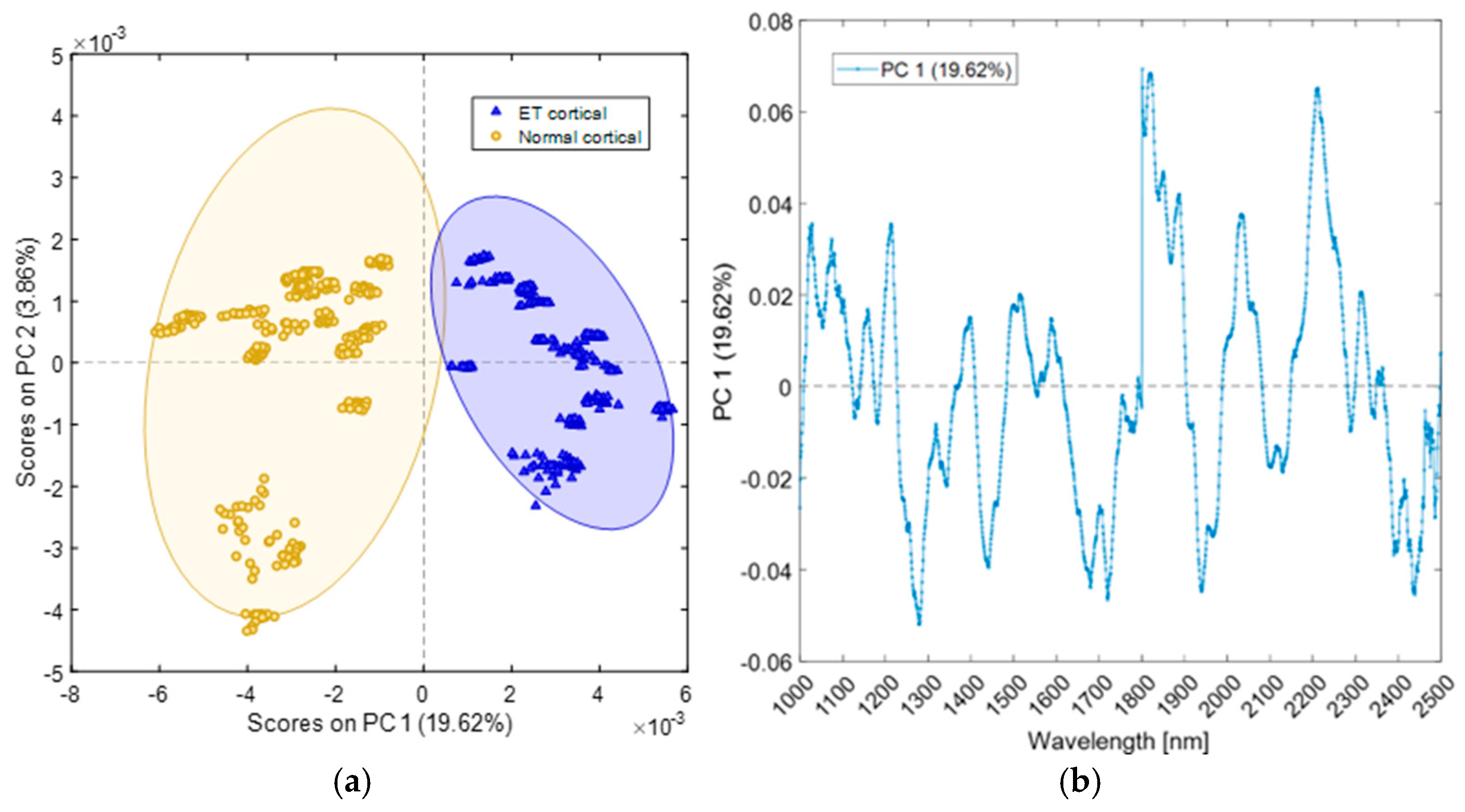
4.2. Cranial Sites
4.3. Influence of Age and BMI in Regression Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torricelli, A.; Pifferi, A.; Taroni, P.; Giambattistelli, E.; Cubeddu, R. In vivo optical characterization of human tissues from 610 to 1010 nm by time-resolved reflectance spectroscopy. Phys. Med. Biol. 2001, 46, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Afara, I.O.; Shaikh, R.; Nippolainen, E.; Querido, W.; Torniainen, J.; Sarin, J.K.; Kandel, S.; Pleshko, N.; Töyräs, J. Characterization of connective tissues using near-infrared spectroscopy and imaging. Nat. Protoc. 2021, 16, 1297–1329. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Currà, A.; Gasbarrone, R.; Cardillo, A.; Trompetto, C.; Fattapposta, F.; Pierelli, F.; Missori, P.; Bonifazi, G.; Serranti, S. Near-infrared spectroscopy as a tool for in vivo analysis of human muscles. Sci. Rep. 2019, 9, 8623. [Google Scholar] [CrossRef]
- Currà, A.; Gasbarrone, R.; Cardillo, A.; Fattapposta, F.; Missori, P.; Marinelli, L.; Bonifazi, G.; Serranti, S.; Trompetto, C. In vivo non-invasive near-infrared spectroscopy distinguishes normal, post-stroke, and botulinum toxin treated human muscles. Sci. Rep. 2021, 11, 17631. [Google Scholar] [CrossRef]
- Haubenberger, D.; Hallett, M. Essential Tremor. N. Engl. J. Med. 2018, 379, 596–597. [Google Scholar] [CrossRef]
- Louis, E.D. Non-motor symptoms in essential tremor: A review of the current data and state of the field. Park. Relat. Disord. 2016, 22 (Suppl. 1), S115–S118. [Google Scholar] [CrossRef]
- Mavroudis, I.; Petridis, F.; Kazis, D. Neuroimaging and neuropathological findings in essential tremor. Acta Neurol. Scand. 2019, 139, 491–496. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, L.; Liu, J.; Wang, X.; Meng, Q. Exploring the diagnostic markers of essential tremor: A study based on machine learning algorithms. Open Life Sci. 2023, 18, 20220622. [Google Scholar] [CrossRef]
- Angelini, L.; Paparella, G.; De Biase, A.; Maraone, A.; Panfili, M.; Berardelli, I.; Cannavacciuolo, A.; Di Vita, A.; Margiotta, R.; Fabbrini, G.; et al. Longitudinal study of clinical and neurophysiological features in essential tremor. Eur. J. Neurol. 2023, 30, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Purrer, V.; Pohl, E.; Lueckel, J.M.; Borger, V.; Sauer, M.; Radbruch, A.; Wüllner, U.; Schmeel, F.C. Artificial-intelligence-based MRI brain volumetry in patients with essential tremor and tremor-dominant Parkinson’s disease. Brain Commun. 2023, 5, fcad271. [Google Scholar] [CrossRef]
- Sharifi, S.; Buijink, A.W.G.; Luft, F.; Scheijbeler, E.P.; Potters, W.V.; van Wingen, G.; Heida, T.; Bour, L.J.; van Rootselaar, A.F. Differences in Olivo-Cerebellar Circuit and Cerebellar Network Connectivity in Essential Tremor: A Resting State fMRI Study. Cerebellum 2023, 22, 1123–1136. [Google Scholar] [CrossRef]
- Saccà, V.; Novellino, F.; Salsone, M.; Abou Jaoude, M.; Quattrone, A.; Chiriaco, C.; Madrigal, J.L.M. Challenging functional connectivity data: Machine learning application on essential tremor recognition. Neurol. Sci. 2023, 44, 199–207. [Google Scholar] [CrossRef]
- Angelini, L.; Terranova, R.; Lazzeri, G.; van den Berg, K.R.; Dirkx, M.F.; Paparella, G. The role of laboratory investigations in the classification of tremors. Neurol. Sci. 2023, 44, 4183–4192. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Tolosa, E.; Marín, C. Clinical rating scale for tremor. Park. Dis. Mov. Disord. 1993, 2, 271–280. [Google Scholar]
