Abstract
A non-intrusive mattress based on an optical-fiber Michelson interferometer is designed for daily sleep monitoring. The optical phase signal of the optical-fiber Michelson interferometer caused by the heartbeat and respiration is demodulated by the phase-generated carrier (PGC) method. The physiological signals and vital indicators including heart rate (HR), respiration rate (RR), and signal energy (SE) are extracted from the optical phase by algorithmic processing. A series of experiments are conducted to confirm the feasibility of the mattress for sleep monitoring. The mattress not only can achieve HR and RR counting, but also can record the waveform of the sleep-induced signal accurately. The body states can also be distinguished by the SE. In an all-night sleep monitoring experiment, the HR measured by the mattress is compared with the HR measured by a commercial smart band, showing a maximum error of 6 bpm (beat per minute). The designed mattress based on an optical-fiber Michelson interferometer shows good performance and great potential in non-intrusive sleep monitoring.
1. Introduction
Sleep is an indispensable physiological process that maintains human physiological functions, promoting cognition recovery and safeguarding mental health. Respiration rate (RR) and heart rate (HR) are two significant indicators that reveal sleep condition, which provide information for health assessment and sleep disorder diagnosis. It is important to develop high-performance monitoring instruments for daily sleep monitoring.
Although the conventional polysomnography (PSG) system is the gold standard for sleep diagnosis in the medical field, it is complicated and uncomfortable to wear the device, which is in direct contact with the human body [1]. Recently, numerous intrusive or indirect sleep monitoring methods have received considerable attention, including wearable watches based on the photoplethysmography (PPG) method [2] or a mattress and belt implanted with piezoelectric thin-film sensors [3,4,5]. Fiber optic sensors are distinguished by their compact structure and high sensitivity and resistance to electromagnetic interference. Therefore, fiber optic sensors have great potential in sleep monitoring [6,7,8].
Various fiber optic sensors have been proposed to monitor respiration and heartbeat signals. Han, P. et al. proposed an isometric cut single-mode plastic fiber sensor to monitor respiration and heartbeat signals by monitoring light intensity [9]. Tan, F. et al. proposed a non-invasive monitoring mattress based on Twin-Core Fiber by detecting the wavelength drift [10]. Foo, S. et al. implanted a fiber optic Bragg grating into the polycarbonate mattress to monitor HR [11]. Recently, sensors based on phase demodulation have been proposed to achieve high-sensitivity monitoring [12,13]. Zhou et al. designed a wearable device based on multimode fiber Mach–Zehnder interferometer to detect sleep signals [12]. Wang et al. proposed a mattress implanted with fiber optic Mach–Zehnder interferometer to assist early diagnosis of respiration and heartbeat abnormality [13]. However, the sensors based on the Mach–Zehnder interferometer are susceptible to polarization perturbation, which may cause signal distortion. Compared to the Mach–Zehnder interferometer, the fiber optic Michelson interferometer combined with Faraday mirrors can effectively mitigate the impact of light polarization perturbation [14,15,16].
In this work, a mattress based on an optical-fiber Michelson interferometer is designed to achieve sleep monitoring. The sensing arm of the interferometer is inserted into the mattress’s latex filling layer as the sensing structure, while the reference arm is isolated to suppress the environment perturbation. The experimental results show the mattress can extract respiration signals, heartbeat signals, and body movement information from the optical phase change induced by the pressure change on the mattress surface. Finally, the all-night sleep monitoring experiment reflects the value of the mattress in daily sleep monitoring. Generally, the mattress is notable for its high sensitivity and capacity of multi-parameter monitoring, which positions it as a suitable device for daily sleep monitoring.
