Fluorometric Mercury (II) Detection Using Heteroatom-Doped Carbon and Graphene Quantum Dots
Abstract
:1. Introduction
2. CQDs and GQDs as Promising Fluorescent Probes
3. Optical Features of Carbon and Graphene Quantum Dots
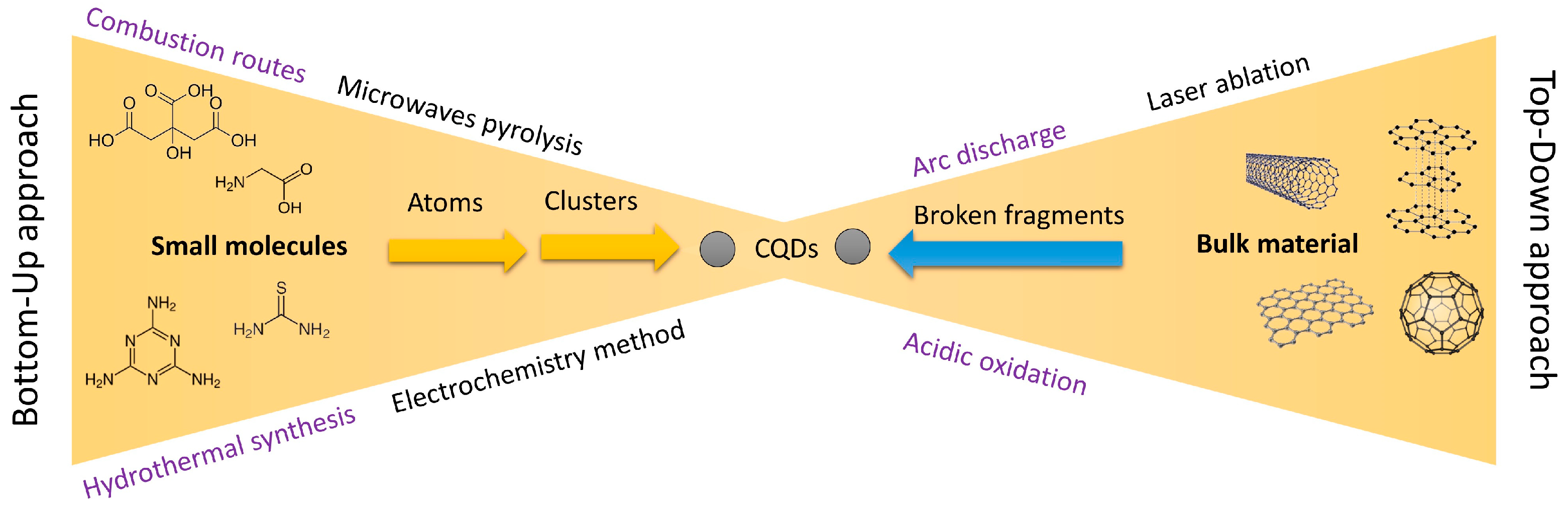
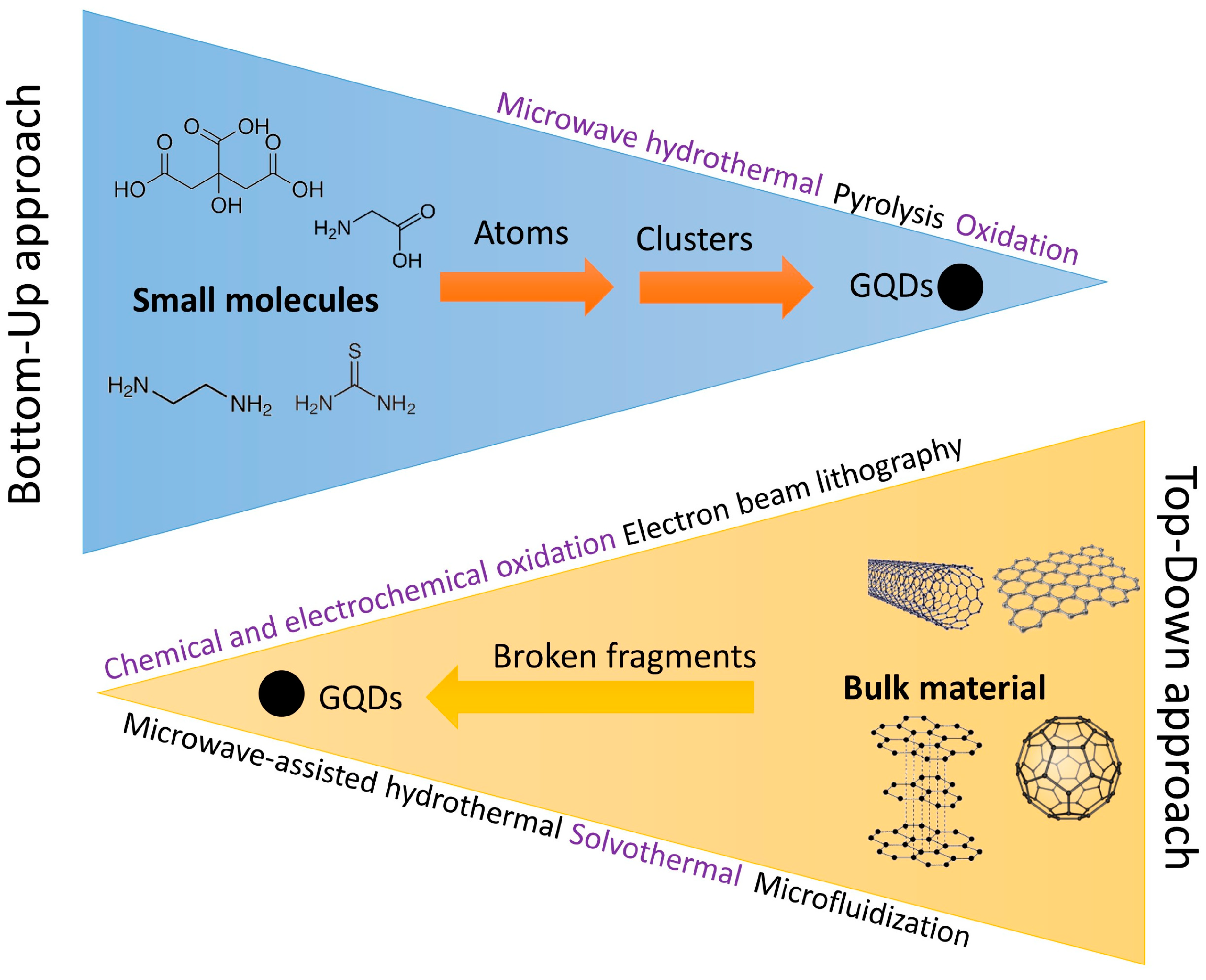
4. Synthesis Methods of Carbon and Graphene Quantum Dots
5. Doping Carbon and Graphene Quantum Dots with Heteroatoms
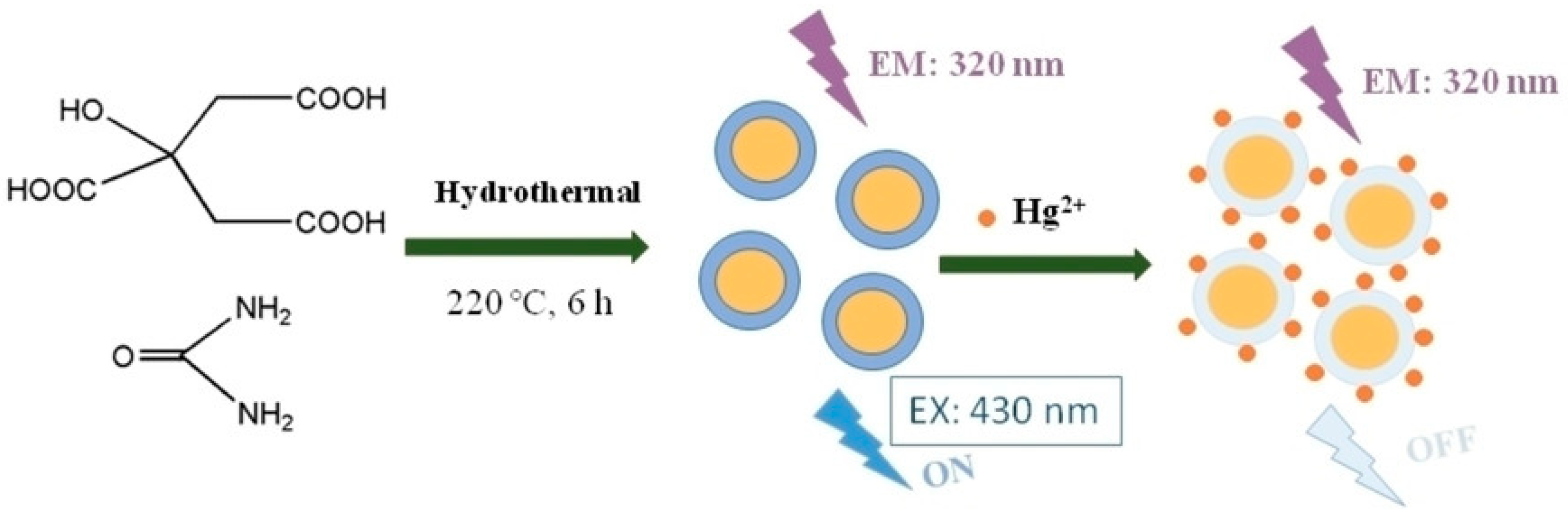
6. Using Carbon and Graphene Quantum Dots for Quantitative Analysis
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Weng, Y.; Zheng, L.; Yao, B.; Weng, W.; Lin, X. Nitrogen-doped carbon quantum dots as fluorescent probe for “off-on” detection of mercury ions, L-cysteine and iodide ions. J. Colloid Interface Sci. 2017, 506, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Guo, Q.; Chen, D.; Liu, Z.; Zheng, X.; Xu, A.; Yang, S.; Ding, G. Facile and Highly Effective Synthesis of Controllable Lattice Sulfur-Doped Graphene Quantum Dots via Hydrothermal Treatment of Durian. ACS Appl. Mater. Interfaces 2018, 10, 5750–5759. [Google Scholar] [CrossRef]
- Pajewska-Szmyt, M.; Buszewski, B.; Gadzała-Kopciuch, R. Carbon dots as rapid assays for detection of mercury (II) ions based on turn-off mode and breast milk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 236, 118320. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Reza Fathi, M.; Shokrollahi, A.; Shajarat, F. Highly selective and sensitive preconcentration of mercury ion and determination by cold vapor atomic absorption spectroscopy. Anal. Lett. 2006, 39, 1171–1185. [Google Scholar] [CrossRef]
- Lee, S.H.; Suh, J.K. Determination of mercury in tuna fish tissue using isotope dilution-inductively coupled plasma mass spectrometry. Microchem. J. 2005, 80, 233–236. [Google Scholar]
- Krata, A.; Vassileva, E.; Bulska, E. Reference measurements for total mercury and methyl mercury content in marine biota samples using direct or species-specific isotope dilution inductively coupled plasma mass spectrometry. Talanta 2016, 160, 562–569. [Google Scholar] [CrossRef]
- KopyŚĆ, E.; PyrzyŃslka, K.; Garbos, S.; Buska, E. Determination of mercury by cold-vapor atomic absorption spectrometry with preconcentration on a gold-trap. Anal. Sci. 2000, 16, 1309–1312. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghanemi, K. Solid phase extraction of cadmium on 2-mercaptobenzothiazole loaded on sulfur powder in the medium of ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate and cold vapor generation–atomic absorption spectrometric determination. J. Hazard. Mater. 2010, 178, 566–571. [Google Scholar] [CrossRef]
- Erxleben, H.; Ruzicka, J. Atomic Absorption Spectroscopy for Mercury, Automated by Sequential Injection and Miniaturized in Lab-on-Valve System. Anal. Chem. 2005, 77, 5124–5128. [Google Scholar] [CrossRef]
- Pfeil, D.L.; Bruce, M.L. Automated determination of mercury cold vapor atomic fluorescence with gold amalgamation. Am. Lab. 2001, 33, 26–33. [Google Scholar]
- Srinivasan, K.; Subramanian, K.; Murugan, K.; Dinakaran, K. Sensitive fluorescence detection of mercury (II) in aqueous solution by the fluorescence quenching effect of MoS2 with DNA functionalized carbon dots. Analyst 2016, 141, 6344–6352. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, W.; Kumar, A.; Jin, S.; Zhao, Y.; Zhang, C.; Zou, G.; Wang, P.C.; Li, F.; Liang, X.-J. Highly sensitive simultaneous detection of mercury and copper ions by ultrasmall fluorescent DNA–Ag nanoclusters. New J. Chem. 2014, 38, 1546–1550. [Google Scholar] [CrossRef]
- Nain, A.; Tseng, Y.-T.; Lin, Y.-S.; Wei, S.-C.; Mandal, R.P.; Unnikrishnan, B.; Huang, C.-C.; Tseng, F.-G.; Chang, H.-T. Tuning the photoluminescence of metal nanoclusters for selective detection of multiple heavy metal ions. Sens. Actuators B Chem. 2020, 321, 128539. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M. Synthesis of metal nanoclusters and their application in Hg2+ ions detection: A review. J. Hazard. Mater. 2022, 424, 127565. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, C.; Zhang, Y.; Rong, X.; Wang, X.; Li, X.; Wang, K.; Zhu, H.; Zhu, B. Rational design of a water-soluble TICT-AIEE-active fluorescent probe for mercury ion detection. Anal. Chim. Acta 2022, 1230, 340337. [Google Scholar] [CrossRef]