- Terravecchia, C.; Mostile, G.; Chisari, C.G.; Rascunà, C.; Terranova, R.; Cicero, C.E.; Giuliano, L.; Donzuso, G.; Sciacca, G.; Luca, A.; et al. Retinal Thickness in Essential Tremor and Early Parkinson Disease: Exploring Diagnostic Insights. J. Neuroophthalmol. 2024, 44, 35–40. [Google Scholar] [CrossRef]
- Lin, S.; Gao, C.; Li, H.; Huang, P.; Ling, Y.; Chen, Z.; Ren, K.; Chen, S. Wearable sensor-based gait analysis to discriminate early Parkinson’s disease from essential tremor. J. Neurol. 2023, 270, 2283–2301. [Google Scholar] [CrossRef]
- Zhao, Y.; Laguna, R.C.; Zhao, Y.; Liu, J.J.; He, X.; Yianni, J.; Sarrigiannis, P.G. A wavelet-based correlation analysis framework to study cerebromuscular activity in essential tremor. Complexity 2018, 2018, 7269494. [Google Scholar] [CrossRef]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G.; et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 75–87. [Google Scholar] [CrossRef]
- Tröster, A.I.; Pahwa, R.; Fields, J.A.; Tanner, C.M.; Lyons, K.E. Quality of life in Essential Tremor Questionnaire (QUEST): Development and initial validation. Park. Relat. Disord. 2005, 11, 367–373. [Google Scholar] [CrossRef]
- Danner, M.; Locherer, M.; Hank, T.; Richter, K. Spectral Sampling with the ASD FIELDSPEC 4; GFZ Data Service. 2015. Available online: https://gfzpublic.gfz-potsdam.de/rest/items/item_1388298/component/file_1388299/content (accessed on 24 October 2024).
- Rinnan, Å.; Berg, F.v.d.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Chong, I.-G.; Jun, C.-H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Abdi, H. Partial least square regression (PLS regression). Encycl. Res. Methods Soc. Sci. 2003, 6, 792–795. [Google Scholar]
- Louis, E.D.; Lee, M.; Babij, R.; Ma, K.; Cortés, E.; Vonsattel, J.P.; Faust, P.L. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 2014, 137, 3142–3148. [Google Scholar] [CrossRef]
- Nicoletti, V.; Cecchi, P.; Pesaresi, I.; Frosini, D.; Cosottini, M.; Ceravolo, R. Cerebello-thalamo-cortical network is intrinsically altered in essential tremor: Evidence from a resting state functional MRI study. Sci. Rep. 2020, 10, 16661. [Google Scholar] [CrossRef]
- Martuscello, R.T.; Kerridge, C.A.; Chatterjee, D.; Hartstone, W.G.; Kuo, S.H.; Sims, P.A.; Louis, E.D.; Faust, P.L. Gene expression analysis of the cerebellar cortex in essential tremor. Neurosci. Lett. 2020, 721, 134540. [Google Scholar] [CrossRef]
- Schreglmann, S.R.; Wang, D.; Peach, R.L.; Li, J.; Zhang, X.; Latorre, A.; Rhodes, E.; Panella, E.; Cassara, A.M.; Boyden, E.S.; et al. Non-invasive suppression of essential tremor via phase-locked disruption of its temporal coherence. Nat. Commun. 2021, 12, 363. [Google Scholar] [CrossRef]
- Shaikh, A.G.; Miura, K.; Optican, L.M.; Ramat, S.; Tripp, R.M.; Zee, D.S. Hypothetical membrane mechanisms in essential tremor. J. Transl. Med. 2008, 6, 68. [Google Scholar] [CrossRef]
- Hor, H.; Francescatto, L.; Bartesaghi, L.; Ortega-Cubero, S.; Kousi, M.; Lorenzo-Betancor, O.; Jiménez-Jiménez, F.J.; Gironell, A.; Clarimón, J.; Drechsel, O.; et al. Missense mutations in TENM4, a regulator of axon guidance and central myelination, cause essential tremor. Hum. Mol. Genet. 2015, 24, 5677–5686. [Google Scholar] [CrossRef] [PubMed]