2. The Principle of the Mattress
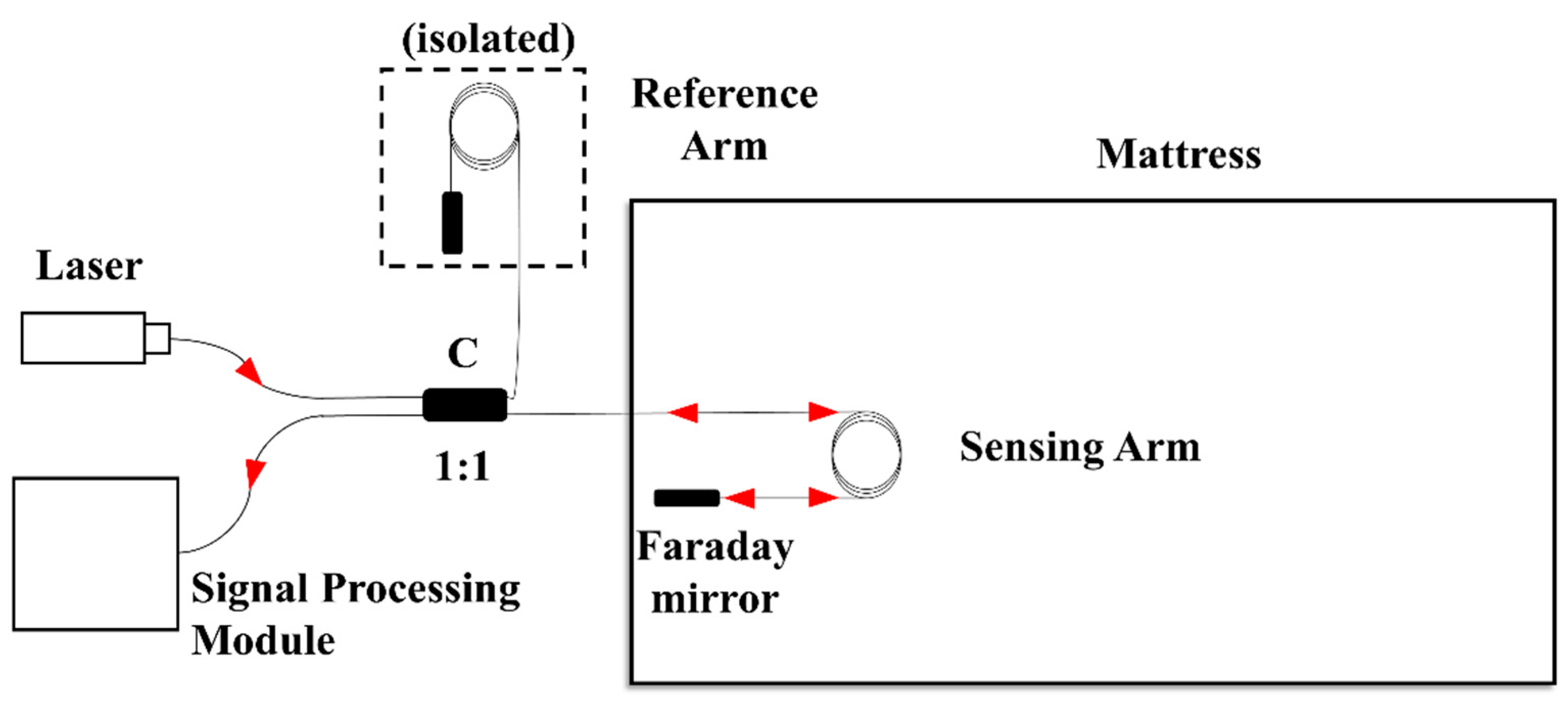
The structure of the proposed mattress is shown in Figure 1. The single frequency light from a narrow-linewidth laser is injected into a Michelson fiber interferometer. The output signal of the interferometer is detected and processed by the signal processing module. One arm of the interferometer is implanted into the mattress’s latex filling layer as the sensing arm, while the other arm is isolated to suppress the environment perturbation. The sensing arm is designed as a circular winding structure to enhance the sensitivity. Two Faraday rotation mirrors are connected at the end of the both arms to suppress the polarization perturbation.
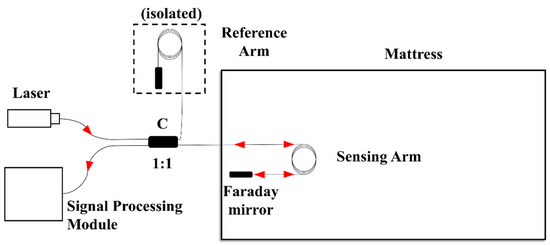
Figure 1.
