Portable Diffuse Optical Tomography for Three-Dimensional Functional Neuroimaging in the Hospital
Abstract
1. Introduction
2. Materials and Methods
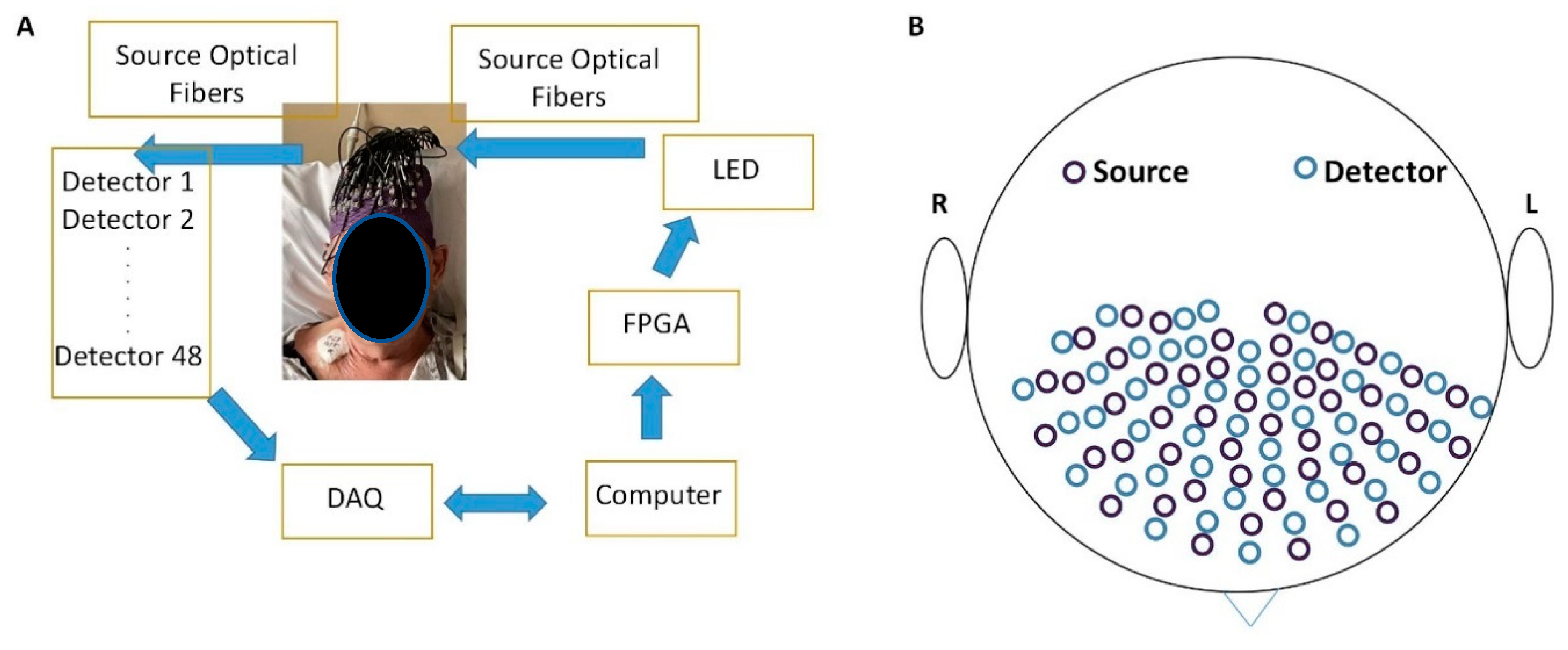
2.1. Portable Diffuse Optical Tomography System
2.2. Light-Emitting Diodes (LEDs)
2.3. Detection Units
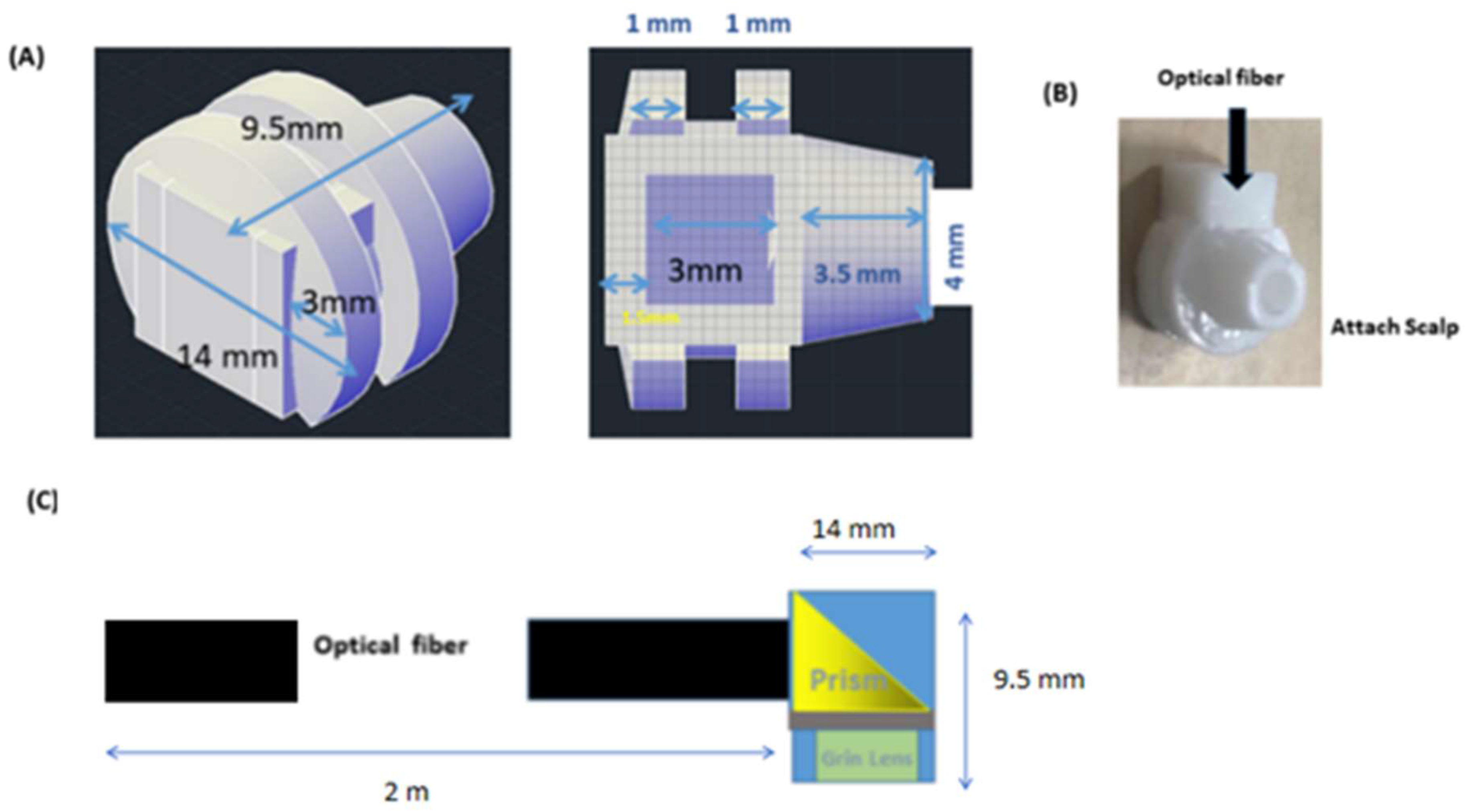
2.4. Optical Fibers
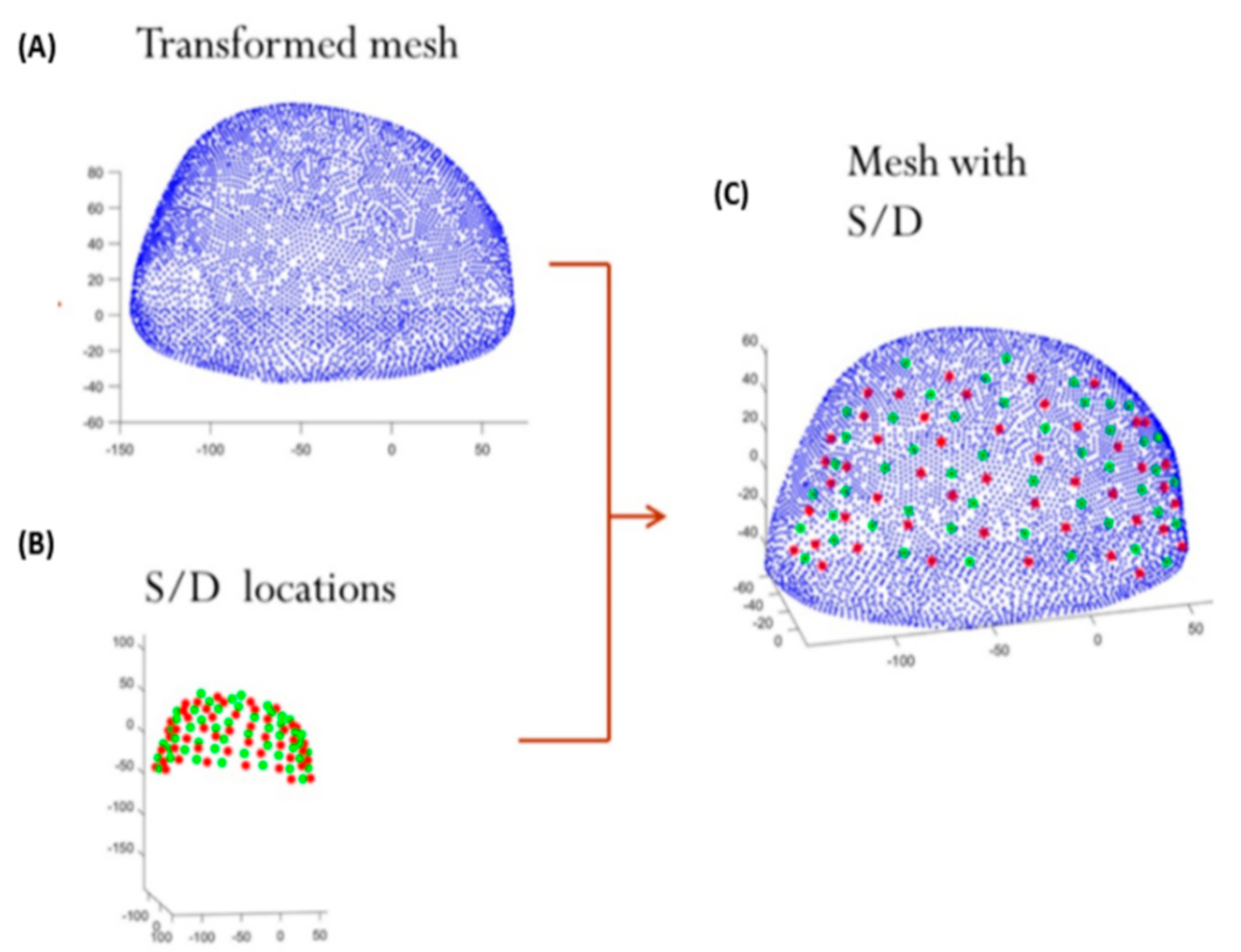
2.5. Mesh Design
2.6. Adapter Design
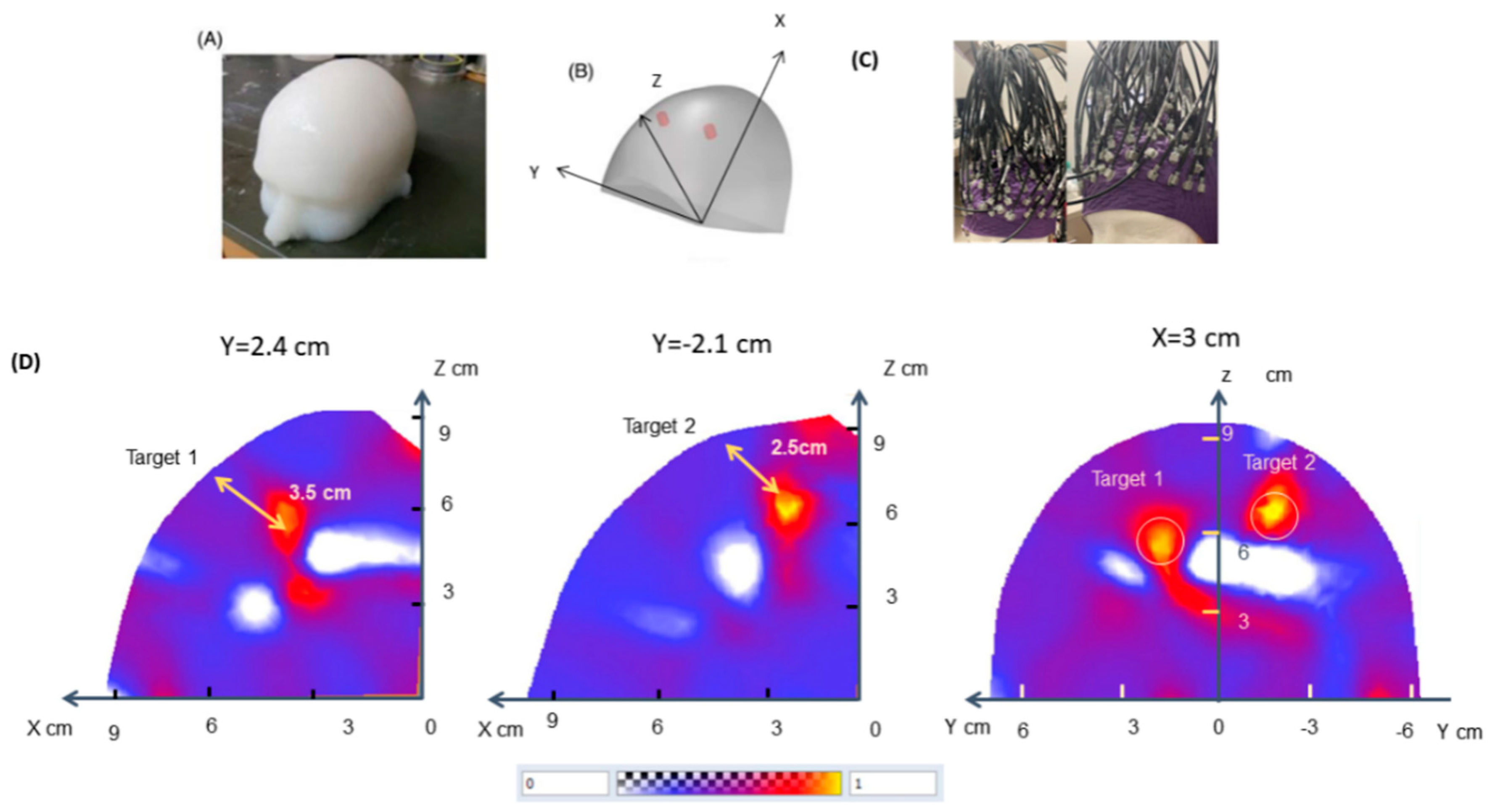
2.7. Phantom Experiments
2.8. DOT Image Reconstruction
2.9. Recruitment
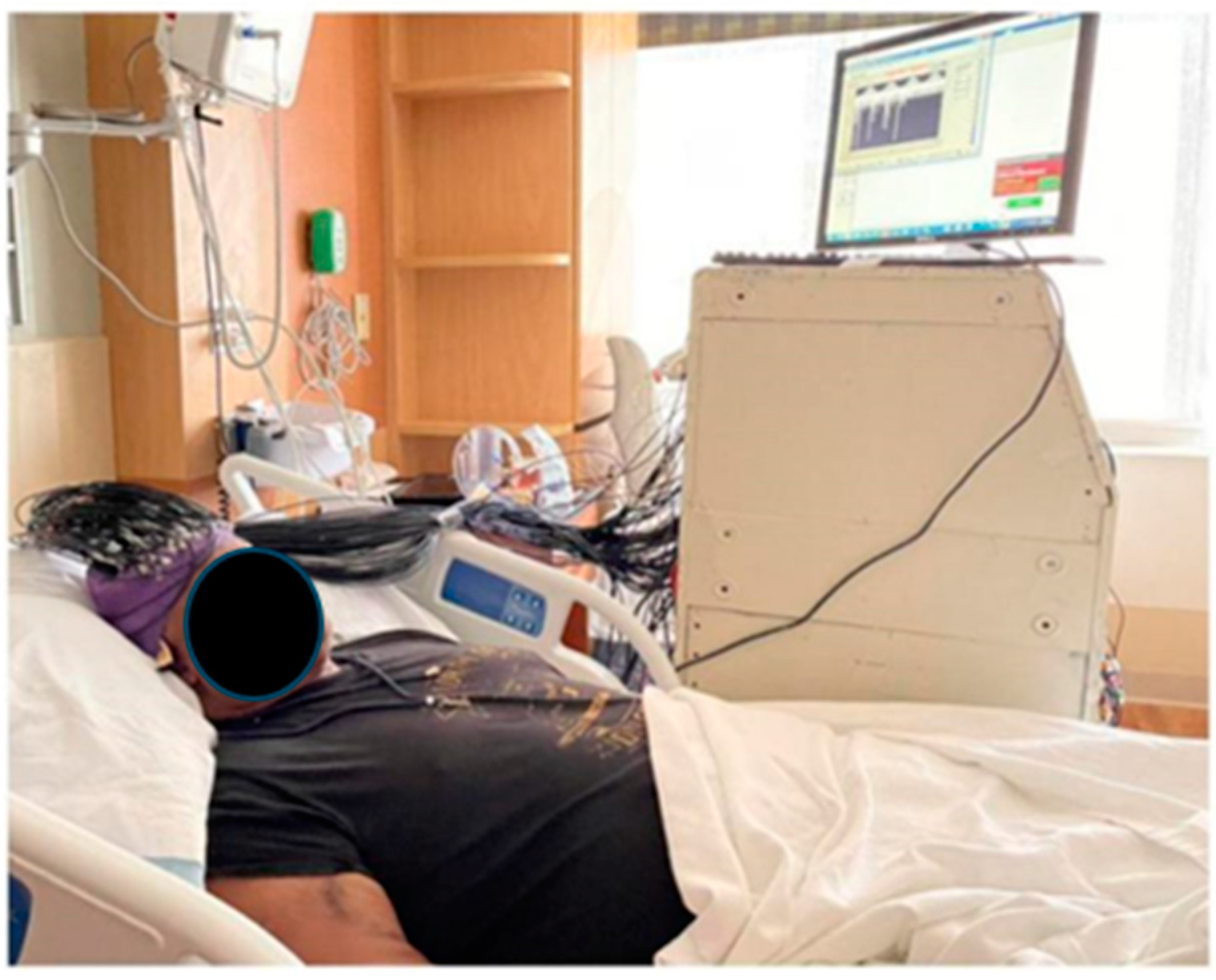
2.10. Imaging Protocol
2.11. Image Analysis and Processing
2.12. Seed-Based Correlation Analysis
2.13. Pearson Correlation Coefficient as a Measure of Connectivity
3. Results
3.1. Demographic Data
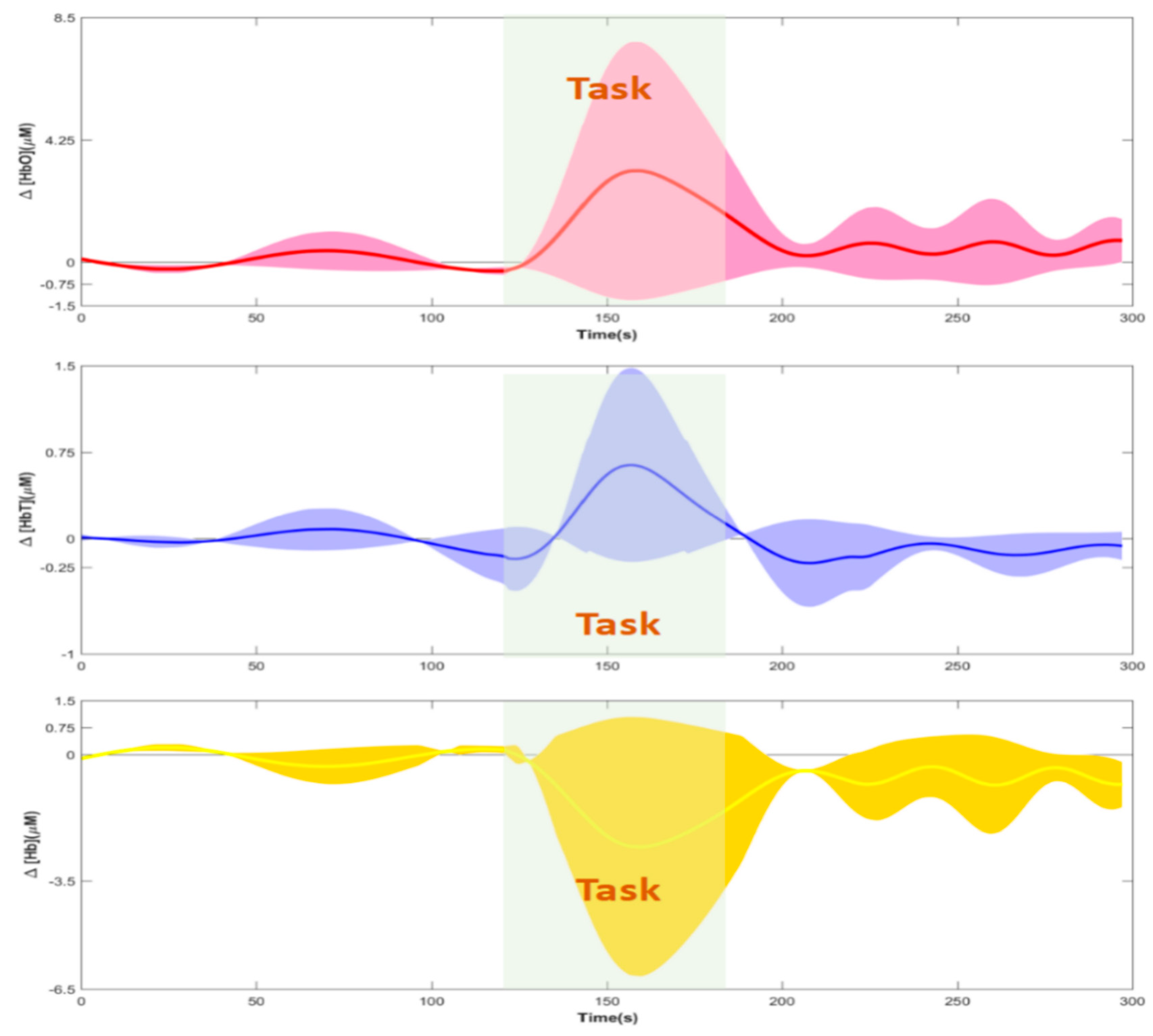
3.2. Hemodynamic Responses and Image Reconstructions
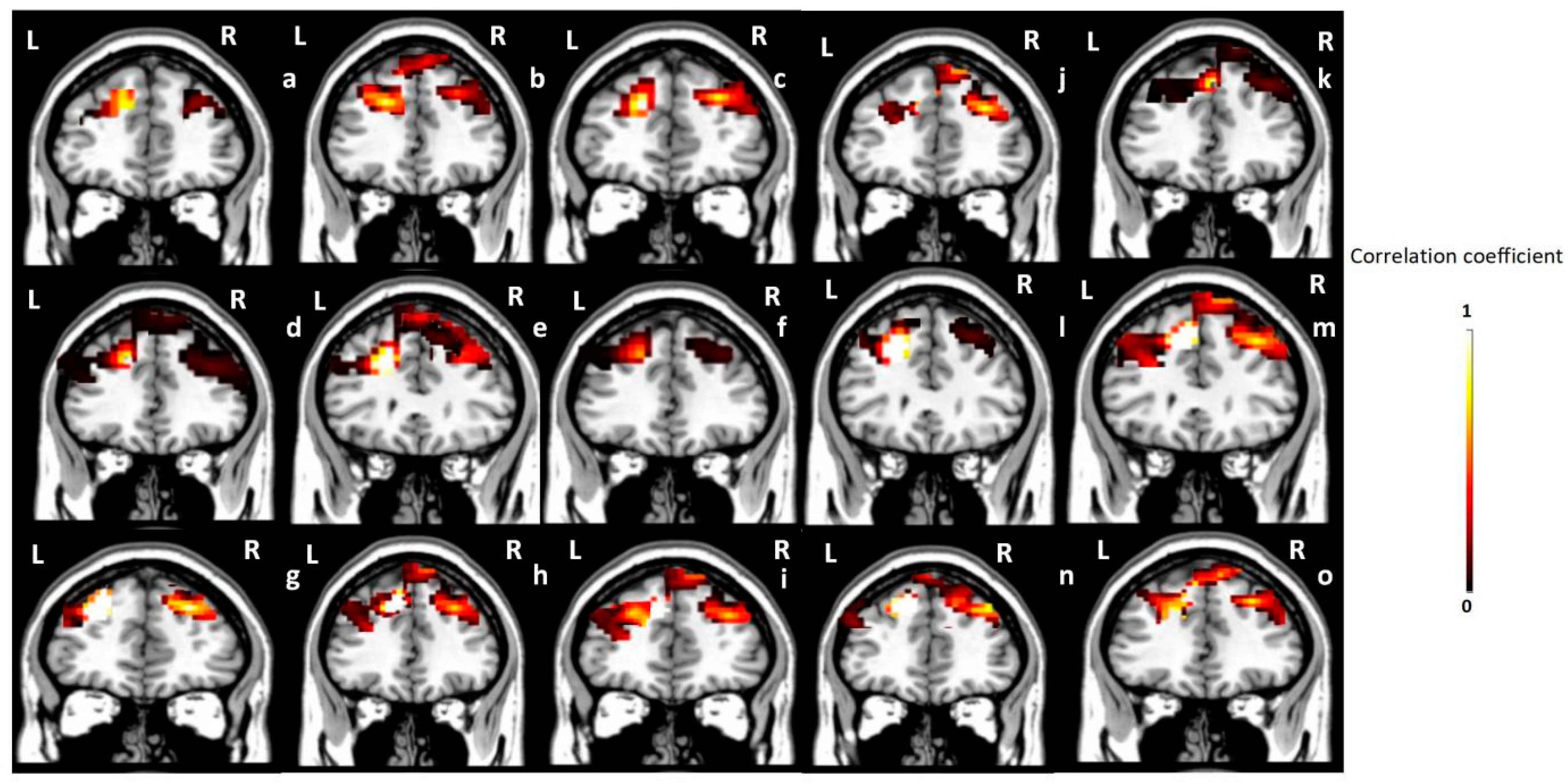
3.3. Seed-Based Correlation Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukadam, N.; Sampson, E.L. A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int. Psychogeriatr. 2011, 23, 344–355. [Google Scholar] [CrossRef]
- Vasilevskis, E.E.; Han, J.H.; Hughes, C.G.; Ely, E.W. Epidemiology and risk factors for delirium across hospital settings. Best Pract. Res. Clin. Anaesthesiol. 2012, 26, 277–287. [Google Scholar] [CrossRef]
- Rabinowitz, A.R.; Levin, H.S. Cognitive sequelae of traumatic brain injury. Psychiatr. Clin. N. Am. 2014, 37, 1–11. [Google Scholar] [CrossRef]
- Pandharipande, P.P.; Girard, T.D.; Ely, E.W. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2014, 370, 185–186. [Google Scholar] [CrossRef][Green Version]
