Sizing Single Gold Nanoparticles with Bright-Field Microscopy
Abstract
:1. Introduction
2. Materials and Methods
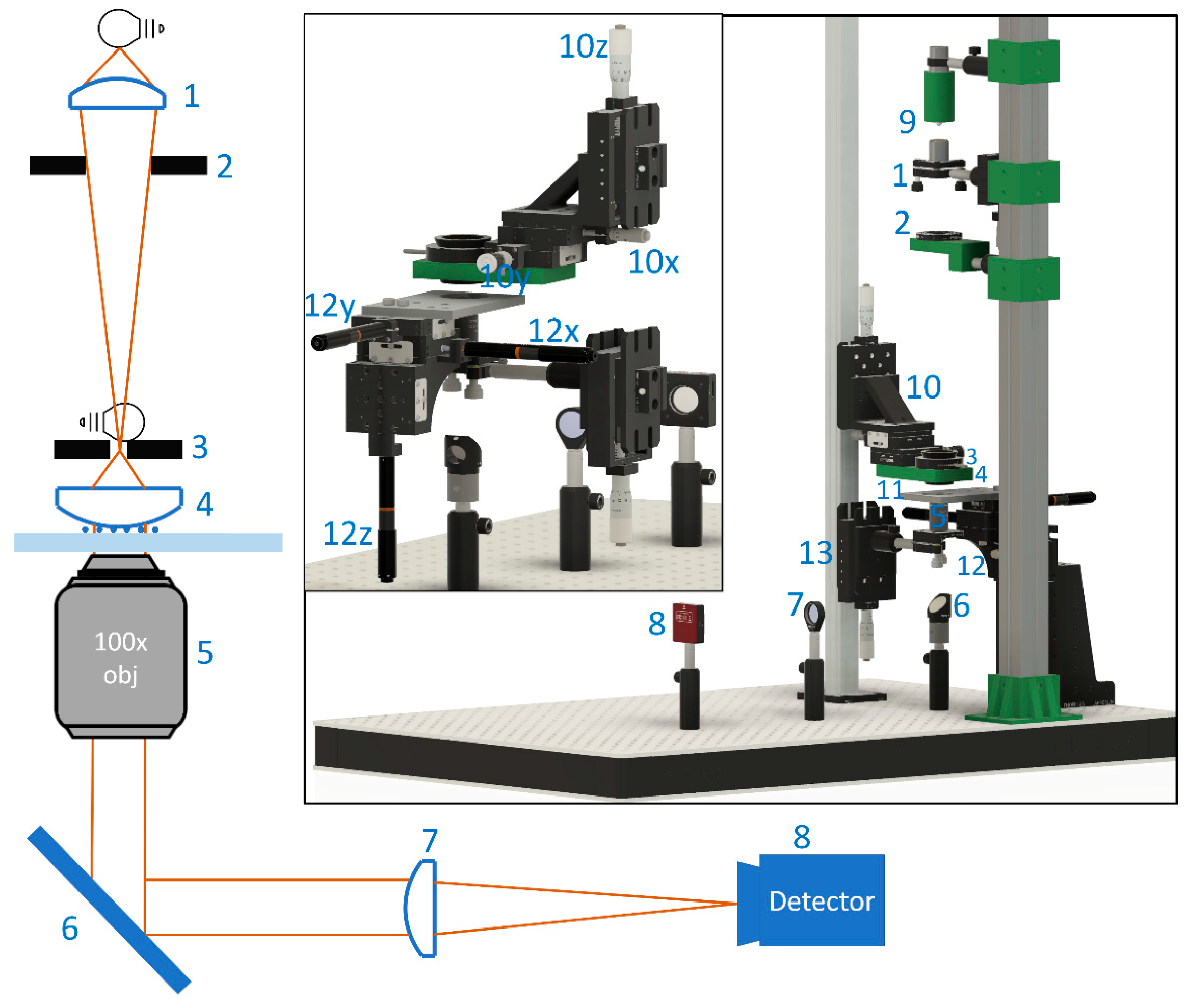
2.1. Microscopy System and Image Acquisition
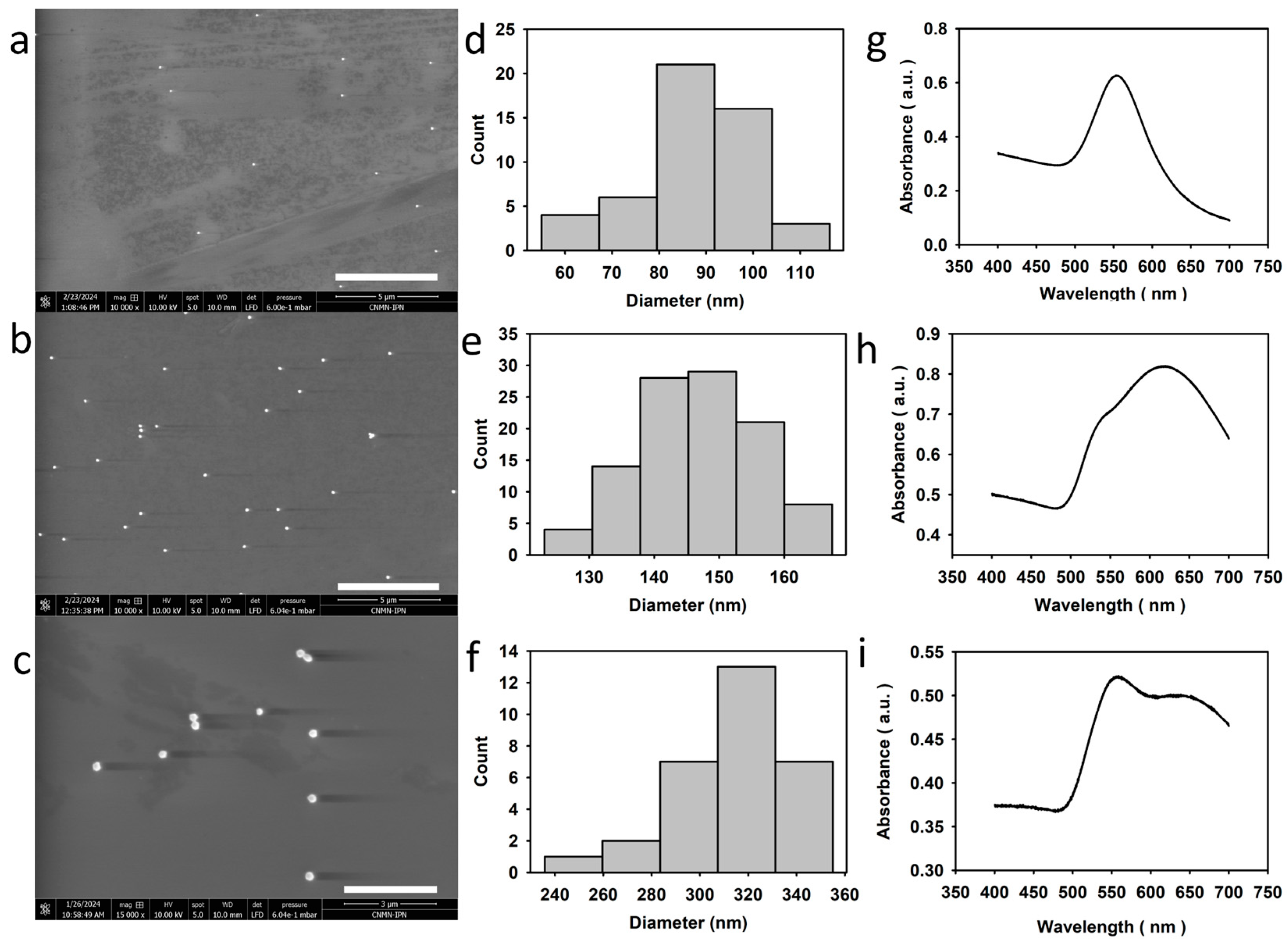
2.2. Nanoparticle Characterization
3. Results
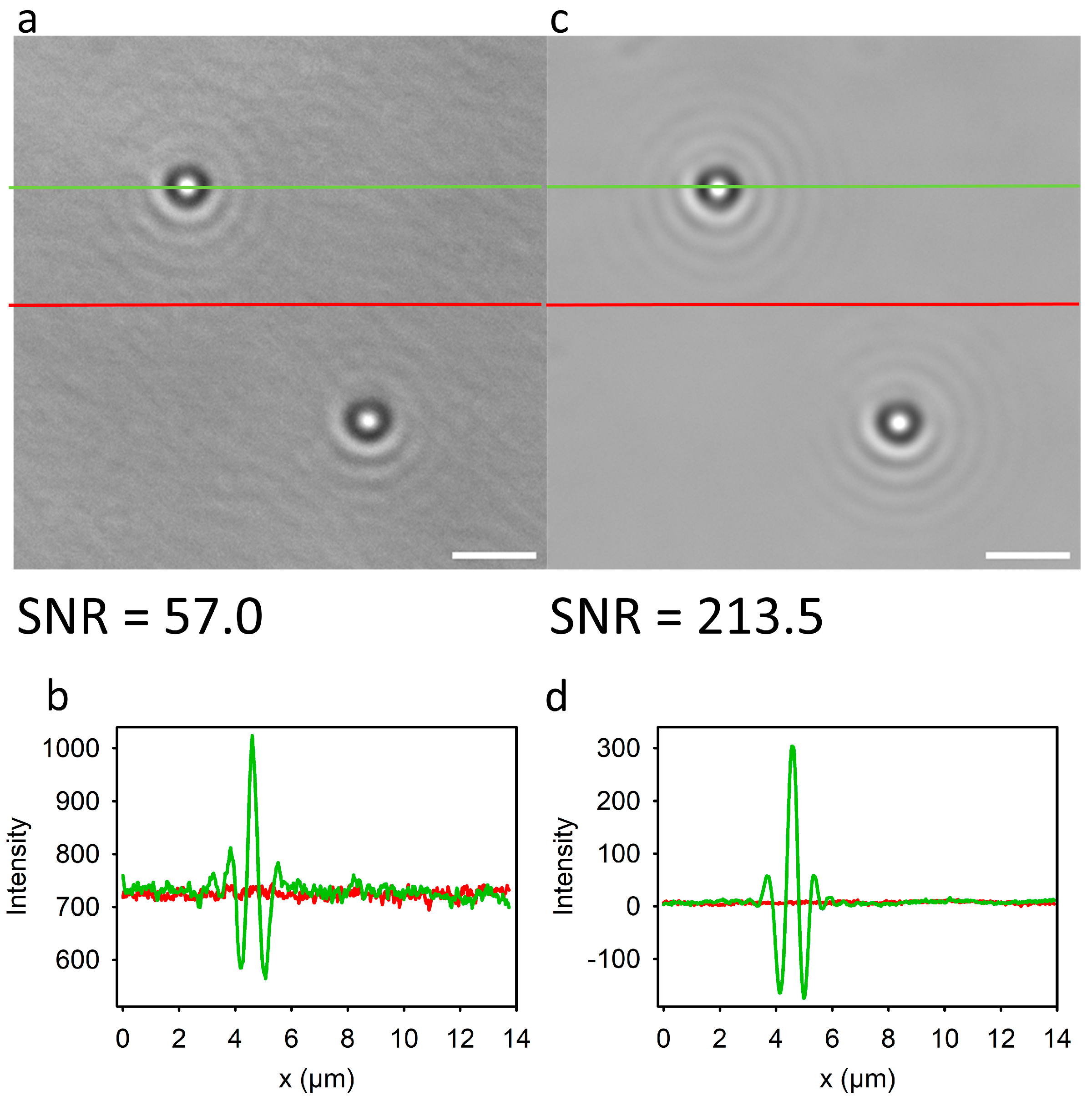
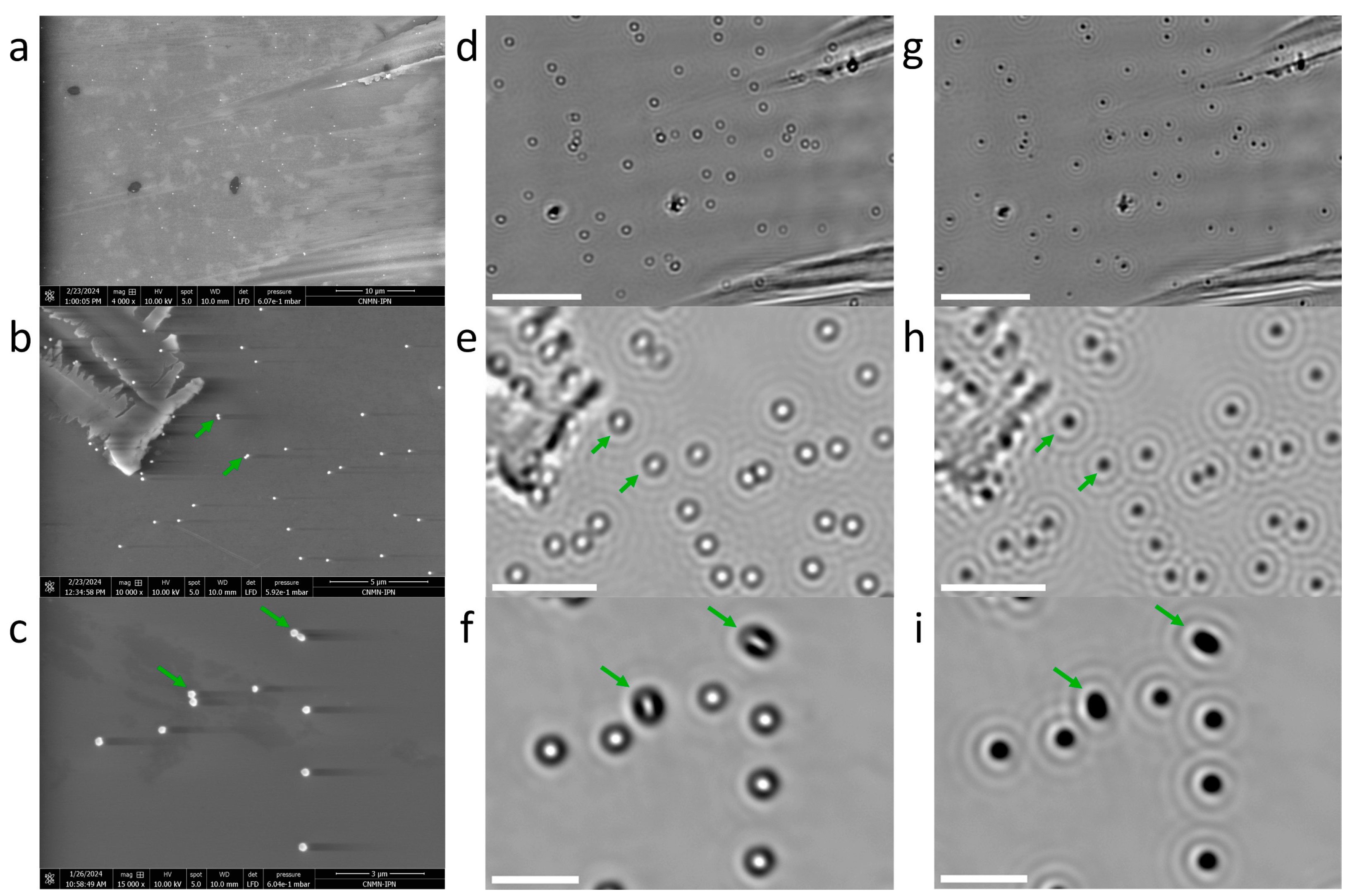
3.1. Signal-to-Noise of AuNPs Visualized by Bright-Field Microscopy
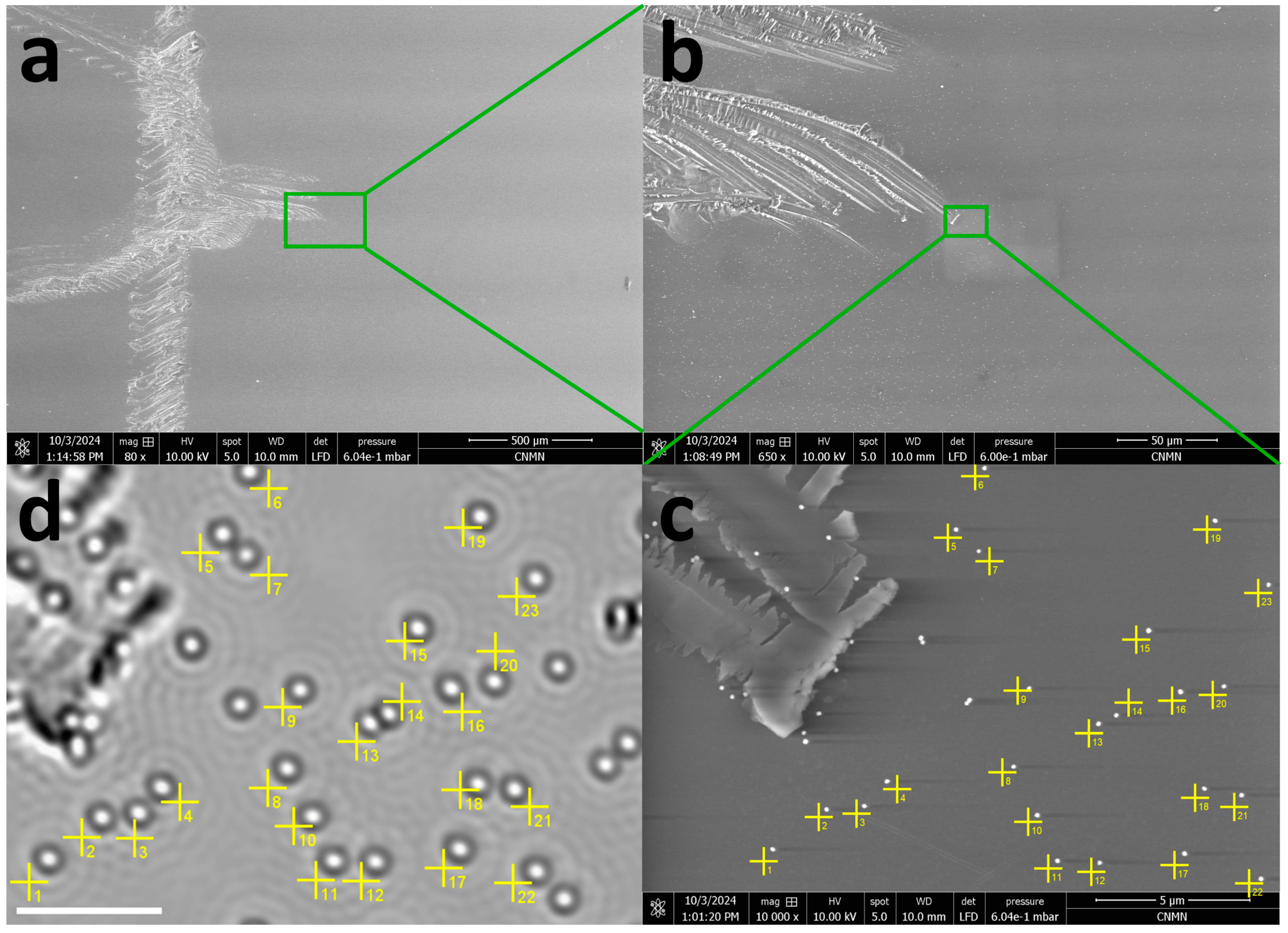
3.2. Sample Alignment in Optical and Electron Microscopies
3.3. Bright-Field Microscopy Signal vs. Size for AuNPs
3.4. Scattering and Absorption Cross-Sections of AuNPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| AFM | Atomic force microscopy |
| UV–Vis | Ultraviolet–visible spectroscopy |
| DLS | Dynamic light scattering |
| PCM | Phase-contrast microscopy |
| DIC | Differential interference contrast microscopy |
| iSCAT | Interferometric scattering microscopy |
| SCS | Scattering cross-section |
| PR | Plasmon resonance |
| NA | Numerical aperture |
| CMOS | Complementary metal–oxide–semiconductor |
| LSPR | Localized surface plasmon resonance |