Structure schematic diagram of the mattress.
When a human lies on the mattress, the phase of light in the sensing arm is modulated by the pressure initiated from the human body. The pressure signal can be obtained by demodulating the phase signal of the fiber optic Michelson interferometer. The phase difference change reflects information such as respiration, heartbeat, and body movement, which can be expressed as follows [17,18]:
where is the equivalent refractive index of the optical fiber, is the wavelength of the light, is the length of the optical fiber, and and represent the change in the equivalent refractive index and optical fiber length, respectively.
One important issue is achieving accurate phase demodulation. In this work, the phase-generated carrier demodulation (PGC) method is adopted [19]. An optical frequency modulation signal with a frequency of is applied to the laser. The output of the interferometer can be expressed as follows:
in which A and B represents the direct current and alternating current amplitude of the interference signal. C is the carrier modulation depth.
Firstly, Equation (2) is multiplexed with and , respectively. After being filtered by a low-pass filter, two orthogonal signals can be obtained as follows:
Then the phase signal of the Michelson interferometer can be expressed as follows:
reflects information such as the respiration, heartbeat and body movement. The next step is to extract the useful information from . Firstly, the demodulated phase signal is processed by a band-pass filter to obtain the respiration and heartbeat signals, respectively. The frequency filtering band range is aligned with the Chinese medical standard [20,21]. Then the filtered time-domain signal of the heartbeat and respiration can be obtained as and , respectively.