- Yoon, S.; Miller, E.W.; He, Q.; Do, P.H.; Chang, C.J. A bright and specific fluorescent sensor for mercury in water, cells, and tissue. Angew. Chem. 2007, 119, 6778–6781. [Google Scholar] [CrossRef]
- Ribeiro, D.S.M.; Castro, R.C.; Páscoa, R.N.M.J.; Soares, J.X.; Rodrigues, S.S.M.; Santos, J.L.M. Tuning CdTe quantum dots reactivity for multipoint detection of mercury (II), silver (I) and copper (II). J. Lumin. 2019, 207, 386–396. [Google Scholar] [CrossRef]
- Labeb, M.; Sakr, A.-H.; Soliman, M.; Abdel-Fattah, T.M.; Ebrahim, S. Effect of capping agent on selectivity and sensitivity of CdTe quantum dots optical sensor for detection of mercury ions. Opt. Mater. 2018, 79, 331–335. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Zhao, Q.; Hou, Y.; Chen, J. Ultrasensitive quantum dot fluorescence quenching assay for selective detection of mercury ions in drinking water. Sci. Rep. 2014, 4, 5624. [Google Scholar] [CrossRef]
- Bano, D.; Kumar, V.; Singh, V.K.; Hasan, S.H. Green synthesis of fluorescent carbon quantum dots for the detection of mercury (II) and glutathione. New J. Chem. 2018, 42, 5814–5821. [Google Scholar] [CrossRef]
- Kou, X.; Jiang, S.; Park, S.-J.; Meng, L.-Y. A review: Recent advances in preparations and applications of heteroatom-doped carbon quantum dots. Dalton Trans. 2020, 49, 6915–6938. [Google Scholar] [CrossRef] [PubMed]
- Azami, M.; Wei, J.; Valizadehderakhshan, M.; Jayapalan, A.; Ayodele, O.O.; Nowlin, K. Effect of Doping Heteroatoms on the Optical Behaviors and Radical Scavenging Properties of Carbon Nanodots. J. Phys. Chem. C 2023, 127, 7360–7370. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, N.; Dong, J.X.; Luo, H.Q.; Li, N.B. Fluorescence detection of mercury ions and cysteine based on magnesium and nitrogen co-doped carbon quantum dots and IMPLICATION logic gate operation. Sens. Actuators B Chem. 2016, 231, 147–153. [Google Scholar] [CrossRef]
- Pajewska-Szmyt, M.; Buszewski, B.; Gadzała-Kopciuch, R. Sulphur and nitrogen doped carbon dots synthesis by microwave assisted method as quantitative analytical nano-tool for mercury ion sensing. Mater. Chem. Phys. 2020, 242, 122484. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hayat, H.; Abbas, A.; Narayan, R.J. Graphene Quantum Dots-Based Electrochemical Biosensing Platform for Early Detection of Acute Myocardial Infarction. Biosensors 2022, 12, 77. [Google Scholar] [CrossRef]
- Javed, N.; O’Carroll, D.M. Carbon dots and stability of their optical properties. Part. Part. Syst. Charact. 2021, 38, 2000271. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Jagannathan, M.; Dhinasekaran, D.; Soundharraj, P.; Rajendran, S.; Vo, D.-V.N.; Prakasarao, A.; Ganesan, S. Green synthesis of white light emitting carbon quantum dots: Fabrication of white fluorescent film and optical sensor applications. J. Hazard. Mater. 2021, 416, 125091. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, S.W.; Kim, M.-K.; Shin, D.Y.; Shin, D.H.; Kim, C.O.; Yang, S.B.; Park, J.H.; Hwang, E.; Choi, S.-H. Anomalous behaviors of visible luminescence from graphene quantum dots: Interplay between size and shape. ACS Nano 2012, 6, 8203–8208. [Google Scholar] [CrossRef]
- Ahirwar, S.; Mallick, S.; Bahadur, D. Electrochemical method to prepare graphene quantum dots and graphene oxide quantum dots. ACS Omega 2017, 2, 8343–8353. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dhand, C.; Dwivedi, N.; Singh, S.; Khan, R.; Verma, S.; Singh, A.; Gupta, M.K.; Kumar, S.; Kumar, R. Graphene quantum dots: A contemporary perspective on scope, opportunities, and sustainability. Renew. Sustain. Energy Rev. 2022, 157, 111993. [Google Scholar] [CrossRef]
- Sheely, A.; Gifford, B.; Tretiak, S.; Bishop, A. Tunable Optical Features of Graphene Quantum Dots from Edge Functionalization. J. Phys. Chem. C 2021, 125, 9244–9252. [Google Scholar] [CrossRef]
- Tang, S.; Chen, D.; Wang, C.; Yang, Y.; Li, X.; Li, T.; Zhang, X. Preparation of concentration dependent nitrogen sulfur Co-doped graphene quantum dots by UV irradiation and reversible optical switch of hydroxypropyl methyl cellulose. Dye. Pigment. 2022, 198, 110022. [Google Scholar] [CrossRef]
- Alas, M.O.; Alkas, F.B.; Aktas Sukuroglu, A.; Genc Alturk, R.; Battal, D. Fluorescent carbon dots are the new quantum dots: An overview of their potential in emerging technologies and nanosafety. J. Mater. Sci. 2020, 55, 15074–15105. [Google Scholar] [CrossRef]