- Kindler, C.; Upadhyay, N.; Purrer, V.; Schmeel, F.C.; Borger, V.; Scheef, L.; Wüllner, U.; Boecker, H. MRgFUS of the nucleus ventralis intermedius in essential tremor modulates functional connectivity within the classical tremor network and beyond. Park. Relat. Disord. 2023, 115, 105845. [Google Scholar] [CrossRef] [PubMed]
- Sakudo, A. Near-infrared spectroscopy for medical applications: Current status and future perspectives. Clin. Chim. Acta 2016, 455, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Gormley, M.E.; Carey, J.R. Muscle fiber orientation in muscles commonly injected with botulinum toxin: An anatomical pilot study. Neurotox. Res. 2006, 9, 115–120. [Google Scholar] [CrossRef]
- Lieber, R.L.; Fridén, J. Clinical significance of skeletal muscle architecture. Clin. Orthop. Relat. Res. 2001, 383, 140–151. [Google Scholar] [CrossRef]
- Jennekens, F.; Tomlinson, B.; Walton, J. Data on the distribution of fibre types in five human limb muscles An autopsy study. J. Neurol. Sci. 1971, 14, 245–257. [Google Scholar] [CrossRef]
- Johnson, M.A.; Polgar, J.; Weightman, D.; Appleton, D. Data on the distribution of fibre types in thirty-six human muscles: An autopsy study. J. Neurol. Sci. 1973, 18, 111–129. [Google Scholar] [CrossRef]
- Felder, A.; Ward, S.R.; Lieber, R.L. Sarcomere length measurement permits high resolution normalization of muscle fiber length in architectural studies. J. Exp. Biol. 2005, 208, 3275–3279. [Google Scholar] [CrossRef]
- Purslow, P.P. The structure and functional significance of variations in the connective tissue within muscle. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Bukharaeva, E.; Skorinkin, A. Cholinergic modulation of acetylcholine secretion at the neuromuscular junction. J. Evol. Biochem. Physiol. 2021, 57, 372–385. [Google Scholar] [CrossRef]
- Harris-Warrick, R.M. Voltage-sensitive ion channels in rhythmic motor systems. Curr. Opin. Neurobiol. 2002, 12, 646–651. [Google Scholar] [CrossRef]
- Rossi, A.E.; Dirksen, R.T. Sarcoplasmic reticulum: The dynamic calcium governor of muscle. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2006, 33, 715–731. [Google Scholar] [CrossRef]
- Protasi, F.; Girolami, B.; Roccabianca, S.; Rossi, D. Store-operated calcium entry: From physiology to tubular aggregate myopathy. Curr. Opin. Pharmacol. 2023, 68, 102347. [Google Scholar] [CrossRef]
- Barclay, C.; Curtin, N. Advances in understanding the energetics of muscle contraction. J. Biomech. 2023, 156, 111669. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; O’connor, P.L.; Zierath, J.R.; O’gorman, D.J. Time course analysis reveals gene-specific transcript and protein kinetics of adaptation to short-term aerobic exercise training in human skeletal muscle. PLoS ONE 2013, 8, e74098. [Google Scholar] [CrossRef] [PubMed]
- Free, D.B.; Syndergaard, I.; Pigg, A.C.; Muceli, S.; Thompson-Westra, J.; Mente, K.; Maurer, C.W.; Haubenberger, D.; Hallett, M.; Farina, D.; et al. Essential Tremor accentuates the pattern of tremor-band coherence between upper-limb muscles. J. Neurophysiol. 2023, 129, 524–540. [Google Scholar] [CrossRef]
- Gallego, J.A.; Dideriksen, J.L.; Holobar, A.; Ibáñez, J.; Glaser, V.; Romero, J.P.; Benito-León, J.; Pons, J.L.; Rocon, E.; Farina, D. The phase difference between neural drives to antagonist muscles in essential tremor is associated with the relative strength of supraspinal and afferent input. J. Neurosci. 2015, 35, 8925–8937. [Google Scholar] [CrossRef]