| APTES | (3-Aminopropyl)triethoxysilane |
References
- Boisselier, E.; Astruc, D. Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Gorth, D.; Rand, D. Silver Nanoparticle Toxicity in Drosophila: Size Does Matter. Int. J. Nanomed. 2011, 2011, 343–350. [Google Scholar] [CrossRef]
- von der Kammer, F.; Legros, S.; Hofmann, T.; Larsen, E.H.; Loeschner, K. Separation and Characterization of Nanoparticles in Complex Food and Environmental Samples by Field-Flow Fractionation. TrAC Trends Anal. Chem. 2011, 30, 425–436. [Google Scholar] [CrossRef]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in Microfluidics for Nanoparticle Separation. Lab Chip 2017, 17, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Dragovic, R. Nanoparticle Tracking Analysis. In Extracellular Vesicles in Health and Disease; Harrison, P., Gardiner, C., Sargent, I.L., Eds.; Jenny Stanford Publishing: Singapore, 2014. [Google Scholar] [CrossRef]
- Lee, K.; Shao, H.; Weissleder, R.; Lee, H. Acoustic Purification of Extracellular Microvesicles. ACS Nano 2015, 9, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Abrica-González, P.; Zamora-Justo, J.A.; Chavez-Sandoval, B.E.; Vázquez-Martínez, G.R.; Balderas-López, J.A. Measurement of the Optical Properties of Gold Colloids by Photoacoustic Spectroscopy. Int. J. Thermophys. 2018, 39, 93. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.Z.; Chin, L.K.; Zhang, J.B.; Tsai, D.P.; Liu, A.Q. Optofluidic Nanoparticles Sorting by Hydrodynamic Optical Force. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; pp. 2122–2125. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Taylor, R.W.; Sandoghdar, V. Interferometric Scattering Microscopy: Seeing Single Nanoparticles and Molecules via Rayleigh Scattering. Nano Lett. 2019, 19, 4827–4835. [Google Scholar] [CrossRef]
- Kukura, P.; Ewers, H.; Müller, C.; Renn, A.; Helenius, A.; Sandoghdar, V. High-Speed Nanoscopic Tracking of the Position and Orientation of a Single Virus. Nat. Methods 2009, 6, 923–927. [Google Scholar] [CrossRef]
- De Brabander, M.; Nuydens, R.; Geuens, G.; Moeremans, M.; Mey, D. The Use of Submicroscopic Gold Particles Combined With Video Contrast Enhancement Living Cell. Cell Motil. 1986, 113, 105–113. [Google Scholar]