- Vibhute, A.; Patil, T.; Gambhir, R.; Tiwari, A.P. Fluorescent carbon quantum dots: Synthesis methods, functionalization and biomedical applications. Appl. Surf. Sci. Adv. 2022, 11, 100311. [Google Scholar] [CrossRef]
- Gulati, S.; Baul, A.; Amar, A.; Wadhwa, R.; Kumar, S.; Varma, R.S. Eco-Friendly and Sustainable Pathways to Photoluminescent Carbon Quantum Dots (CQDs). Nanomaterials 2023, 13, 554. [Google Scholar] [CrossRef]
- Xu, A.; Wang, G.; Li, Y.; Dong, H.; Yang, S.; He, P.; Ding, G. Carbon-based quantum dots with solid-state photoluminescent: Mechanism, implementation, and application. Small 2020, 16, 2004621. [Google Scholar] [CrossRef]
- Gaurav, A.; Jain, A.; Tripathi, S.K. Review on Fluorescent Carbon/Graphene Quantum Dots: Promising Material for Energy Storage and Next-Generation Light-Emitting Diodes. Materials 2022, 15, 7888. [Google Scholar] [CrossRef]
- Magesh, V.; Sundramoorthy, A.K.; Ganapathy, D. Recent advances on synthesis and potential applications of carbon quantum dots. Front. Mater. 2022, 9, 906838. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Zhao, Y.; Sun, R.-C.; Zhong, L.; Peng, X. Facile and high-yield synthesis of carbon quantum dots from biomass-derived carbons at mild condition. ACS Sustain. Chem. Eng. 2019, 7, 7833–7843. [Google Scholar] [CrossRef]
- Kalluri, A.; Debnath, D.; Dharmadhikari, B.; Patra, P. Chapter Twelve—Graphene Quantum Dots: Synthesis and Applications. In Methods in Enzymology; Kumar, C.V., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 335–354. [Google Scholar]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining carbon dots: Synthesis and biomedical and optoelectronic applications. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Fernando, K.A.S.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.-P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Qi, G.; Chen, K.; Zou, M.; Yuwen, L.; Zhang, X.; Huang, W.; Wang, L. Microwave-assisted preparation of white fluorescent graphene quantum dots as a novel phosphor for enhanced white-light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2739–2744. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.-T. Upconversion and downconversion fluorescent graphene quantum dots: Ultrasonic preparation and photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef]
- Li, L.L.; Ji, J.; Fei, R.; Wang, C.Z.; Lu, Q.; Zhang, J.R.; Jiang, L.P.; Zhu, J.J. A facile microwave avenue to electrochemiluminescent two-color graphene quantum dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Rajender, G.; Giri, P.K. Formation mechanism of graphene quantum dots and their edge state conversion probed by photoluminescence and Raman spectroscopy. J. Mater. Chem. C 2016, 4, 10852–10865. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, C.; Zheng, X.; Gao, L.; Cui, Z.; Yang, H.; Guo, C.; Chi, Y.; Li, C.M. One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J. Mater. Chem. 2012, 22, 8764–8766. [Google Scholar] [CrossRef]
- Jin, H.; Huang, H.; He, Y.; Feng, X.; Wang, S.; Dai, L.; Wang, J. Graphene Quantum Dots Supported by Graphene Nanoribbons with Ultrahigh Electrocatalytic Performance for Oxygen Reduction. J. Am. Chem. Soc. 2015, 137, 7588–7591. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Tabish, T.A.; Bull, S.J.; Lim, T.M.; Phan, A.N. High yield synthesis of graphene quantum dots from biomass waste as a highly selective probe for Fe3+ sensing. Sci. Rep. 2020, 10, 21262. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarkhah, A.; Hosseini, E.; Kamkar, M.; Sehat, A.A.; Dordanihaghighi, S.; Allahbakhsh, A.; van der Kuur, C.; Arjmand, M. Synthesis, applications, and prospects of graphene quantum dots: A comprehensive review. Small 2022, 18, 2102683. [Google Scholar] [CrossRef] [PubMed]
- Somaraj, G.; Mathew, S.; Abraham, T.; Ambady, K.G.; Mohan, C.; Mathew, B. Nitrogen and Sulfur Co-Doped Carbon Quantum Dots for Sensing Applications: A Review. ChemistrySelect 2022, 7, e202200473. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Li, B.; Fan, X.; Wang, W.; Liu, P.; Xu, S.; Luo, X. Nitrogen doped carbon dots: Mechanism investigation and their application for label free CA125 analysis. J. Mater. Chem. B 2019, 7, 3053–3058. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Baragau, I.-A.; Gromicova, R.; Nicolaev, A.; Thomson, S.A.J.; Rennie, A.; Power, N.P.; Sajjad, M.T.; Kellici, S. Investigating the effect of N-doping on carbon quantum dots structure, optical properties and metal ion screening. Sci. Rep. 2022, 12, 13806. [Google Scholar] [CrossRef]