- Boyas, S.; Guével, A. Neuromuscular fatigue in healthy muscle: Underlying factors and adaptation mechanisms. Ann. Phys. Rehabil. Med. 2011, 54, 88–108. [Google Scholar] [CrossRef]
- Ruonala, V.; Meigal, A.; Rissanen, S.M.; Airaksinen, O.; Kankaanpää, M.; Karjalainen, P.A. EMG signal morphology and kinematic parameters in essential tremor and Parkinson’s disease patients. J. Electromyogr. Kinesiol. 2014, 24, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Stavusis, J.; Lace, B.; Schäfer, J.; Geist, J.; Inashkina, I.; Kidere, D.; Pajusalu, S.; Wright, N.T.; Saak, A.; Weinhold, M.; et al. Novel mutations in MYBPC1 are associated with myogenic tremor and mild myopathy. Ann. Neurol. 2019, 86, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, C.A.; Hemstreet, T.M.; Jöbsis-Vandervliet, F.F. Near-infrared spectrophotometric monitoring of oxygen distribution to intact brain and skeletal muscle tissues. Crit. Care Med. 1986, 14, 698–706. [Google Scholar] [CrossRef]
- Hazeki, O.; Seiyama, A.; Tamura, M. Near-infrared spectrophotometric monitoring of haemoglobin and cytochrome a, a 3 in situ. Oxyg. Transp. Tissue IX 1987, 215, 283–289. [Google Scholar]
- Fox, E.; Jöbsis-Vander Vliet, F.F.; Mitnick, M.H. Monitoring cerebral oxygen sufficiency in anesthesia and surgery. Oxyg. Transp. Tissue VII 1985, 191, 849–854. [Google Scholar]
- Brazy, J.E.; Lewis, D.V.; Mitnick, M.H.; vander Vliet, F.F.J.b. Noninvasive monitoring of cerebral oxygenation in preterm infants: Preliminary observations. Pediatrics 1985, 75, 217–225. [Google Scholar] [CrossRef]
- Ferrari, M.; De Marchis, C.; Giannini, I.; Di Nicola, A.; Agostino, R.; Nodari, S.; Bucci, G. Cerebral blood volume and hemoglobin oxygen saturation monitoring in neonatal brain by near IR spectroscopy. Oxyg. Transp. Tissue VIII 1986, 200, 203–211. [Google Scholar]
- Ferrari, M.; Zanette, E.; Giannini, I.; Sideri, G.; Fieschi, C.; Carpi, A. Effects of carotid artery compression test on regional cerebral blood volume, hemoglobin oxygen saturation and cytochrome-c-oxidase redox level in cerebrovascular patients. In Oxygen Transport to Tissue VIII; Springer: Berlin/Heidelberg, Germany, 1986; pp. 213–221. [Google Scholar]
- Louis, E.D.; Faust, P.L. Essential tremor pathology: Neurodegeneration and reorganization of neuronal connections. Nat. Rev. Neurol. 2020, 16, 69–83. [Google Scholar] [CrossRef]
- Shill, H.A.; Adler, C.H.; Sabbagh, M.N.; Connor, D.J.; Caviness, J.N.; Hentz, J.G.; Beach, T.G. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology 2008, 70, 1452–1455. [Google Scholar] [CrossRef]
- Farrell, K.; Cosentino, S.; Iida, M.A.; Chapman, S.; Bennett, D.A.; Faust, P.L.; Louis, E.D.; Crary, J.F. Quantitative Assessment of Pathological Tau Burden in Essential Tremor: A Postmortem Study. J. Neuropathol. Exp. Neurol. 2019, 78, 31–37. [Google Scholar] [CrossRef]
- Bagepally, B.S.; Bhatt, M.D.; Chandran, V.; Saini, J.; Bharath, R.D.; Vasudev, M.K.; Prasad, C.; Yadav, R.; Pal, P.K. Decrease in cerebral and cerebellar gray matter in essential tremor: A voxel-based morphometric analysis under 3T MRI. J. Neuroimaging 2012, 22, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.; Peller, M.; Wolff, S.; Alfke, K.; Witt, K.; Gaser, C.; Jansen, O.; Siebner, H.R.; Deuschl, G. Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology 2006, 67, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Moll, N.M.; Rietsch, A.M.; Thomas, S.; Ransohoff, A.J.; Lee, J.C.; Fox, R.; Chang, A.; Ransohoff, R.M.; Fisher, E. Multiple sclerosis normal-appearing white matter: Pathology–imaging correlations. Ann. Neurol. 2011, 70, 764–773. [Google Scholar] [CrossRef]
