Illuminating Life Sciences: A Biophysical Guide to the Use of Chromatic and White Light Sources in Photobiology
Abstract
:1. Introduction
2. Physical Perspective on Relevant Quantities
2.1. Present Energy and Photons
2.1.1. Irradiance
2.1.2. Photon Flux Density
2.2. Present Spectral Power Distribution
2.2.1. Chromatic Light Sources
2.2.2. White Light Sources
3. Materials and Methods
4. Results
4.1. Chromatic Light Sources
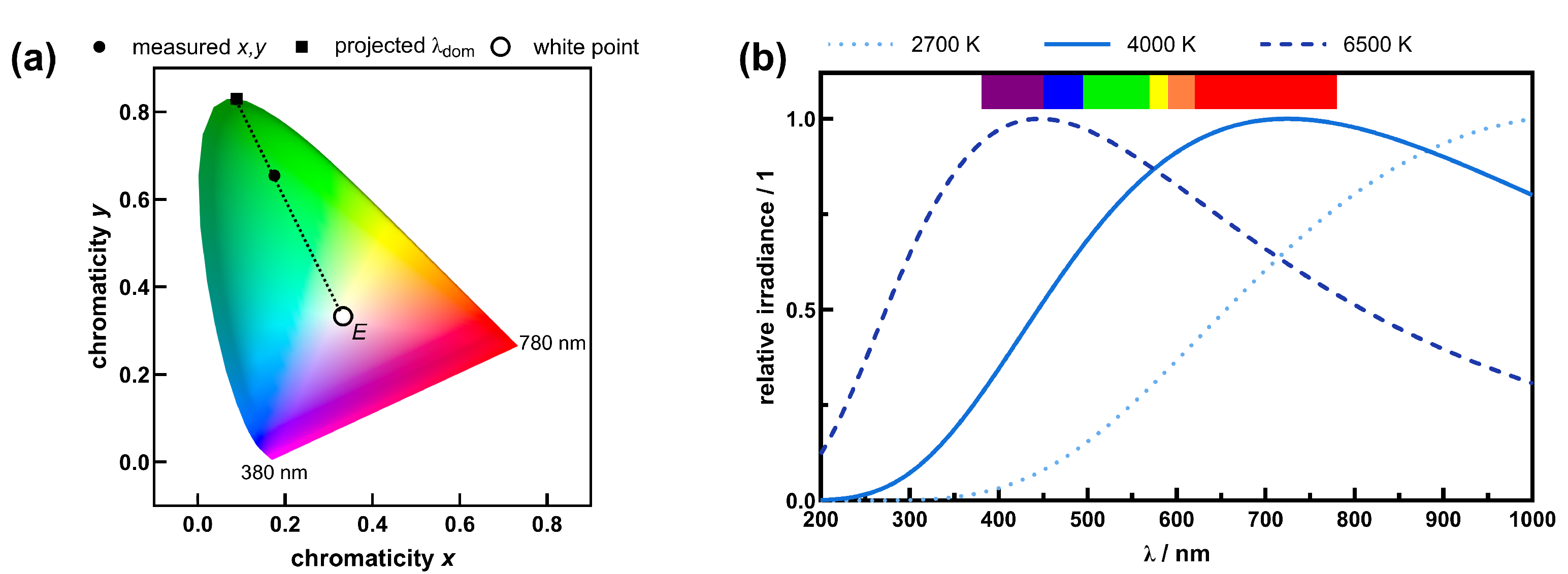
4.2. White Light Sources
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leister, D. Photobiology: Introduction, overview and challenges. Front. Photobiol. 2023, 1, 1253330. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-S.; Hitti, Y.; MacPherson, S.; Orsat, V.; Lefsrud, M.G. Comparison and perspective of conventional and LED lighting for photobiology and industry applications. Environ. Exp. Bot. 2020, 171, 103953. [Google Scholar] [CrossRef]
- Parrine, D.; Wu, B.-S.; Muhammad, B.; Rivera, K.; Pappin, D.; Zhao, X.; Lefsrud, M. Proteome modifications on tomato under extreme high light induced-stress. Proteome Sci. 2018, 16, 20. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.; He, S.; Shi, L.; Song, Y.; Lou, X.; He, D. LED-Supplied Red and Blue Light Alters the Growth, Antioxidant Status, and Photochemical Potential of in Vitro-Grown Gerbera jamesonii Plantlets. HST 2019, 37, 473–489. [Google Scholar] [CrossRef]
- Miyashita, K.; Tanakamaru, S.; Maitani, T.; Kimura, K. Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Environ. Exp. Bot. 2005, 53, 205–214. [Google Scholar] [CrossRef]
- Gutu, A.; Kehoe, D.M. Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol. Plant 2012, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod Control of Plant Growth: Flowering Time Genes Beyond Flowering. Front. Plant Sci. 2021, 12, 805635. [Google Scholar] [CrossRef]
- Hörnlein, C.; Confurius-Guns, V.; Stal, L.J.; Bolhuis, H. Daily rhythmicity in coastal microbial mats. npj Biofilms Microbiomes 2018, 4, 11. [Google Scholar] [CrossRef]
- Nitschke, S.; Cortleven, A.; Iven, T.; Feussner, I.; Havaux, M.; Riefler, M.; Schmülling, T. Circadian Stress Regimes Affect the Circadian Clock and Cause Jasmonic Acid-Dependent Cell Death in Cytokinin-Deficient Arabidopsis Plants. Plant Cell 2016, 28, 1616–1639. [Google Scholar] [CrossRef]