- Yang, S.; Sun, J.; Li, X.; Zhou, W.; Wang, Z.; He, P.; Ding, G.; Xie, X.; Kang, Z.; Jiang, M. Large-scale fabrication of heavy doped carbon quantum dots with tunable-photoluminescence and sensitive fluorescence detection. J. Mater. Chem. A 2014, 2, 8660–8667. [Google Scholar] [CrossRef]
- Cheng, Z.; Du, F.; Sun, L.; Jiang, L.; Ruan, G.; Li, J. Nitrogen-Doped Carbon Quantum Dots as a “Turn-Off” Fluorescent Probes for Highly Selective and Sensitive Detection of Mercury (II) Ions. ChemistrySelect 2019, 4, 2122–2128. [Google Scholar] [CrossRef]
- Luo, L.; Wang, P.; Wang, Y.; Wang, F. pH assisted selective detection of Hg (II) and Ag (I) based on nitrogen-rich carbon dots. Sens. Actuators B Chem. 2018, 273, 1640–1647. [Google Scholar] [CrossRef]
- Guo, H.; Raj, J.; Wang, Z.; Zhang, T.; Wang, K.; Lin, L.; Hou, W.; Zhang, J.; Wu, M.; Wu, J. Synergistic Effects of Amine Functional Groups and Enriched-Atomic-Iron Sites in Carbon Dots for Industrial-Current–Density CO2 Electroreduction. Small 2024, 20, 2311132. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z.; Song, L.; Guo, W.; Gao, W.; Ci, L.; Rao, A.; Quan, W.; Vajtai, R.; Ajayan, P.M. Synthesis of S-doped graphene by liquid precursor. Nanotechnology 2012, 23, 275605. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Jia, Q.; Liu, W.; Guo, L.; Liu, Q.; Lan, M.; Zhang, H.; Meng, X.; Wang, P. Red-emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Adv. Mater. 2015, 27, 4169–4177. [Google Scholar] [CrossRef]
- Wu, F.; Yang, M.; Zhang, H.; Zhu, S.; Zhu, X.; Wang, K. Facile synthesis of sulfur-doped carbon quantum dots from vitamin B1 for highly selective detection of Fe3+ ion. Opt. Mater. 2018, 77, 258–263. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Cao, J.; Zhu, J.; Fan, L.; Li, X. Sulfur-Doped Graphene Quantum Dots as a Novel Fluorescent Probe for Highly Selective and Sensitive Detection of Fe3+. Anal. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yang, H.; Lee, J.; Cho, H.-H.; Kim, D.; Lee, D.C.; Kim, B.J. Multicolor Emitting Block Copolymer-Integrated Graphene Quantum Dots for Colorimetric, Simultaneous Sensing of Temperature, pH, and Metal Ions. Chem. Mater. 2015, 27, 5288–5294. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Z.; Guo, H.; Lei, Z.; Liu, Z.; Wang, L. Solvent engineering of oxygen-enriched carbon dots for efficient electrochemical hydrogen peroxide production. Small 2023, 19, 2303156. [Google Scholar] [CrossRef]
- Hu, B.; Huang, K.; Tang, B.; Lei, Z.; Wang, Z.; Guo, H.; Lian, C.; Liu, Z.; Wang, L. Graphene quantum dot-mediated atom-layer semiconductor electrocatalyst for hydrogen evolution. Nano-Micro Lett. 2023, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Yarur, F.; Macairan, J.-R.; Naccache, R. Ratiometric detection of heavy metal ions using fluorescent carbon dots. Environ. Sci. Nano 2019, 6, 1121–1130. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Ren, Y.; Sun, X.; Pan, W.; Wang, J. The selectivity of the carboxylate groups terminated carbon dots switched by buffer solutions for the detection of multi-metal ions. Sens. Actuators B Chem. 2017, 240, 941–948. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Park, T.J.; Kailasa, S.K. Glutathione-capped Syzygium cumini carbon dot-amalgamated agarose hydrogel film for naked-eye detection of heavy metal ions. J. Anal. Sci. Technol. 2020, 11, 13. [Google Scholar] [CrossRef]
- Amjadi, M.; Jalili, R. A molecularly imprinted dual-emission carbon dot-quantum dot mesoporous hybrid for ratiometric determination of anti-inflammatory drug celecoxib. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 345–351. [Google Scholar] [CrossRef]
- Shahshahanipour, M.; Rezaei, B.; Ensafi, A.A.; Etemadifar, Z. An ancient plant for the synthesis of a novel carbon dot and its applications as an antibacterial agent and probe for sensing of an anti-cancer drug. Mater. Sci. Eng. C 2019, 98, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.B.; Contreras-Cáceres, R.; Bandosz, T.J.; Jiménez-Jiménez, J.; Rodríguez-Castellón, E.; da Silva, J.C.E.; Algarra, M. Carbon dots as fluorescent sensor for detection of explosive nitrocompounds. Carbon 2016, 106, 171–178. [Google Scholar] [CrossRef]