- Taylor, R.W.; Sandoghdar, V. Interferometric Scattering (ISCAT) Microscopy and Related Techniques. In Biological and Medical Physics, Biomedical Engineering; Astratov, V., Ed.; Biological and Medical Physics, Biomedical Engineering; Springer International Publishing: Cham, Switzerland, 2019; pp. 25–65. ISBN 978-3-030-21721-1. [Google Scholar]
- Ortega-Arroyo, J.; Kukura, P. Interferometric Scattering Microscopy (ISCAT): New Frontiers in Ultrafast and Ultrasensitive Optical Microscopy. Phys. Chem. Chem. Phys. 2012, 14, 15625. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Maestre, D.; Conrad-Billroth, C.; Juffmann, T. Fundamental Bounds on the Precision of ISCAT, COBRI and Dark-Field Microscopy for 3D Localization and Mass Photometry. J. Phys. D Appl. Phys. 2021, 54, 394002. [Google Scholar] [CrossRef]
- Sun, W.; Wang, G.; Fang, N.; Yeung, E.S. Wavelength-Dependent Differential Interference Contrast Microscopy: Selectively Imaging Nanoparticle Probes in Live Cells. Anal. Chem. 2009, 81, 9203–9208. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.; Zilli, A.; Wang, Y.; Langbein, W.; Borri, P. Quantitative High-Throughput Optical Sizing of Individual Colloidal Nanoparticles by Wide-Field Imaging Extinction Microscopy. In Proceedings of the Colloidal Nanoparticles for Biomedical Applications XIV, San Francisco, CA, USA, 2–4 February 2019; Parak, W.J., Osiński, M., Eds.; SPIE: Bellingham, WA, USA, 2019; p. 17. [Google Scholar]
- Gutierrez-Medina, B. Optical Sectioning of Unlabeled Samples Using Bright-Field Microscopy. Proc. Natl. Acad. Sci. USA 2022, 119, e2122937119. [Google Scholar] [CrossRef]
- Yahaya, M.L.; Zakaria, N.D.; Noordin, R.; Abdul Razak, K. Synthesis of Large and Stable Colloidal Gold Nanoparticles (AuNPs) by Seeding-Growth Method. Mater. Today Proc. 2022, 66, 2943–2947. [Google Scholar] [CrossRef]
- Abrica-González, P.; Zamora-Justo, J.A.; Sotelo-López, A.; Vázquez-Martínez, G.R.; Balderas-López, J.A.; Muñoz-Diosdado, A.; Ibáñez-Hernández, M. Gold Nanoparticles with Chitosan, N-Acylated Chitosan, and Chitosan Oligosaccharide as DNA Carriers. Nanoscale Res. Lett. 2019, 14, 258. [Google Scholar] [CrossRef]