- Nitschke, S.; Cortleven, A.; Schmülling, T. Novel Stress in Plants by Altering the Photoperiod. Trends Plant Sci. 2017, 22, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Abuelsoud, W.; Cortleven, A.; Schmülling, T. Photoperiod stress induces an oxidative burst-like response and is associated with increased apoplastic peroxidase and decreased catalase activities. J. Plant Physiol. 2020, 253, 153252. [Google Scholar] [CrossRef] [PubMed]
- Cortleven, A.; Roeber, V.M.; Frank, M.; Bertels, J.; Lortzing, V.; Beemster, G.T.S.; Schmülling, T. Photoperiod Stress in Arabidopsis thaliana Induces a Transcriptional Response Resembling That of Pathogen Infection. Front. Plant Sci. 2022, 13, 838284. [Google Scholar] [CrossRef]
- Kehoe, D.M. Chromatic adaptation and the evolution of light color sensing in cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 9029–9030. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.L. Seeing new light: Recent insights into the occurrence and regulation of chromatic acclimation in cyanobacteria. Curr. Opin. Plant Biol. 2017, 37, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Colleselli, L.; Siewert, B.; Vrabl, P.; Schöbel, H. Optical simulations in life-sciences: Benefiting from ray-tracing in biotechnology and photobiology. Opt. Commun. 2024, 552, 130028. [Google Scholar] [CrossRef]
- Björn, L.O. Photobiology: The Science of Light and Life, 3rd ed.; Springer: New York, NY, USA, 2015; ISBN 978-1-4939-1467-8. [Google Scholar]
- Pearcy, R.W. Radiation and light measurements. In Plant Physiological Ecology: Field Methods and Instrumentation, 1st ed.; Pearcy, R.W., Ehleringer, J.R., Eds.; Chapman and Hall: London, UK, 1989; pp. 97–116. ISBN 978-0-412-40730-7. [Google Scholar]
- Arecchi, A. Field Guide to Illumination; SPIE: Bellingham, WA, USA, 2007; ISBN 9780819481221. [Google Scholar]
- Smith, T.; Guild, J. The C.I.E. colorimetric standards and their use. Trans. Opt. Soc. 1931, 33, 73–134. [Google Scholar] [CrossRef]
- CIE|International Commission on Illumination/Comission Internationale de l’Eclairage/Internationale Beleuchtungskommission. Available online: https://cie.co.at/ (accessed on 25 April 2024).