- Babar, D.G.; Garje, S.S. Nitrogen and phosphorus co-doped carbon dots for selective detection of nitro explosives. ACS Omega 2020, 5, 2710–2717. [Google Scholar] [CrossRef]
- Li, J.; Du, B.; Li, Y.; Wang, Y.; Wu, D.; Wei, Q. A turn-on fluorescent sensor for highly sensitive mercury (II) detection based on a carbon dot-labeled oligodeoxyribonucleotide and MnO2 nanosheets. New J. Chem. 2018, 42, 1228–1234. [Google Scholar] [CrossRef]
- Mandal, P.; Sahoo, D.; Sarkar, P.; Chakraborty, K.; Das, S. Fluorescence turn-on and turn-off sensing of pesticides by carbon dot-based sensor. New J. Chem. 2019, 43, 12137–12151. [Google Scholar] [CrossRef]
- Ashrafi Tafreshi, F.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS ONE 2020, 15, e0230646. [Google Scholar] [CrossRef] [PubMed]
- Moonrinta, S.; Kwon, B.; In, I.; Kladsomboon, S.; Sajomsang, W.; Paoprasert, P. Highly biocompatible yogurt-derived carbon dots as multipurpose sensors for detection of formic acid vapor and metal ions. Opt. Mater. 2018, 81, 93–101. [Google Scholar] [CrossRef]
- Gu, J.; Hu, D.; Wang, W.; Zhang, Q.; Meng, Z.; Jia, X.; Xi, K. Carbon dot cluster as an efficient “off–on” fluorescent probe to detect Au (III) and glutathione. Biosens. Bioelectron. 2015, 68, 27–33. [Google Scholar] [CrossRef]
- Maiti, S.; Das, K.; Das, P.K. Label-free fluorimetric detection of histone using quaternized carbon dot–DNA nanobiohybrid. Chem. Commun. 2013, 49, 8851–8853. [Google Scholar] [CrossRef]
- Freire, R.; Le, N.D.; Jiang, Z.; Kim, C.S.; Rotello, V.M.; Fechine, P. NH2-rich Carbon Quantum Dots: A protein-responsive probe for detection and identification. Sens. Actuators B Chem. 2018, 255, 2725–2732. [Google Scholar] [CrossRef]
- Godavarthi, S.; Kumar, K.M.; Vélez, E.V.; Hernandez-Eligio, A.; Mahendhiran, M.; Hernandez-Como, N.; Aleman, L.; Gomez, L.M. Nitrogen doped carbon dots derived from Sargassum fluitans as fluorophore for DNA detection. J. Photochem. Photobiol. B Biol. 2017, 172, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Nandi, S.; Jelinek, R. Carbon-dot–hydrogel for enzyme-mediated bacterial detection. RSC Adv. 2017, 7, 588–594. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Ghanbari, D.; Salavati-Niasari, M. Photoluminescence carbon dot as a sensor for detecting of Pseudomonas aeruginosa bacteria: Hydrothermal synthesis of magnetic hollow NiFe2O4-carbon dots nanocomposite material. Compos. Part B Eng. 2019, 161, 564–577. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, H.; Nian, Q.; Xu, Q. Electrospun chitosan/polyethylene oxide nanofibers mat loaded with copper (II) as a new sensor for colorimetric detection of tetracycline. Int. J. Biol. Macromol. 2022, 212, 527–535. [Google Scholar] [CrossRef]
- Msto, R.K.; Othman, H.O.; Al-Hashimi, B.R.; Salahuddin Ali, D.; Hassan, D.H.; Hassan, A.Q.; Smaoui, S. Fluorescence Turns on-off-on Sensing of Ferric Ion and L-Ascorbic Acid by Carbon Quantum Dots. J. Food Qual. 2023, 2023, 5555608. [Google Scholar] [CrossRef]
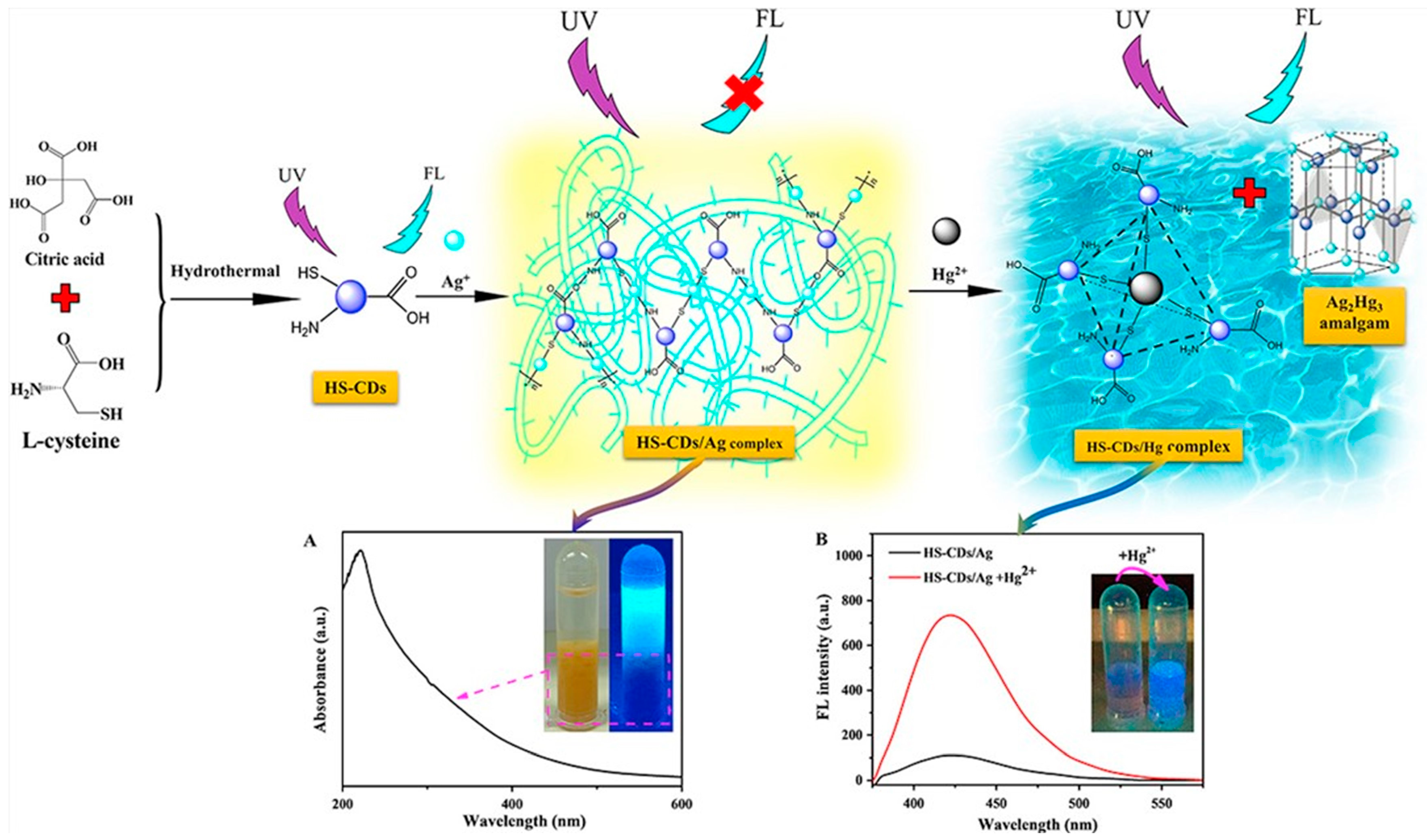
- Han, Y.; Shi, L.; Luo, X.; Chen, X.; Yang, W.; Tang, W.; Wang, J.; Yue, T.; Li, Z. A signal-on fluorescent sensor for ultra-trace detection of Hg2+ via Ag+ mediated sulfhydryl functionalized carbon dots. Carbon 2019, 149, 355–363. [Google Scholar] [CrossRef]
- Gan, Z.; Hu, X.; Huang, X.; Li, Z.; Zou, X.; Shi, J.; Zhang, W.; Li, Y.; Xu, Y. A dual-emission fluorescence sensor for ultrasensitive sensing mercury in milk based on carbon quantum dots modified with europium (III) complexes. Sens. Actuators B Chem. 2021, 328, 128997. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Ghanbari, D.; Amiri, O.; Salavati-Niasari, M. Electro-spinning of cellulose acetate nanofibers/Fe/carbon dot as photoluminescence sensor for mercury (II) and lead (II) ions. Carbohydr. Polym. 2020, 229, 115428. [Google Scholar] [CrossRef]
- Ghanem, A.; Al-Marjeh, R.A.-Q.B.; Atassi, Y. Novel nitrogen-doped carbon dots prepared under microwave-irradiation for highly sensitive detection of mercury ions. Heliyon 2020, 6, e03750. [Google Scholar] [CrossRef]