- Bujak, L.; Piliarik, M. Interferometric Scattering (ISCAT) Microscopy for High Fidelity Tracking at Microseconds Timescales. In Nanoimaging and Nanospectroscopy VI; SPIE: Bellingham, WA, USA, 2018; Volume 10726, pp. 53–60. [Google Scholar] [CrossRef]
- Kyaw, H.H.; Al-Harthi, S.H.; Sellai, A.; Dutta, J. Self-Organization of Gold Nanoparticles on Silanated Surfaces. Beilstein J. Nanotechnol. 2015, 6, 2345–2353. [Google Scholar] [CrossRef]
- Gutiérrez-Medina, B.; de J. Sánchez Miranda, M. Quantitative Image Restoration in Bright Field Optical Microscopy. Biophys. J. 2017, 113, 1916–1919. [Google Scholar] [CrossRef]
- Streibl, N. Three-Dimensional Imaging by a Microscope. J. Opt. Soc. Am. A 1985, 2, 121. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer: Berlin/Heidelberg, Germany, 2013; Volume 25, ISBN 3662091097. [Google Scholar]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 3527618163. [Google Scholar]
- Olson, J.; Dominguez-Medina, S.; Hoggard, A.; Wang, L.Y.; Chang, W.S.; Link, S. Optical Characterization of Single Plasmonic Nanoparticles. Chem. Soc. Rev. 2015, 44, 40–57. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.-W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370. [Google Scholar] [CrossRef]