- Fogg, C.; Griffiths, P.; Meredith, P.; Bridges, J. Hospital outcomes of older people with cognitive impairment: An integrative review. Int. J. Geriatr. Psychiatry 2018, 33, 1177–1197. [Google Scholar] [CrossRef]
- Zhang, T.; Ren, Y.; Shen, P.; Jiang, S.; Yang, Y.; Wang, Y.; Li, Z.; Yang, Y. Prevalence and Associated Risk Factors of Cognitive Frailty: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 13, 755926. [Google Scholar] [CrossRef]
- Fong, T.G.; Davis, D.; Growdon, M.E.; Albuquerque, A.; Inouye, S.K. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015, 14, 823–832, Erratum in Lancet Neurol. 2015, 14, 788. [Google Scholar] [CrossRef]
- Howieson, D. Current limitations of neuropsychological tests and assessment procedures. Clin. Neuropsychol. 2019, 33, 200–208. [Google Scholar] [CrossRef]
- Helfand, B.K.I.; D’Aquila, M.L.; Tabloski, P.; Erickson, K.; Yue, J.; Fong, T.G.; Hshieh, T.T.; Metzger, E.D.; Schmitt, E.M.; Boudreaux, E.D.; et al. Detecting Delirium: A Systematic Review of Identification Instruments for Non-ICU Settings. J. Am. Geriatr. Soc. 2021, 69, 547–555. [Google Scholar] [CrossRef]
- Jones, R.N.; Cizginer, S.; Pavlech, L.; Albuquerque, A.; Daiello, L.A.; Dharmarajan, K.; Gleason, L.J.; Helfand, B.; Massimo, L.; Oh, E.; et al. Better Assessment of Illness (BASIL) Study Group. Assessment of Instruments for Measurement of Delirium Severity: A Systematic Review. JAMA Intern. Med. 2019, 179, 231–239. [Google Scholar] [CrossRef]
- Sheehan, B. Assessment scales in dementia. Ther. Adv. Neurol. Disord. 2012, 5, 349–358. [Google Scholar] [CrossRef]