- Prodoehl, J.; Li, H.; Planetta, P.J.; Goetz, C.G.; Shannon, K.M.; Tangonan, R.; Comella, C.L.; Simuni, T.; Zhou, X.J.; Leurgans, S.; et al. Diffusion tensor imaging of Parkinson’s disease, atypical parkinsonism, and essential tremor. Mov. Disord. 2013, 28, 1816–1822. [Google Scholar] [CrossRef]
- Sengul, Y.; Temur, H.O.; Corakcı, Z.; Sengul, H.S.; Dowd, H.; Ustun, I.; Alkan, A.; Louis, E.D. Brain microstructural changes and cognitive function in non-demented essential tremor patients: A diffusion tensor imaging study. Int. J. Neurosci. 2022, 132, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.G.; Quattrone, A.; Sarica, A.; Aracri, F.; Calomino, C.; Caligiuri, M.E.; Novellino, F.; Nisticò, R.; Buonocore, J.; Crasà, M.; et al. Cortical involvement in essential tremor with and without rest tremor: A machine learning study. J. Neurol. 2023, 270, 4004–4012. [Google Scholar] [CrossRef]
- Younger, E.; Ellis, E.G.; Parsons, N.; Pantano, P.; Tommasin, S.; Caeyenberghs, K.; Benito-León, J.; Romero, J.P.; Joutsa, J.; Corp, D.T. Mapping Essential Tremor to a Common Brain Network Using Functional Connectivity Analysis. Neurology 2023, 101, e1483–e1494. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, B.; Häussler, S.; Schelter, B.; Lauk, M.; Guschlbauer, B.; Timmer, J.; Lücking, C.H. Tremor-correlated cortical activity in essential tremor. Lancet 2001, 357, 519–523. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.M.; Lu, M.K.; Tsai, C.H.; Chiou, J.C.; Liao, J.R.; Duann, J.R. VBM Reveals Brain Volume Differences between Parkinson’s Disease and Essential Tremor Patients. Front. Hum. Neurosci. 2013, 7, 247. [Google Scholar] [CrossRef]
- Halliday, D.M.; Conway, B.A.; Farmer, S.F.; Shahani, U.; Russell, A.J.; Rosenberg, J.R. Coherence between low-frequency activation of the motor cortex and tremor in patients with essential tremor. Lancet 2000, 355, 1149–1153. [Google Scholar] [CrossRef]
- Hellriegel, H.; Schulz, E.M.; Siebner, H.R.; Deuschl, G.; Raethjen, J.H. Continuous theta-burst stimulation of the primary motor cortex in essential tremor. Clin. Neurophysiol. 2012, 123, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Batra, D.; Kamble, N.; Bhattacharya, A.; Sahoo, L.; Yadav, R.; Pal, P.K. Modulatory effect of continuous theta burst stimulation in patients with essential tremor. Park. Relat. Disord. 2022, 94, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Benito-León, J.; Serrano, J.I.; Louis, E.D.; Holobar, A.; Romero, J.P.; Povalej-Bržan, P.; Kranjec, J.; Bermejo-Pareja, F.; Del Castillo, M.D.; Posada, I.J.; et al. Essential tremor severity and anatomical changes in brain areas controlling movement sequencing. Ann. Clin. Transl. Neurol. 2018, 6, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Seufert, J.; Bogdahn, U.; Reichmann, H.; Reiners, K. Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology 1995, 45, 182–184. [Google Scholar] [CrossRef]
- Stockner, H.; Sojer, M.; K, K.S.; Mueller, J.; Wenning, G.K.; Schmidauer, C.; Poewe, W. Midbrain sonography in patients with essential tremor. Mov. Disord. 2007, 22, 414–417. [Google Scholar] [CrossRef]