- Meyer-Arendt, J.R. Radiometry and photometry: Units and conversion factors. Appl. Opt. 1968, 7, 2081. [Google Scholar] [CrossRef] [PubMed]
- Einstein, A. Über einen die Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichtspunkt. Ann. Phys. 1905, 322, 132–148. [Google Scholar] [CrossRef]
- McCree, K.J. The measurement of photosynthetically active radiation. Sol. Energy 1973, 15, 83–87. [Google Scholar] [CrossRef]
- Walter, A.; Schöbel, H. Shed light on photosynthetic organisms: A physical perspective to correct light measurements. Photosynth. Res. 2023, 156, 325–336. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulae, 2nd ed.; Wiley Classics Library, Ed.; Wiley: New York, NY, USA; Weinheim, Germany, 2000; ISBN 9780471399186. [Google Scholar]
- Schanda, J. Colorimetry: Understanding the CIE System; CIE/Commission Internationale de l’Eclairage: Vienna, Austria, 2007; ISBN 9780470175620. [Google Scholar]
- Yao, Q.; Ju, J.; Liang, R.; Chen, D.; Zhao, H. Relationship between Peak Wavelength and Dominant Wavelength of Light Sources Based on Vector-Based Dominant Wavelength Calculation Method. LEUKOS 2014, 10, 11–18. [Google Scholar] [CrossRef]
- Billmeyer, F.W.; Fairman, H.S. CIE Method for Calculating Tristimulus Values. Color Res. Appl. 1987, 12, 27–36. [Google Scholar] [CrossRef]
- CIE. CIE 1931 Colour-Matching Functions, 2 Degree Observer; International Commission on Illumination (CIE): Vienna, Austria, 2019. [Google Scholar]
- Planck, M. Ueber das Gesetz der Energieverteilung im Normalspectrum. Ann. Der Phys. 1901, 309, 553–563. [Google Scholar] [CrossRef]
- Schanda, J.; Danyi, M. Correlated Color-Temperature Calculations in the CIE 1976 Chromaticity Diagram. Color Res. Appl. 1977, 2, 161–163. [Google Scholar] [CrossRef]
- The Mathworks, I. MATLAB R2024b; MathWorks: Natick, MA, USA, 2024. [Google Scholar]
- PhotochemCAD Chemicals. Available online: https://www.photochemcad.com/ (accessed on 15 May 2024).