- Wiegand, T.L.T.; Rémi, J.; Dimitriadis, K. Electroencephalography in delirium assessment: A scoping review. BMC Neurol. 2022, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Pauli, R.; O’Donnell, A.; Cruse, D. Resting-State Electroencephalography for Prognosis in Disorders of Consciousness Following Traumatic Brain Injury. Front. Neurol. 2020, 11, 586945. [Google Scholar] [CrossRef] [PubMed]
- Cassani, R.; Estarellas, M.; San-Martin, R.; Fraga, F.J.; Falk, T.H. Systematic Review on Resting-State EEG for Alzheimer’s Disease Diagnosis and Progression Assessment. Dis. Markers 2018, 2018, 5174815. [Google Scholar] [CrossRef] [PubMed]
- Deiner, S.; Luo, X.; Silverstein, J.H.; Sano, M. Can Intraoperative Processed EEG Predict Postoperative Cognitive Dysfunction in the Elderly? Clin. Ther. 2015, 37, 2700–2705. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Brunet, D. EEG Source Imaging: A Practical Review of the Analysis Steps. Front. Neurol. 2019, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.E.; Cooper, C.E. Making light work: Illuminating the future of biomedical optics. Philos. Trans. R. Soc. A Math Phys. Eng. Sci. 2011, 369, 4358–4379. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delpy, D.T.; Cope, M. Quantification in tissue near-infrared spectroscopy. Philos. Trans. R. Soc. B Biol. Sci. 1997, 352, 649–659. [Google Scholar] [CrossRef]
- Hoshi, Y.; Yamada, Y. Overview of diffuse optical tomography and its clinical applications. J. Biomed. Opt. 2016, 21, 091312. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, J.; Jiang, R.; Yang, H.; Carney, P.R.; Jiang, H. Pre-seizure state identified by diffuse optical tomography. Sci. Rep. 2014, 4, 3798. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Q.; Li, C.; Grobmyer, S.R.; Fajardo, L.L.; Jiang, H. Phase-contrast diffuse optical tomography pilot results in the breast. Acad. Radiol. 2008, 15, 859–866. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, Q.; Sobel, E.S.; Jiang, H. Tomographic x-ray-guided three-dimensional diffuse optical tomography of osteoarthritis in the finger joints. J. Biomed. Opt. 2008, 13, 044006. [Google Scholar] [CrossRef]
- Zeff, B.W.; White, B.R.; Dehghani, H.; Schlaggar, B.L.; Culver, J.P. Retinotopic mapping of adult human visual cortex with high-density diffuse optical tomography. Proc. Natl. Acad. Sci. USA 2007, 104, 12169–12174. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, S.; Wagoner, R.; Yang, H.; Currier, G.; Jiang, H. Three-dimensional optical imaging of brain activation during transcranial magnetic stimulation. J. X-ray Sci. Technol. 2021, 29, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Huang, J.; Yang, H.; Wagoner, R.; Kozel, F.A.; Currier, G.; Jiang, H. Neuroimaging of depression with diffuse optical tomography during repetitive transcranial magnetic stimulation. Sci. Rep. 2021, 11, 7328. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Carpenter, L.L.; Jiang, H. Optical neuroimaging: Advancing transcranial magnetic stimulation treatments of psychiatric disorders. Vis. Comput. Ind. Biomed. Art 2022, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, T.; Yang, H.; Tang, J.; Carney, P.R.; Jiang, H. Fast noninvasive functional diffuse optical tomography for brain imaging. J. Biophotonics 2017, 11, e201600267. [Google Scholar] [CrossRef]