- Berg, D.; Siefker, C.; Ruprecht-Dörfler, P.; Becker, G. Relationship of substantia nigra echogenicity and motor function in elderly subjects. Neurology 2001, 56, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, F.S.; Wurster, I.; Seppi, K.; Stockner, H.; Scherfler, C.; Sojer, M.; Schmidauer, C.; Berg, D.; Poewe, W. Substantia nigra hyperechogenicity and Parkinson’s disease risk in patients with essential tremor. Mov. Disord. 2016, 31, 579–583. [Google Scholar] [CrossRef]
- Budisic, M.; Trkanjec, Z.; Bosnjak, J.; Lovrencic-Huzjan, A.; Vukovic, V.; Demarin, V. Distinguishing Parkinson’s disease and essential tremor with transcranial sonography. Acta Neurol. Scand. 2009, 119, 17–21. [Google Scholar] [CrossRef]
- Wills, A.J.; Jenkins, I.H.; Thompson, P.D.; Findley, L.J.; Brooks, D.J. Red nuclear and cerebellar but no olivary activation associated with essential tremor: A positron emission tomographic study. Ann. Neurol. 1994, 36, 636–642. [Google Scholar] [CrossRef]
- Klein, J.C.; Lorenz, B.; Kang, J.S.; Baudrexel, S.; Seifried, C.; van de Loo, S.; Steinmetz, H.; Deichmann, R.; Hilker, R. Diffusion tensor imaging of white matter involvement in essential tremor. Hum. Brain Mapp. 2011, 32, 896–904. [Google Scholar] [CrossRef]
- Nicoletti, G.; Manners, D.; Novellino, F.; Condino, F.; Malucelli, E.; Barbiroli, B.; Tonon, C.; Arabia, G.; Salsone, M.; Giofre’, L.; et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology 2010, 74, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Han, B.S.; Kim, H.S.; Lee, P.H. Diffusion tensor imaging in patients with essential tremor. AJNR Am. J. Neuroradiol. 2008, 29, 151–153. [Google Scholar] [CrossRef]
- Jia, L.; Jia-Lin, S.; Qin, D.; Qing, L.; Yan, Z. A diffusion tensor imaging study in essential tremor. J. Neuroimaging 2011, 21, 370–374. [Google Scholar] [CrossRef]
- Shill, H.A.; Adler, C.H.; Beach, T.G.; Lue, L.F.; Caviness, J.N.; Sabbagh, M.N.; Sue, L.I.; Walker, D.G. Brain biochemistry in autopsied patients with essential tremor. Mov. Disord. 2012, 27, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Boecker, H.; Weindl, A.; Brooks, D.J.; Ceballos-Baumann, A.O.; Liedtke, C.; Miederer, M.; Sprenger, T.; Wagner, K.J.; Miederer, I. GABAergic dysfunction in essential tremor: An 11C-flumazenil PET study. J. Nucl. Med. 2010, 51, 1030–1035. [Google Scholar] [CrossRef]
- Chuang, W.L.; Huang, Y.Z.; Lu, C.S.; Chen, R.S. Reduced cortical plasticity and GABAergic modulation in essential tremor. Mov. Disord. 2014, 29, 501–507. [Google Scholar] [CrossRef]
- Wichmann, T.; DeLong, M.R. Neurotransmitters and disorders of the basal ganglia. In Basic Neurochemistry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 856–871. [Google Scholar]
- Rossi, R.; Arjmand, S.; Bærentzen, S.L.; Gjedde, A.; Landau, A.M. Synaptic vesicle glycoprotein 2A: Features and functions. Front. Neurosci. 2022, 16, 864514. [Google Scholar] [CrossRef]
- Bae, J.R.; Lee, W.; Jo, Y.O.; Han, S.; Koh, S.; Song, W.K.; Kim, S.H. Distinct synaptic vesicle recycling in inhibitory nerve terminals is coordinated by SV2A. Progress. Neurobiol. 2020, 194, 101879. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, C.; Chen, B.; Hernandez, N.C.; Faust, P.L.; Cai, Z.; Louis, E.D.; Matuskey, D. Decreased Synaptic Vesicle Glycoprotein 2A Binding in the Human Postmortem Essential Tremor Cerebellum: Evidence of Reduction in Synaptic Density. Cerebellum 2024, 23, 1053–1060. [Google Scholar] [CrossRef]
- Louis, E.D.; Marder, K.; Jurewicz, E.C.; Watner, D.; Levy, G.; Mejia-Santana, H. Body mass index in essential tremor. Arch. Neurol. 2002, 59, 1273–1277. [Google Scholar] [CrossRef]