- Muthurasu, A.; Ganesh, V. Tuning optical properties of nitrogen-doped carbon dots through fluorescence resonance energy transfer using Rhodamine B for the ratiometric sensing of mercury ions. Anal. Methods 2021, 13, 1857–1865. [Google Scholar] [CrossRef]
- Yahyazadeh, E.; Shemirani, F. Easily synthesized carbon dots for determination of mercury (II) in water samples. Heliyon 2019, 5, e01596. [Google Scholar] [PubMed]
- He, J.H.; Cheng, Y.Y.; Yang, T.; Zou, H.Y.; Huang, C.Z. Functional preserving carbon dots-based fluorescent probe for mercury (II) ions sensing in herbal medicines via coordination and electron transfer. Anal. Acta 2018, 1035, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhang, L.; Li, S.; Yue, S.; Wang, G. Selective detection of mercury ions via single and dual signals by silicon-doped carbon quantum dots. New J. Chem. 2023, 47, 14242–14248. [Google Scholar] [CrossRef]
- Chaghaghazardi, M.; Kashanian, S.; Nazari, M.; Omidfar, K.; Joseph, Y.; Rahimi, P. Nitrogen and sulfur co-doped carbon quantum dots fluorescence quenching assay for detection of mercury (II). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 293, 122448. [Google Scholar] [CrossRef]
- Omer, K.M.; Aziz, K.H.H.; Mohammed, S.J. Improvement of selectivity via the surface modification of carbon nanodots towards the quantitative detection of mercury ions. New J. Chem. 2019, 43, 12979–12986. [Google Scholar] [CrossRef]
- Lu, C.; Ding, H.; Wang, Y.; Xiong, C.; Wang, X. Colorimetric and turn-on fluorescence determination of mercury (II) by using carbon dots and gold nanoparticles. Nanotechnology 2021, 32, 155501. [Google Scholar] [CrossRef]
- Li, B.; Ma, H.; Zhang, B.; Qian, J.; Cao, T.; Feng, H.; Li, W.; Dong, Y.; Qin, W. Dually emitting carbon dots as fluorescent probes for ratiometric fluorescent sensing of pH values, mercury (II), chloride and Cr (VI) via different mechanisms. Microchim. Acta 2019, 186, 341. [Google Scholar] [CrossRef]
- Ren, H.; Labidi, A.; Sun, J.; Allam, A.A.; Ajarem, J.S.; Abukhadra, M.R.; Wang, C. Facile synthesis of nitrogen, sulfur co-doped carbon quantum dots for selective detection of mercury (II). Environ. Chem. Lett. 2024, 22, 35–41. [Google Scholar] [CrossRef]
- Hao, X.; Dai, S.; Wang, J.; Fang, Z. Synthesis of blue fluorescent carbon dots and their application in detecting mercury and iodine based on “off–on” mode. Luminescence 2021, 36, 721–732. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Xie, H.; Wei, L.; Xu, L.; Zhang, L.; Lan, W.; Zhou, C.; She, Y.; Fu, H. A novel thioctic acid-carbon dots fluorescence sensor for the detection of Hg2+ and thiophanate methyl via S-Hg affinity. Food Chem. 2021, 346, 128923. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Hu, G.; Zhao, F.; Wang, X.; Ma, Z.; Liu, R. Carbon dots prepared by thermal reactions and selective detections of copper and mercury ions in visible spectrum. Appl. Phys. A 2021, 127, 388. [Google Scholar] [CrossRef]
- Tang, W.; Wang, Y.; Wang, P.; Di, J.; Yang, J.; Wu, Y. Synthesis of strongly fluorescent carbon quantum dots modified with polyamidoamine and a triethoxysilane as quenchable fluorescent probes for mercury (II). Microchim. Acta 2016, 183, 2571–2578. [Google Scholar] [CrossRef]
- Achadu, O.J.; Revaprasadu, N. Microwave-assisted synthesis of thymine-functionalized graphitic carbon nitride quantum dots as a fluorescent nanoprobe for mercury (II). Microchim. Acta 2018, 185, 461. [Google Scholar] [CrossRef] [PubMed]
- Karami, C.; Taher, M.A.; Shahlaei, M. A simple method for determination of mercury (II) ions by PNBS-doped carbon dots as a fluorescent probe. J. Mater. Sci. Mater. Electron. 2020, 31, 5975–5983. [Google Scholar] [CrossRef]
- Lan, M.; Zhang, J.; Chui, Y.-S.; Wang, P.; Chen, X.; Lee, C.-S.; Kwong, H.-L.; Zhang, W. Carbon nanoparticle-based ratiometric fluorescent sensor for detecting mercury ions in aqueous media and living cells. ACS Appl. Mater. Interfaces 2014, 6, 21270–21278. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Omer, K.M.; Hamarawf, R.F. Lowering the detection limit towards nanomolar mercury ion detection via surface modification of N-doped carbon quantum dots. New J. Chem. 2019, 43, 8677–8683. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Wu, N.; Xu, M.; Wang, M.; Zhang, L.; Yang, W. In-situ synthesis of carbon dots-embedded europium metal-organic frameworks for ratiometric fluorescence detection of Hg2+ in aqueous environment. Anal. Acta 2021, 1141, 13–20. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, D.; Liu, X.; Han, A. Green hydrothermal synthesis of N-doped carbon dots from biomass highland barley for the detection of Hg2+. Sensors 2019, 19, 3169. [Google Scholar] [CrossRef]