- Jiang, H. Diffuse Optical Tomography: Principles and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Meagher, J.; Leonard, M.; Donoghue, L.; O’regan, N.; Timmons, S.; Exton, C.; Cullen, W.; Dunne, C.; Adamis, D.; Maclullich, A.J.; et al. Months backward test: A review of its use in clinical studies. World J. Psychiatry 2015, 5, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Wildgruber, D.; Kischka, U.; Ackermann, H.; Klose, U.; Grodd, W. Dynamic pattern of brain activation during sequencing of word strings evaluated by fMRI. Brain Res. Cogn. Brain Res. 1999, 7, 285–294. [Google Scholar] [CrossRef]
- Marinus, J.; Visser, M.; Verwey, N.A.; Verhey, F.R.; Middelkoop, H.A.; Stiggelbout, A.M.; van Hilten, J.J. Assessment of cognition in Parkinson’s disease. Neurology 2003, 61, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Tardiff, M.; Roy, M.; Remi, B.; Laforce, R. Months backward test as a reliable predictor of cognitive decline in mild Alzheimer’s disease. Alzheimer’s Dement. 2013, 9, S741–S742. [Google Scholar] [CrossRef]
- Bellelli, G.; Morandi, A.; Davis, D.H.; Mazzola, P.; Turco, R.; Gentile, S.; Ryan, T.; Cash, H.; Guerini, F.; Torpilliesi, T.; et al. Validation of the 4AT, a new instrument for rapid delirium screening: A study in 234 hospitalised older people. Age Ageing 2014, 43, 496–502, Erratum in Age Ageing 2015, 44, 175. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, A.Y.; Stewart, M.; Lasker, J.; Abdoulaev, G.S.; Hielscher, A.H. Three-dimensional optical tomographic brain imaging in small animals, part 1: Hypercapnia. J. Biomed. Opt. 2004, 9, 1046–1062. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gagnon, L.; Yücel, M.A.; Dehaes, M.; Cooper, R.J.; Perdue, K.L.; Selb, J.; Boas, D.A. Quantification of the cortical contribution to the NIRS signal over the motor cortex using concurrent NIRS-fMRI measurements. Neuroimage 2012, 59, 3933–3940. [Google Scholar] [CrossRef]
- Turnbull, L.; Hütt, M.-T.; Ioannides, A.A.; Kininmonth, S.; Poeppl, R.; Tockner, K.; Bracken, L.J.; Keesstra, S.; Liu, L.; Masselink, R.; et al. Connectivity and complex systems: Learning from a multi-disciplinary perspective. Appl. Netw. Sci. 2018, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Radicchi, F.; van den Heuvel, M.P.; Sporns, O. Discordant attributes of structural and functional brain connectivity in a two-layer multiplex network. Sci. Rep. 2019, 9, 2885. [Google Scholar] [CrossRef]
- Wu, L.; Caprihan, A.; Bustillo, J.; Mayer, A.; Calhoun, V. An approach to directly link ICA and seed-based functional connectivity: Application to schizophrenia. Neuroimage 2018, 179, 448–470. [Google Scholar] [CrossRef]
- Dennis, E.L.; Thompson, P.M. Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol. Rev. 2014, 24, 49–62. [Google Scholar] [CrossRef]
- Lu, C.M.; Zhang, Y.J.; Biswal, B.B.; Zang, Y.F.; Peng, D.L.; Zhu, C.Z. Use of fNIRS to assess resting state functional connectivity. J. Neurosci. Methods 2010, 186, 242–249. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.J.; Lu, C.M.; Ma, S.Y.; Zang, Y.F.; Zhu, C.Z. Functional connectivity as revealed by independent component analysis of resting-state fNIRS measurements. Neuroimage 2010, 51, 1150–1161. [Google Scholar] [CrossRef]