| ID | Condition | Age [yrs] | Sex | Weight [kg] | Height [cm] | BMI | FMT | QUEST |
|---|---|---|---|---|---|---|---|---|
| B01 | ET | 86 | 0 | 98 | 170 | 34 | 54 | 52 |
| B02 | ET | 81 | 1 | 51 | 160 | 20 | 32 | 11 |
| B03 | ET | 21 | 1 | 58 | 167 | 21 | 23 | 0 |
| B04 | ET | 86 | 0 | 80 | 177 | 26 | 54 | 36 |
| B05 | ET | 77 | 1 | 56 | 160 | 22 | 24 | 6 |
| B06 | ET | 74 | 1 | 53 | 155 | 22 | 21 | 183 |
| B07 | ET | 76 | 0 | 57 | 160 | 22 | 15 | 28 |
| B08 | ET | 81 | 0 | 81 | 179 | 25 | 12 | 6 |
| B09 | ET | 68 | 0 | 73 | 173 | 24 | 2 | 0 |
| B10 | ET | 86 | 0 | 78 | 175 | 25 | 9 | 0 |
| B11 | ET | 72 | 0 | 75 | 177 | 24 | 18 | 14 |
| B12 | ET | 89 | 1 | 72 | 163 | 27 | 25 | 69 |
| B13 | ET | 54 | 1 | 62 | 164 | 23 | 5 | 106 |
| B14 | ET | 80 | 0 | 70 | 171 | 24 | 17 | 30 |
| B15 | ET | 76 | 0 | 89 | 173 | 30 | 14 | 3 |
| B16 | ET | 74 | 1 | 78 | 152 | 34 | 30 | 172 |
| NT01 | Normal | 74 | 1 | 58 | 157 | 24 | - | - |
| NT02 | Normal | 74 | 0 | 70 | 175 | 23 | - | - |
| NT03 | Normal | 55 | 1 | 58 | 168 | 21 | - | - |
| NT04 | Normal | 27 | 1 | 54 | 160 | 21 | - | - |
| NT05 | Normal | 24 | 1 | 58 | 167 | 21 | - | - |
| NT06 | Normal | 24 | 1 | 47 | 165 | 17 | - | - |
| NT07 | Normal | 36 | 0 | 75 | 174 | 25 | - | - |
| NT08 | Normal | 21 | 0 | 90 | 185 | 26 | - | - |
| NT09 | Normal | 59 | 0 | 102 | 178 | 32 | - | - |
| NT10 | Normal | 66 | 0 | 74 | 168 | 26 | - | - |
| NT11 | Normal | 53 | 1 | 75 | 156 | 31 | - | - |
| NT12 | Normal | 50 | 0 | 68 | 170 | 24 | - | - |
| NT13 | Normal | 58 | 0 | 83 | 173 | 28 | - | - |
| NT14 | Normal | 26 | 0 | 72 | 178 | 23 | - | - |
| NT15 | Normal | 27 | 0 | 80 | 177 | 26 | - | - |
| NT16 | Normal | 33 | 0 | 57 | 160 | 22 | - | - |
| Model | Model Phase | Class | Sensitivity | Specificity | Number of Spectra | Error Rate | Precision | Accuracy |
|---|---|---|---|---|---|---|---|---|
| ET biceps/ Normal biceps | C | ET biceps | 1 | 1 | 534 | 0 | 1 | 1 |
| Normal biceps | 1 | 1 | 586 | 0 | 1 | 1 | ||
| CV | ET biceps | 1 | 1 | 534 | 0 | 1 | 1 | |
| Normal biceps | 1 | 1 | 586 | 0 | 1 | 1 | ||
| P | ET biceps | 1 | 1 | 266 | 0 | 1 | 1 | |
| Normal biceps | 1 | 1 | 214 | 0 | 1 | 1 | ||
| ET triceps/ Normal triceps | C | ET Triceps | 1 | 1 | 544 | 0 | 1 | 1 |
| Normal triceps | 1 | 1 | 576 | 0 | 1 | 1 | ||
| CV | ET Triceps | 1 | 1 | 544 | 0 | 1 | 1 | |
| Normal triceps | 1 | 1 | 576 | 0 | 1 | 1 | ||
| P | ET Triceps | 1 | 1 | 256 | 0 | 1 | 1 | |
| Normal triceps | 1 | 1 | 224 | 0 | 1 | 1 | ||
| ET cortical/ Normal cortical | C | ET cortical | 1 | 1 | 224 | 0 | 1 | 1 |
| Normal cortical | 1 | 1 | 224 | 0 | 1 | 1 | ||
| CV | ET cortical | 1 | 1 | 224 | 0 | 1 | 1 | |
| Normal cortical | 1 | 1 | 224 | 0 | 1 | 1 | ||
| P | ET cortical | 1 | 1 | 96 | 0 | 1 | 1 | |
| Normal cortical | 1 | 1 | 96 | 0 | 1 | 1 | ||
| ET brainstem/ Normal brainstem | C | ET brainstem | 1 | 1 | 205 | 0 | 1 | 1 |
| Normal brainstem | 1 | 1 | 243 | 0 | 1 | 1 | ||
| C | ET brainstem | 1 | 1 | 205 | 0 | 1 | 1 | |
| Normal brainstem | 1 | 1 | 243 | 0 | 1 | 1 | ||
| P | ET brainstem | 1 | 1 | 115 | 0 | 1 | 1 | |
| Normal brainstem | 1 | 1 | 77 | 0 | 1 | 1 |
| Dataset | PLS | LV | RMSEC | RMSECV | C Bias | CV Bias | R2C | R2CV |
|---|---|---|---|---|---|---|---|---|
| ET biceps/Normal biceps | Age | 4 | 5 | 5 | 0 | 0.006 | 0.959 | 0.958 |
| BMI | 4 | 1.62 | 1.65 | 0 | −0.001 | 0.827 | 0.821 | |