- Payne, L.M.; Langbein, W.; Borri, P. Polarization-Resolved Extinction and Scattering Cross-Sections of Individual Gold Nanoparticles Measured by Wide-Field Microscopy on a Large Ensemble. Appl. Phys. Lett. 2013, 102, 131107. [Google Scholar] [CrossRef]
- Ando, J.; Nakamura, A.; Visootsat, A.; Yamamoto, M.; Song, C.; Murata, K.; Iino, R. Single-Nanoparticle Tracking with Angstrom Localization Precision and Microsecond Time Resolution. Biophys. J. 2018, 115, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Kefer, P.; Iqbal, F.; Locatelli, M.; Lawrimore, J.; Zhang, M.; Bloom, K.; Bonin, K.; Vidi, P.-A.; Liu, J. Performance of deep learning restoration methods for the extraction of particle dynamics in noisy microscopy image sequences. Mol. Biol. Cell 2021, 32, 903–914. [Google Scholar] [CrossRef] [PubMed]
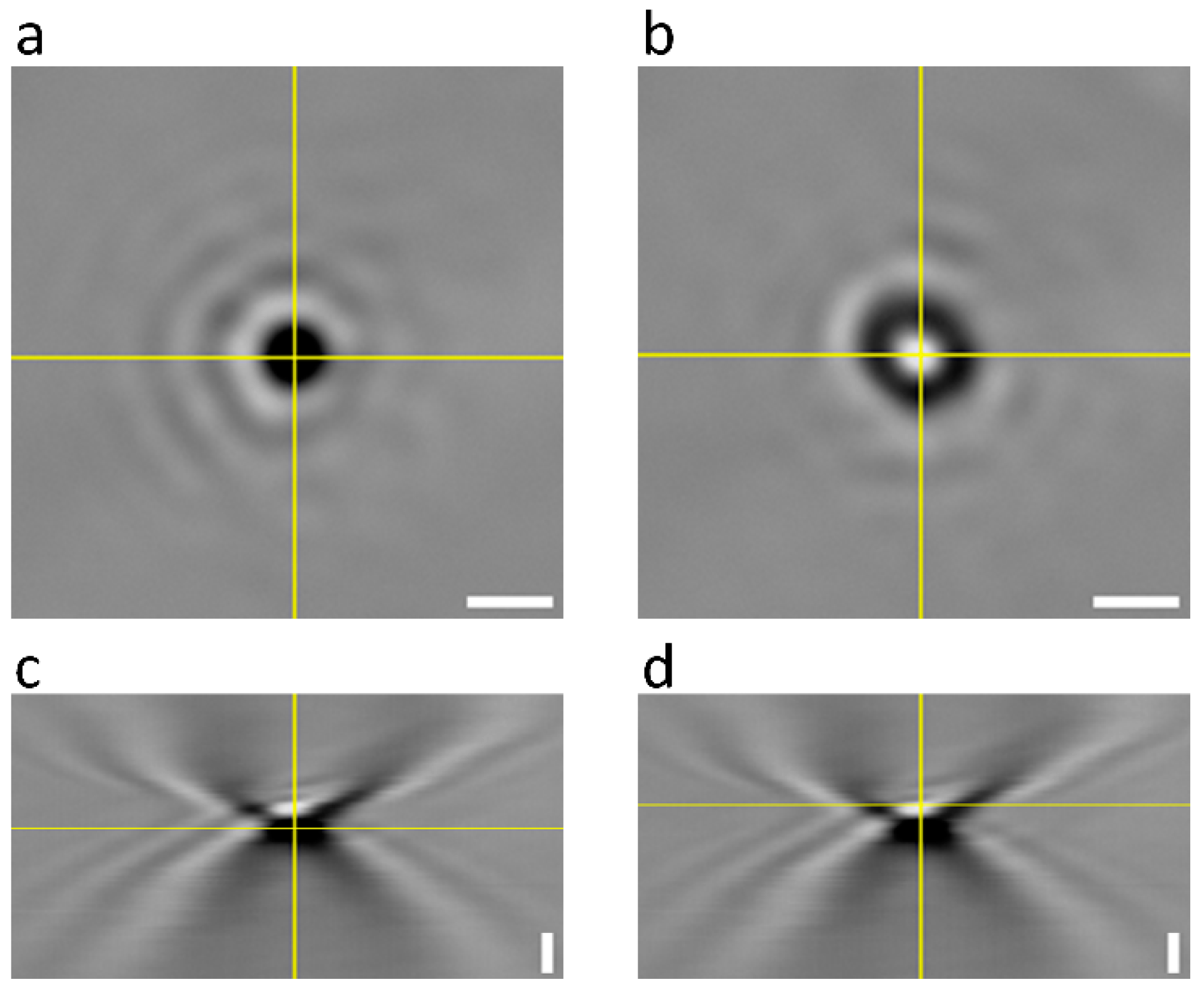


| 80 nm | 150 nm | 300 nm | |
|---|---|---|---|
| Cmin, λ = 460 nm | −51.8 ± 11.8 | −137.2 ± 15.8 | −346.1 ± 39.1 |
| Cmax, λ = 460 nm | 4.3 ± 5.0 | 13.3 ± 7.9 | 123.0 ± 78.8 |
| Cmin, λ = 520 nm | −122.7 ± 21.0 | −235.7 ± 23.6 | −545.7 ± 42.9 |
| Cmax, λ = 520 nm | 75.4 ± 22.9 | 201.5 ± 30.4 * | 204.4 ± 62.7 * |