- White, B.R.; Snyder, A.Z.; Cohen, A.L.; Petersen, S.E.; Raichle, M.E.; Schlaggar, B.L.; Culver, J.P. Resting-state functional connectivity in the human brain revealed with diffuse optical tomography. Neuroimage 2009, 47, 148–156. [Google Scholar] [CrossRef]
- Šverko, Z.; Vrankić, M.; Vlahinić, S.; Rogelj, P. Complex Pearson Correlation Coefficient for EEG Connectivity Analysis. Sensors 2022, 22, 1477. [Google Scholar] [CrossRef] [PubMed]
- Joel, S.E.; Caffo, B.S.; van Zijl, P.C.; Pekar, J.J. On the relationship between seed-based and ICA-based measures of functional connectivity. Magn. Reson. Med. 2011, 66, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Gerton, B.K.; Brown, T.T.; Meyer-Lindenberg, A.; Kohn, P.; Holt, J.L.; Olsen, R.K.; Berman, K.F. Shared and distinct neurophysiological components of the digits forward and backward tasks as revealed by functional neuroimaging. Neuropsychologia 2004, 42, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Xiong, G.; Elkind, J.A.; Putnam, B.; Cohen, A.S. Brain Injury Impairs Working Memory and Prefrontal Circuit Function. Front. Neurol. 2015, 6, 240. [Google Scholar] [CrossRef] [PubMed]
- Haggstrom, L.; Welschinger, R.; Caplan, G.A. Functional neuroimaging offers insights into delirium pathophysiology: A systematic review. Australas. J. Ageing 2017, 36, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dang, M.; Zhang, Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: A systematic review of symptom-general and -specific lesion patterns. Mol. Neurodegener. 2021, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Barbey, A.K.; Koenigs, M.; Grafman, J. Dorsolateral prefrontal contributions to human working memory. Cortex 2013, 49, 1195–1205. [Google Scholar] [CrossRef]