- Gómez Pineros, B.S.; Granados-Oliveros, G. L-cysteine modified N-doped carbon quantum dots derived from peach palm (Bactris gasipaes) peels for detection of mercury ions. J. Mol. Struct. 2024, 1317, 138990. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Wang, X.; Routh, P.; Sana, B.; Lim, S.; Kim, D.H.; Lim, K.H.; Li, J.; Chen, P. Facile synthesis of graphene quantum dots from 3D graphene and their application for Fe3+ sensing. Adv. Funct. Mater. 2014, 24, 3021–3026. [Google Scholar] [CrossRef]
- Gao, B.; Chen, D.; Gu, B.; Wang, T.; Wang, Z.; Yang, Y.; Guo, Q.; Wang, G. Facile and highly effective synthesis of nitrogen-doped graphene quantum dots as a fluorescent sensing probe for Cu2+ detection. Curr. Appl. Phys. 2020, 20, 538–544. [Google Scholar] [CrossRef]
- Kadian, S.; Sethi, S.K.; Manik, G. Recent advancements in synthesis and property control of graphene quantum dots for biomedical and optoelectronic applications. Mater. Chem. Front. 2021, 5, 627–658. [Google Scholar] [CrossRef]
- Kongsanan, N.; Pimsin, N.; Keawprom, C.; Sricharoen, P.; Areerob, Y.; Nuengmatcha, P.; Oh, W.-C.; Chanthai, S.; Limchoowong, N. A fluorescence switching sensor for sensitive and selective detections of cyanide and ferricyanide using mercuric cation-graphene quantum dots. ACS Omega 2021, 6, 14379–14393. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Yang, J.; Xia, X.; Yi, R.; Jiang, J.; Liu, W.; Chen, J.; Chen, L.; Xu, J. “On-off-on” fluorescence switch of graphene quantum dots: A cationic control strategy. Appl. Surf. Sci. 2021, 546, 149110. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.-T.; Tsai, Y.-Y.; Ashraf Gandomi, Y.; Yeom, S.; Kihm, K.D.; Fu, C.-C.; Juang, R.-S. Sulfur and nitrogen co-doped graphene quantum dots as a fluorescent quenching probe for highly sensitive detection toward mercury Ions. ACS Appl. Nano Mater. 2019, 2, 790–798. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, X.; Deng, M.; Cao, Y.; Li, Y.; Zheng, H.; Li, F.; Yan, F.; Lan, T.; Shi, L. Nitrogen doped graphene quantum dots as a fluorescent probe for mercury (II) ions. Microchim. Acta 2019, 186, 140. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Chowdhury, A.D.; Doong, R.-A. Highly sensitive and selective detection of mercury ions using N, S-codoped graphene quantum dots and its paper strip based sensing application in wastewater. Sens. Actuators B Chem. 2017, 252, 1169–1178. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, X.; Xing, X.; Wang, Z.; Zou, T.; Wang, Z.; Zhao, R.; Wang, Y. One-pot synthesis of N-doped graphene quantum dots as highly sensitive fluorescent sensor for detection of mercury ions water solutions. Mater. Res. Express 2019, 6, 095615. [Google Scholar] [CrossRef]
- Zhu, Q.; Mao, H.; Li, J.; Hua, J.; Wang, J.; Yang, R.; Li, Z. A glycine-functionalized graphene quantum dots synthesized by a facile post-modification strategy for a sensitive and selective fluorescence sensor of mercury ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119090. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. Rapid detection of mercury ions based on nitrogen-doped graphene quantum dots accelerating formation of manganese porphyrin. ACS Sens. 2018, 3, 1040–1047. [Google Scholar] [CrossRef]
- Chini, M.K.; Kumar, V.; Javed, A.; Satapathi, S. Graphene quantum dots and carbon nano dots for the FRET based detection of heavy metal ions. Nano-Struct. Nano-Objects 2019, 19, 100347. [Google Scholar] [CrossRef]

| CQDs Precursor | Synthesis Method | QY | LOD | Linear Range | Reference |
|---|---|---|---|---|---|
| Citric acid, tartaric acid, ethanediamine | Solvothermal | - | 83.5 nM | 0–18 µM | [1] |
| Citric acid, melamine | Solid thermal method | - | 0.44 μM | 2–14 μM | [3] |
| Citric acid, ethylenediamine, Mg(OH)2 | Hydrothermal | - | 0.02 µM | 0.05–5 µM | [24] |
| Citric acid, glutathione or thiourea | Microwave hydrothermal | 26% | 5.4 µM | 5–50 µM | [25] |