- Bruno, T.J. CRC Handbook of Fundamental Spectroscopic Correlation Charts; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429129780. [Google Scholar]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Schuurmans, J.M.; Verschoor, A.M.; Hellingwerf, K.J.; Huisman, J.; Matthijs, H.C.P. Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynth. Res. 2018, 138, 177–189. [Google Scholar] [CrossRef]
- Pagels, F.; Lopes, G.; Vasconcelos, V.; Guedes, A.C. White and red LEDs as two-phase batch for cyanobacterial pigments production. Bioresour. Technol. 2020, 307, 123105. [Google Scholar] [CrossRef]
- Fuente, D.; Keller, J.; Conejero, J.A.; Rögner, M.; Rexroth, S.; Urchueguía, J.F. Light distribution and spectral composition within cultures of micro-algae: Quantitative modelling of the light field in photobioreactors. Algal Res. 2017, 23, 166–177. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, N.; Sidiroglou, F.; Fraser, S.; Husaric, M.; Kiatos, D.; Apostolopoulos, V.; Feehan, J. The effects of polarized photobiomodulation on cellular viability, proliferation, mitochondrial membrane potential and apoptosis in human fibroblasts: Potential applications to wound healing. J. Photochem. Photobiol. B 2022, 236, 112574. [Google Scholar] [CrossRef]
- Volkov, V. Discovering electrophysiology in photobiology: A brief overview of several photobiological processes with an emphasis on electrophysiology. Commun. Integr. Biol. 2014, 7, e28423. [Google Scholar] [CrossRef]
- Polyakov, S.; Yoshino, F.; Liu, M.; Stegeman, G. Nonlinear refraction and multiphoton absorption in polydiacetylenes from 1200 to 2200 nm. Phys. Rev. B 2004, 69, 115421. [Google Scholar] [CrossRef]
- Stamford, J.D.; Stevens, J.; Mullineaux, P.M.; Lawson, T. LED Lighting: A Grower’s Guide to Light Spectra. HortScience 2023, 58, 180–196. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Livadariu, O.; Maximilian, C.; Rahmanifar, B.; Cornea, C.P. LED Technology Applied to Plant Development for Promoting the Accumulation of Bioactive Compounds: A Review. Plants 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Royer, M.; Murdoch, M.J.; Smet, K.; Whitehead, L.; David, A.; Houser, K.; Esposito, T.; Livingston, J.; Ohno, Y. Improved Method for Evaluating and Specifying the Chromaticity of Light Sources. LEUKOS 2023, 19, 35–52. [Google Scholar] [CrossRef]