- Jobson, D.D.; Hase, Y.; Clarkson, A.N.; Kalaria, R.N. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun. 2021, 3, fcab125. [Google Scholar] [CrossRef]
- Ford, J.H.; Kensinger, E.A. Older adults recruit dorsomedial prefrontal cortex to decrease negativity during retrieval of emotionally complex real-world events. Neuropsychologia 2019, 135, 107239. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Jiang, S.; Yang, H.; Czuma, R.; Yang, Y.; Kozel, F.A.; Jiang, H. Portable Diffuse Optical Tomography for Three-Dimensional Functional Neuroimaging in the Hospital. Photonics 2024, 11, 238. https://doi.org/10.3390/photonics11030238
Huang J, Jiang S, Yang H, Czuma R, Yang Y, Kozel FA, Jiang H. Portable Diffuse Optical Tomography for Three-Dimensional Functional Neuroimaging in the Hospital. Photonics. 2024; 11(3):238. https://doi.org/10.3390/photonics11030238
Chicago/Turabian StyleHuang, Jingyu, Shixie Jiang, Hao Yang, Richard Czuma, Ying Yang, F. Andrew Kozel, and Huabei Jiang. 2024. "Portable Diffuse Optical Tomography for Three-Dimensional Functional Neuroimaging in the Hospital" Photonics 11, no. 3: 238. https://doi.org/10.3390/photonics11030238
APA StyleHuang, J., Jiang, S., Yang, H., Czuma, R., Yang, Y., Kozel, F. A., & Jiang, H. (2024). Portable Diffuse Optical Tomography for Three-Dimensional Functional Neuroimaging in the Hospital. Photonics, 11(3), 238. https://doi.org/10.3390/photonics11030238