Based on the respiration and heartbeat signals of the mattress, HR and RR can be calculated by the following formulas:
where FFT represents Fast Fourier Transform.
Body movement monitoring is important for sleep assessment. The body movement leads to a change in the pressure on the sensor, which leads to the amplitude of changing. In this work, the spectrum signal energy (SE) of the respiration and heartbeat is treated as an important indicator of the body movement information [22]. The signal energy is defined as follows:
3. Experimental Results and Discussion
3.1. Heartbeat and Respiration Monitoring
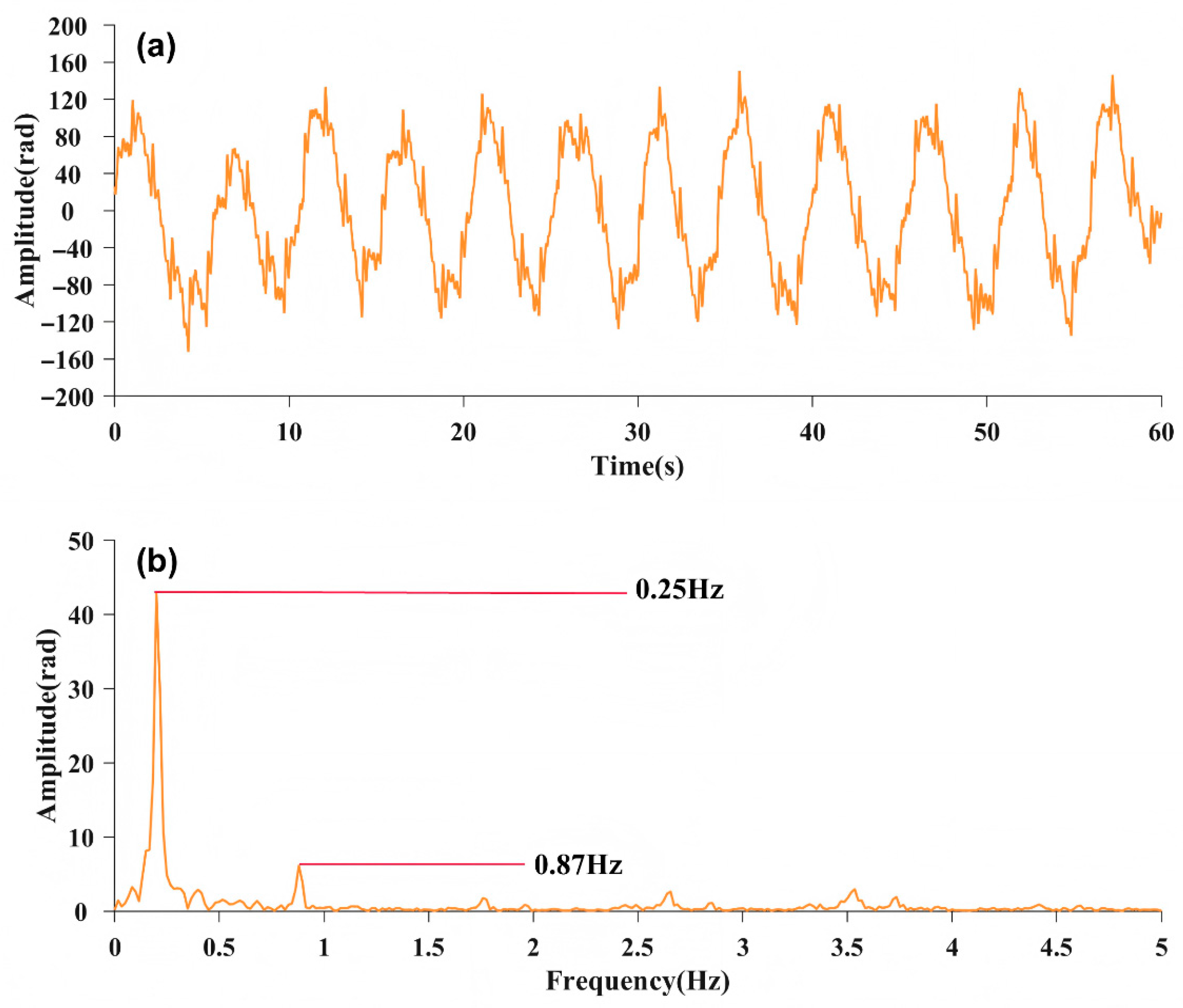
In this experiment, a subject lies on a bed where the fiber optic sensor is placed under the mattress. The fiber optic sensor is below the chest of the subject. Figure 2 shows a 60 s phase signal captured by the interferometer. Figure 2a shows the time-domain signal. Figure 2b shows the frequency-domain spectrum, which features two discernible spikes. The corresponding frequencies, and , are, respectively, 0.25 Hz and 0.87 Hz. These can be identified as the signal frequencies associated with respiration and heartbeat based on the diagnostic criteria of medical signals [23].
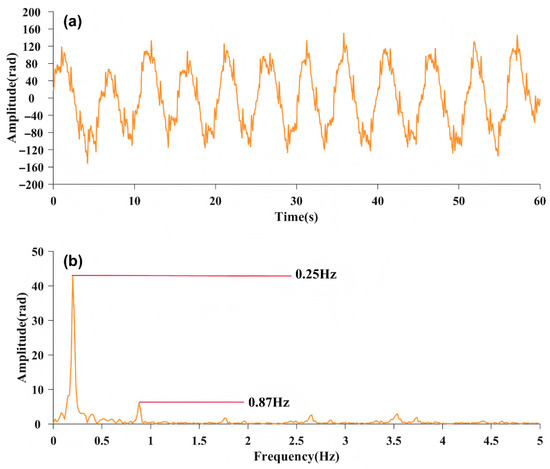
Figure 2.
(a) 60 s time-domain image of phase signal; (b) Frequency-domain spectrum of the phase signal.