| Cmin, λ = 620 nm | −177.4 ± 49.0 | −360.4 ± 25.5 | −589.4 ± 89.9 |
| Cmax, λ = 620 nm | 87.7 ± 32.7 | 265.0 ± 18.1 ‡ | 272.1 ± 27.0 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotelo-López, A.; Mendoza-Acevedo, S.; Balderas-López, J.A.; Gutiérrez-Medina, B. Sizing Single Gold Nanoparticles with Bright-Field Microscopy. Photonics 2025, 12, 314. https://doi.org/10.3390/photonics12040314
Sotelo-López A, Mendoza-Acevedo S, Balderas-López JA, Gutiérrez-Medina B. Sizing Single Gold Nanoparticles with Bright-Field Microscopy. Photonics. 2025; 12(4):314. https://doi.org/10.3390/photonics12040314
Chicago/Turabian StyleSotelo-López, Antonio, Salvador Mendoza-Acevedo, José Abraham Balderas-López, and Braulio Gutiérrez-Medina. 2025. "Sizing Single Gold Nanoparticles with Bright-Field Microscopy" Photonics 12, no. 4: 314. https://doi.org/10.3390/photonics12040314
APA StyleSotelo-López, A., Mendoza-Acevedo, S., Balderas-López, J. A., & Gutiérrez-Medina, B. (2025). Sizing Single Gold Nanoparticles with Bright-Field Microscopy. Photonics, 12(4), 314. https://doi.org/10.3390/photonics12040314