| ET triceps/Normal triceps | Age | 5 | 4 | 4 | 0 | −0.001 | 0.971 | 0.967 |
| BMI | 6 | 1.51 | 1.59 | 0 | 0.013 | 0.851 | 0.833 | |
| ET cortical/Normal cortical | Age | 4 | 4 | 4 | 0 | 0.027 | 0.97 | 0.966 |
| BMI | 5 | 0.841 | 0.914 | 0 | −0.001 | 0.954 | 0.954 | |
| ET brainstem /Normal brainstem | Age | 5 | 4 | 5 | 0 | −0.002 | 0.964 | 0.955 |
| BMI | 6 | 1.172 | 1.385 | 0 | −0.002 | 0.91 | 0.875 |
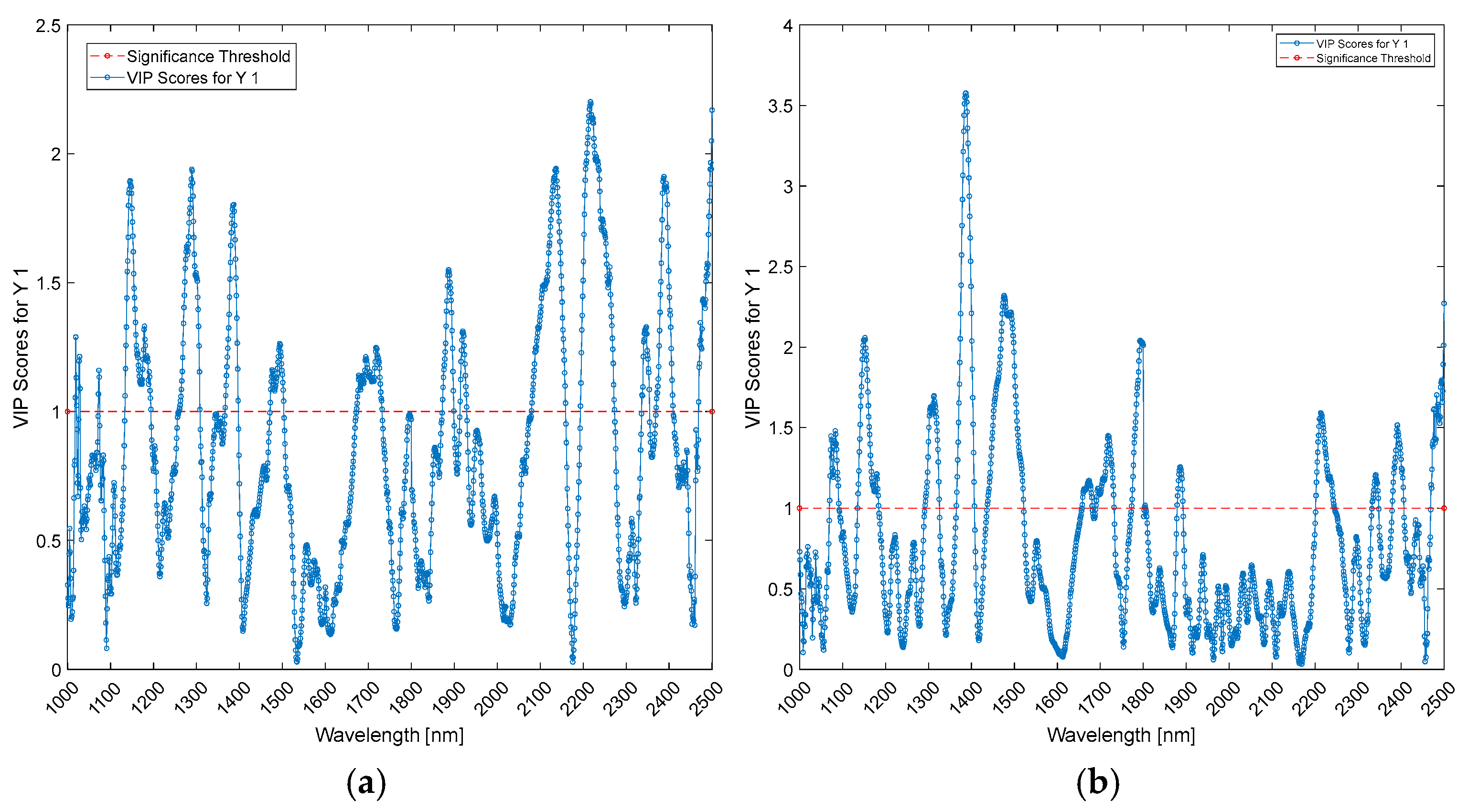
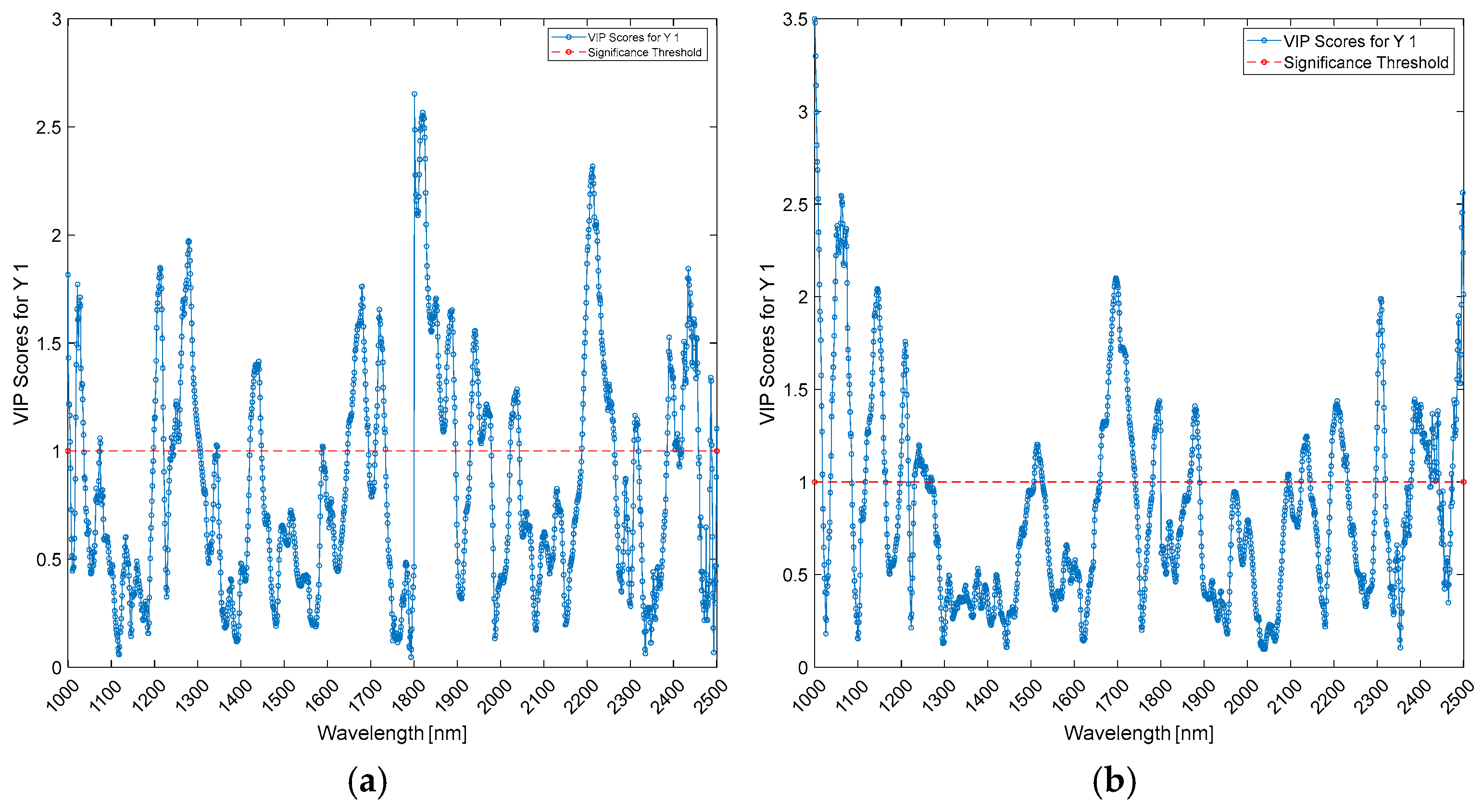
| Wavelengths (nm) | Biceps | Triceps | ||||
|---|---|---|---|---|---|---|
| PLS-DA | PLS (Age) | PLS (BMI) | PLS-DA | PLS (Age) | PLS (BMI) | |
| 1000–1100 | 1000 | |||||
| 1100–1200 | 1100; 1150–1200 | 1150 | 1150 | 1150–1200 | ||
| 1200–1300 | 1200 | 1200 | ||||
| 1300–1400 | 1350 | 1350–1400 | 1350–1400 | |||
| 1400–1500 | 1450–1500 | 1450–1500 | ||||
| 1500–1600 | ||||||
| 1600–1700 | ||||||
| 1700–1800 | ||||||
| 1800–1900 | 1800 | |||||
| 1900–2000 | 1900 | 1900 | ||||
| 2000–2100 | ||||||
| 2100–2200 | ||||||
| 2200–2300 | 2200–2250 | |||||
| 2300–2400 | ||||||
| 2400–2500 | 2500 | 2500 | 2500 | 2500 | ||
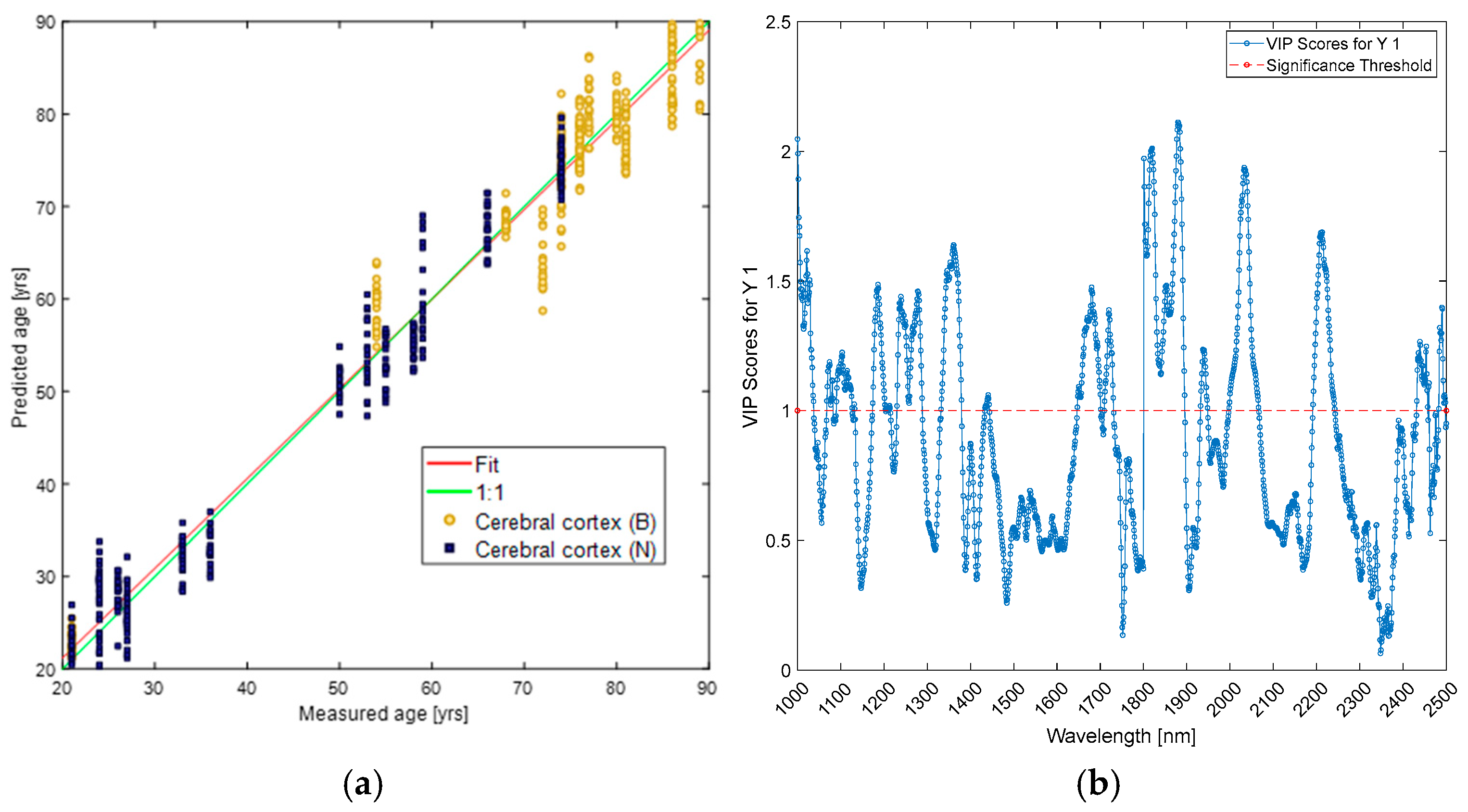
| Wavelengths (nm) | Cerebral Cortex | Mid-Brain | ||||
|---|---|---|---|---|---|---|
| PLS-DA | PLS (Age) | PLS (BMI) | PLS-DA | PLS (Age) | PLS (BMI) | |
| 1000–1100 | 1000 | 1000 | 1000; 1050 | 1000; 1050–1100 | 1000 | |
| 1100–1200 | 1150 | 1150–1200 | ||||
| 1200–1300 | ||||||
| 1300–1400 | ||||||
| 1400–1500 | ||||||
| 1500–1600 | ||||||
| 1600–1700 | 1650 | |||||
| 1700–1800 | 1700 | |||||
| 1800–1900 | 1800–1850 | 1800; 1850–1900 | 1850–1900 | |||
| 1900–2000 | ||||||
| 2000–2100 | ||||||
| 2100–2200 | ||||||
| 2200–2300 | 2200–2250 | |||||
| 2300–2400 | 2300 | |||||
| 2400–2500 | 2500 | 2500 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Currà, A.; Gasbarrone, R.; Gattabria, D.; Bonifazi, G.; Serranti, S.; Greco, D.; Missori, P.; Fattapposta, F.; Picciano, A.; Maffucci, A.; et al. In Vivo Insights: Near-Infrared Photon Sampling of Reflectance Spectra from Cranial and Extracranial Sites in Healthy Individuals and Patients with Essential Tremor. Photonics 2024, 11, 1025. https://doi.org/10.3390/photonics11111025
Currà A, Gasbarrone R, Gattabria D, Bonifazi G, Serranti S, Greco D, Missori P, Fattapposta F, Picciano A, Maffucci A, et al. In Vivo Insights: Near-Infrared Photon Sampling of Reflectance Spectra from Cranial and Extracranial Sites in Healthy Individuals and Patients with Essential Tremor. Photonics. 2024; 11(11):1025. https://doi.org/10.3390/photonics11111025
Chicago/Turabian StyleCurrà, Antonio, Riccardo Gasbarrone, Davide Gattabria, Giuseppe Bonifazi, Silvia Serranti, Daniela Greco, Paolo Missori, Francesco Fattapposta, Alessandra Picciano, Andrea Maffucci, and et al. 2024. "In Vivo Insights: Near-Infrared Photon Sampling of Reflectance Spectra from Cranial and Extracranial Sites in Healthy Individuals and Patients with Essential Tremor" Photonics 11, no. 11: 1025. https://doi.org/10.3390/photonics11111025
APA StyleCurrà, A., Gasbarrone, R., Gattabria, D., Bonifazi, G., Serranti, S., Greco, D., Missori, P., Fattapposta, F., Picciano, A., Maffucci, A., & Trompetto, C. (2024). In Vivo Insights: Near-Infrared Photon Sampling of Reflectance Spectra from Cranial and Extracranial Sites in Healthy Individuals and Patients with Essential Tremor. Photonics, 11(11), 1025. https://doi.org/10.3390/photonics11111025