- Martines-Arano, H.; Valdivia-Flores, A.; Castillo-Cruz, J.; García-Pérez, B.E.; Torres-Torres, C. Spatially modulated ablation driven by chaotic attractors in human lung epithelial cancer cells. Biomed. Phys. Eng. Express 2024, 10, 35041. [Google Scholar] [CrossRef]
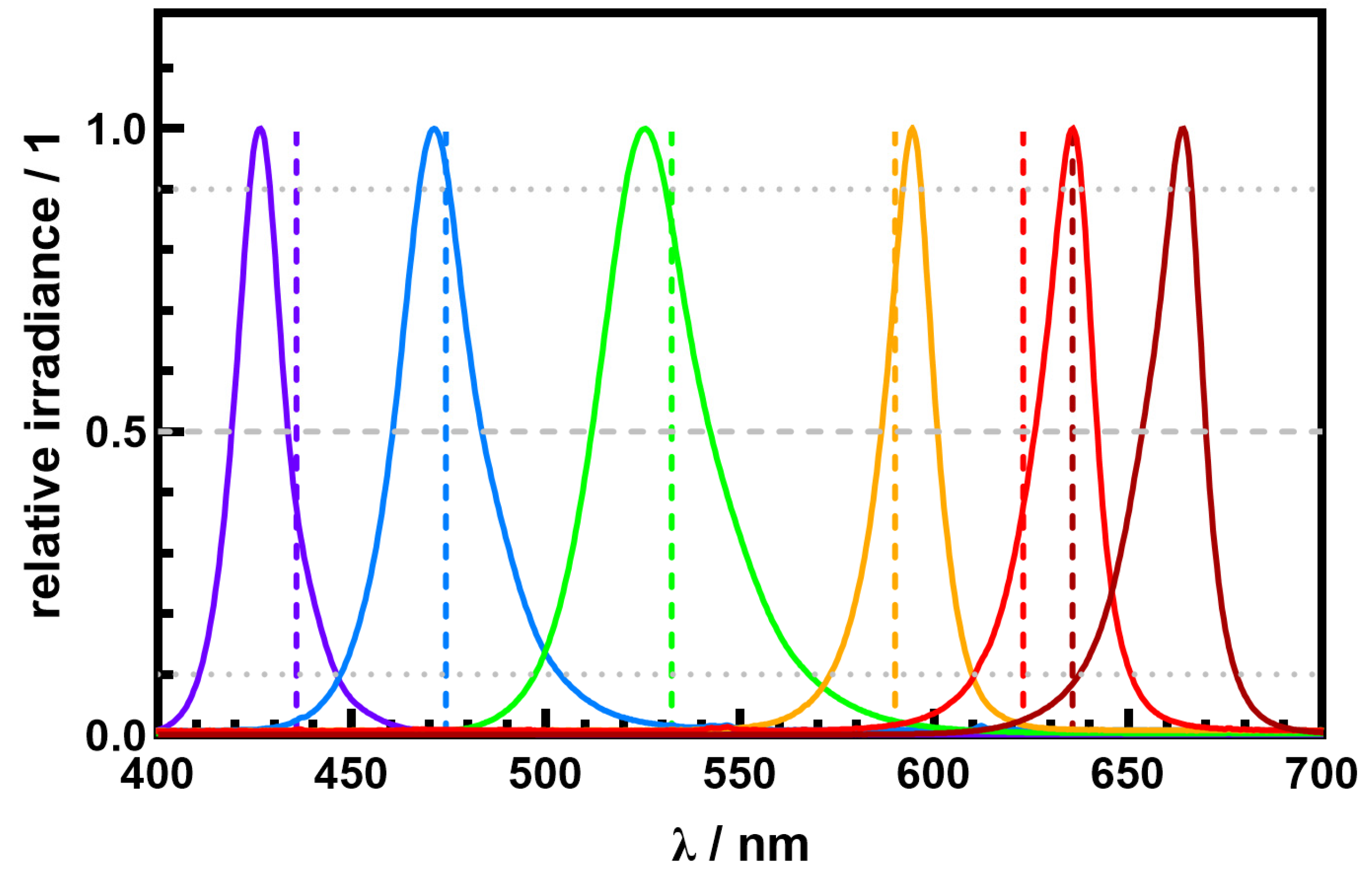


| Color | LED Product Family | ||||
|---|---|---|---|---|---|
| violet | Luxeon CZ | 426 | 436 | 356 | 364 |
| blue | Oslon SSL | 441 | 447 | 369 | 374 |
| blue | Luxeon 3535L | 471 | 474 | 384 | 397 |
| green | Luxeon 3535L | 521 | 526 | 435 | 440 |
| green | Oslon SSL | 520 | 525 | 435 | 439 |
| amber | Oslon SSL | 594 | 590 | 496 | 494 |
| red | Luxeon 3535L | 635 | 623 | 531 | 521 |
| hyper red | Oslon SSL | 663 | 636 | 554 | 531 |
| CCT Nominal | Model | Chromaticity Coordinates | CCT Measured | ||
|---|---|---|---|---|---|
| x | y | ||||
| 2700 K | LED | 0.471 | 0.419 | 2620 K | 500 |
| 2700 K | fluorescent tube | 0.465 | 0.422 | 2710 K | 505 |
| 3000 K | LED | 0.441 | 0.405 | 2940 K | 494 |
| 3000 K | fluorescent tube | 0.440 | 0.402 | 2930 K | 495 |
| 4000 K | LED | 0.390 | 0.387 | 3830 K | 481 |
| 4000 K | fluorescent tube | 0.386 | 0.385 | 3970 K | 481 |
| 6500 K | LED | 0.317 | 0.337 | 6250 K | 461 |
| 6500 K | fluorescent tube | 0.320 | 0.345 | 6080 K | 466 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutschlechner, M.; Schöbel, H. Illuminating Life Sciences: A Biophysical Guide to the Use of Chromatic and White Light Sources in Photobiology. Photonics 2024, 11, 487. https://doi.org/10.3390/photonics11060487
Mutschlechner M, Schöbel H. Illuminating Life Sciences: A Biophysical Guide to the Use of Chromatic and White Light Sources in Photobiology. Photonics. 2024; 11(6):487. https://doi.org/10.3390/photonics11060487
Chicago/Turabian StyleMutschlechner, Mira, and Harald Schöbel. 2024. "Illuminating Life Sciences: A Biophysical Guide to the Use of Chromatic and White Light Sources in Photobiology" Photonics 11, no. 6: 487. https://doi.org/10.3390/photonics11060487