| Citric acid, urea | Hydrothermal | - | 1.3 nM | 0.005–250 μM | [59] |
| Citric acid, L-cysteine, | Hydrothermal | - | 4.2 pM | 0.01–0.75 nM | [88] |
| Citric acid, triethylenetetramine, (TETA) | Thermal | 54% | 0.2 nM | 1–20 nM | [89] |
| Citric acid, ethylene diamine | Hydrothermal | - | - | - | [90] |
| Citric acid, 2,2-dimethyl-1,3-propanediamine | Microwave-assisted synthesis | 51.20% | 7.63 nM | 0–4.2 μM | [91] |
| Citric acid, urea | Hydrothermal | - | 8.7 μM | 10–70 μM | [92] |
| Citric acid, | Hydrothermal | - | 38 ppb | 0.12–2 ppm | [93] |
| glycine | |||||
| Citric acid, spermine | Hydrothermal | - | 2.2 nM | 0.01–1.0 µM | [94] |
| Citric acid, aminopropyltriethoxysilane (APTEOS) | Hydrothermal | 0.015 μM | 0.02–5.0 μM | [95] | |
| Citric acid, chitosan, thiourea | Hydrothermal | 33.00% | 4 nM | 5–160 nM | [96] |
| 2,4,6-Triaminopyrimidine | Hydrothermal metho. | - | 11.4 nM | - | [60] |
| Xylose | Solvothermal | - | 10 nM | 50–800 nM | [97] |
| Glutathione | Solvothermal | 41.90% | 0.5 μM | 0.5–15.0 μM | [98] |
| Ortho-phenylenediamine (OPDA) | Solvothermal | - | 60 nM | 30–60 µM | [99] |
| Methyl orange | - | 29.4 | 237 nM | - | [100] |
| Malic acid, urea | Microwave-assisted hydrothermal synthesis | - | 0.90 μM | 0–40 μM | [101] |
| Trisodium citrate dihydrate, DL-thioctic acid, | Hydrothermal | - | 33.3 nM | 0.05–5.8 μM | [102] |
| Aconitic acid, oligomeric polyethyleneimine | Thermal | 44.20% | 84 nM | 0–800 μM | [103] |
| Polyamidoamine (PAMAM), and (3-aminopropyl)triethoxysilane (APTES) | Hydrothermal | - | 87 fM | 0.2 nM–10 µM | [104] |
| Diaminomaleonitrile (DAMN), thymine-1-acetic acid | Microwave-assisted hydrothermal synthesis | - | 0.15 nM | 1.0–500 nM | [105] |
| Glucose, HAuCl4, reduced glutathione | Microwaving | - | 8.7 nM | 50–1000 nM | [2] |
| Glucose, boric acid, thiourea, phosphoric | Hydrothermal | - | 16.5 μM | 25.0 μM–1500.0 mM | [106] |
| Trisodium citrate dihydrate, melamine | Microwave-assisted hydrothermal | - | 42 nM | 0–6 μM | [107] |
| Methyl glycine diacetic acid trisodium salt (MGDA), m-phenylenediamine (MPD) | Hydrothermal | 63.8 | 0.9 μM | 0–100 μM | [68] |
| D-Glucose, aspartic acid, and | Hydrothermal | - | 10 nM | 20–800 nM | [108] |
| branched polyethyleneimine | |||||
| Honey | Hydrothermal | - | 1.02 nM | 0–10 nM | [11] |
| Tamarindus indica leaves | - | - | 6 nM | 0–0.1 µM | [20] |
| Hongcaitai | Hydrothermal | - | 0.06 µM | 0.2–15 µM | [72] |
| Black wolfberry | Hydrothermal | - | 0.12 nM | 0–300 μM | [109] |
| Highland barley, Ethylenediamine | Hydrothermal | 14.40% | 0.48 µM | 10–160 µM | [110] |
| Peach palm (Bactris gasipaes) peels | Microwave assisted | 25.4 | 0.19 μM | - | [111] |
| GQDs Precursor | Synthesis Method | QY | LOD | Linear Range | Reference |
|---|---|---|---|---|---|
| Glucose, urea, and ammonia sulfate | Infrared (IR)-assisted pyrolysis | - | 10 ppb | 10 ppb–10 ppm | [117] |
| Citric acid and Thiourea | Hydrothermal | 41.90% | 0.14 nM | 0.1–15 µM | [119] |
| Citric acid and ethylene diamine | Hydrothermal | - | 0.45 nM | 100–1000 nM | [120] |
| Citric acid and Gly | Hydrothermal | 35.50% | 8.3 nM | 0–3.0 μM | [121] |
| Graphene oxide | Electrochemical | - | 2.5 µM | 2.5–800 µM | [118] |
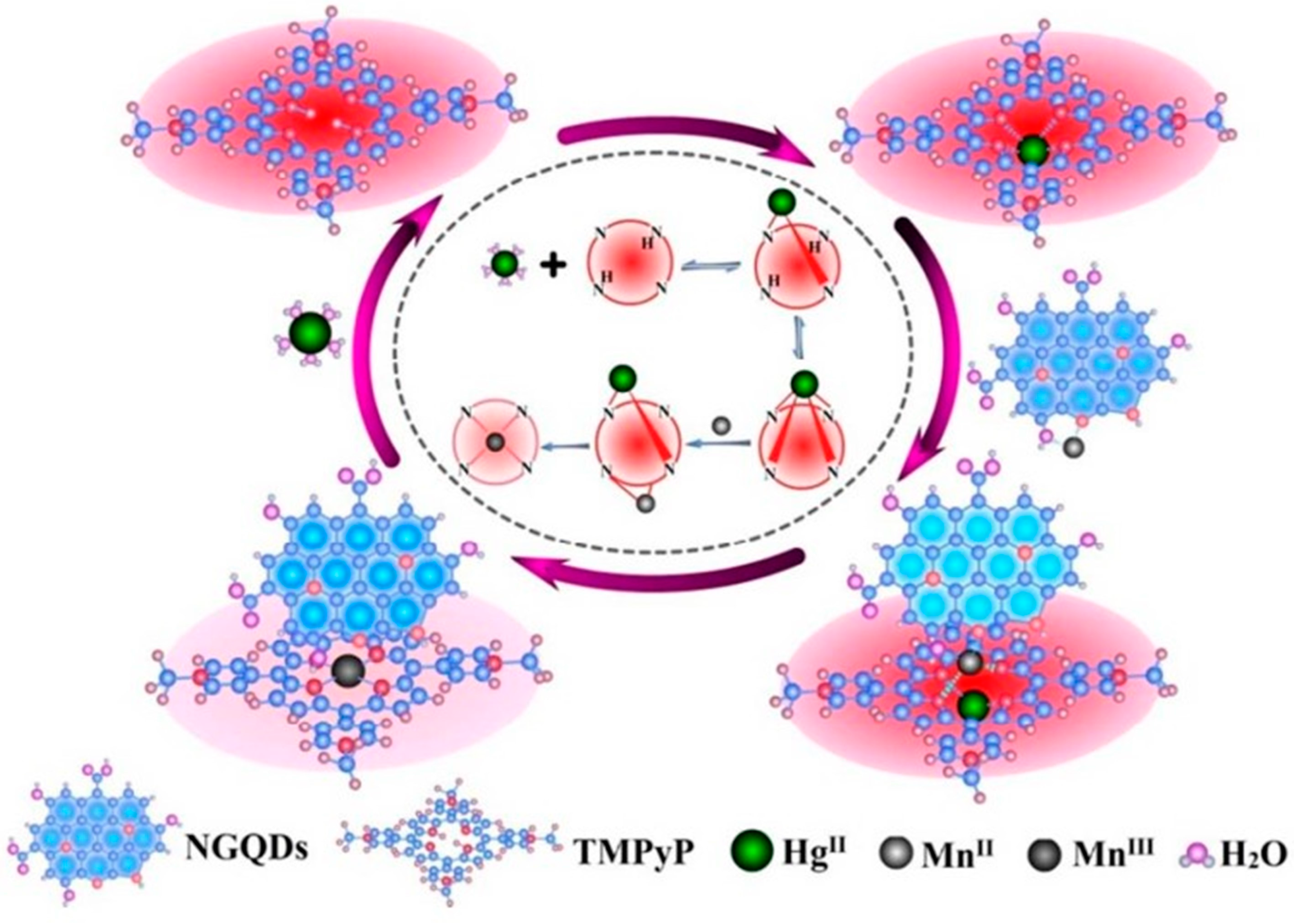
| Graphene oxide and 5,10,15,20-tetrakis(1-methyl-4-pyridinio)porphyrin tetra(p-toluenesulfonate) | Two-step hydrothermal method | - | 0.32 nM | 2–200 nM | [122] |
| (TMPyP) | |||||
| Graphene oxide, urea, citric acid | Solvotermal | - | - | - | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaghazardi, M.; Kashanian, S.; Nazari, M.; Omidfar, K.; Joseph, Y.; Rahimi, P. Fluorometric Mercury (II) Detection Using Heteroatom-Doped Carbon and Graphene Quantum Dots. Photonics 2024, 11, 841. https://doi.org/10.3390/photonics11090841
Chaghazardi M, Kashanian S, Nazari M, Omidfar K, Joseph Y, Rahimi P. Fluorometric Mercury (II) Detection Using Heteroatom-Doped Carbon and Graphene Quantum Dots. Photonics. 2024; 11(9):841. https://doi.org/10.3390/photonics11090841
Chicago/Turabian StyleChaghazardi, Mosayeb, Soheila Kashanian, Maryam Nazari, Kobra Omidfar, Yvonne Joseph, and Parvaneh Rahimi. 2024. "Fluorometric Mercury (II) Detection Using Heteroatom-Doped Carbon and Graphene Quantum Dots" Photonics 11, no. 9: 841. https://doi.org/10.3390/photonics11090841