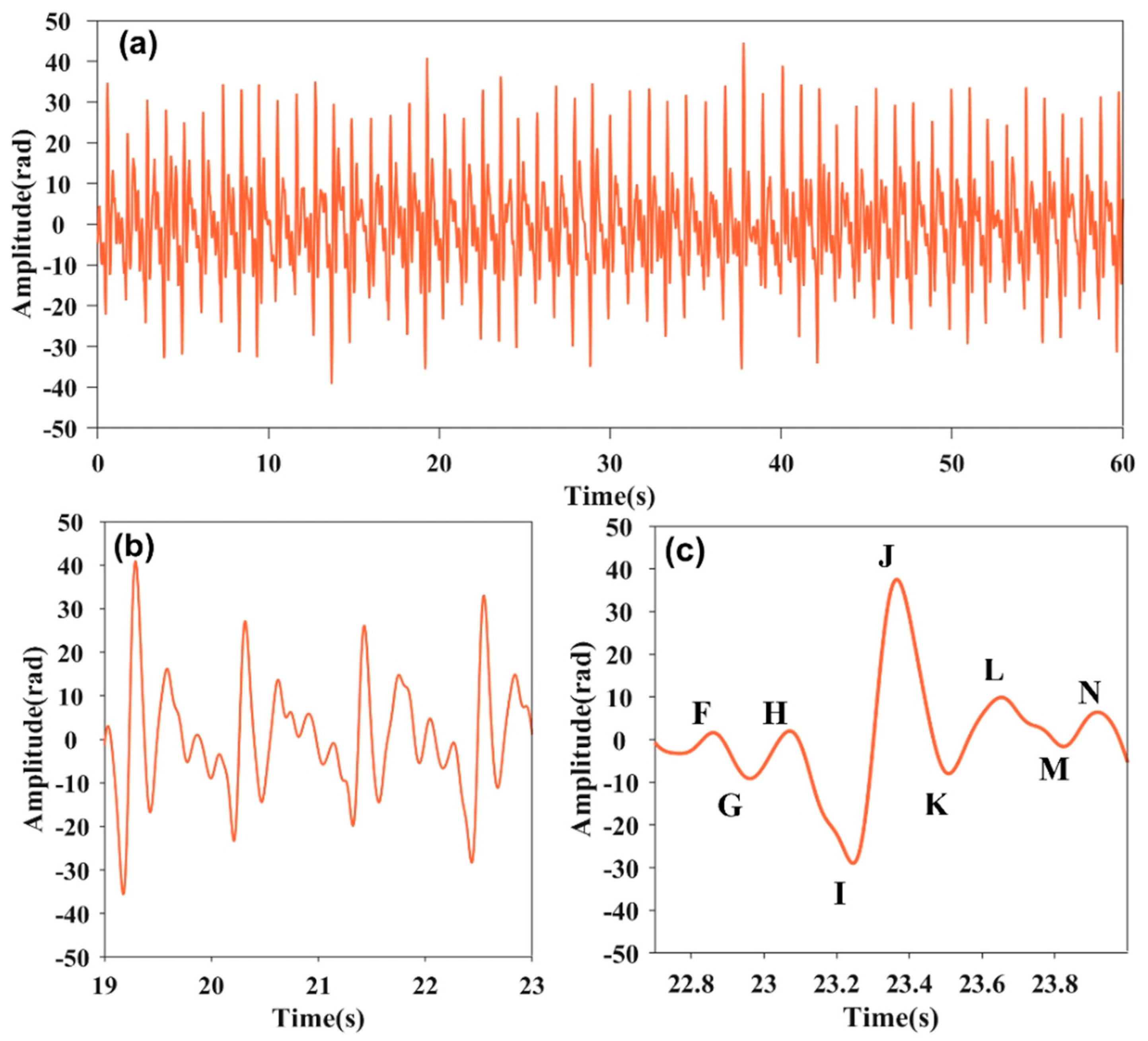
By band-pass filtering, the heartbeat signal is obtained as Figure 3. The 60 s heartbeat signal is shown as Figure 3a. Figure 3b provides more details about the heartbeat signal. The frequency of the heartbeat signal is about 0.87 Hz, corresponding to the second frequency spikes in Figure 2b. As is illustrated in Figure 3c, the heartbeat signal exhibits consistent characteristics to the standard Ballistocardiogram (BCG) shown in reference [24]. Its single-cycle order encompasses fluctuations of FGHIJKLMN. FGH, IJK and LMN, respectively, correspond to the pre-systolic phases, systolic phases, and diastolic phases [25,26]. The results confirm the proposed sensor can not only count the heartbeat rate, but can also accurately restore the details in the cardiac cycle. The details are very helpful for health assessment and sleep disorder diagnosis.
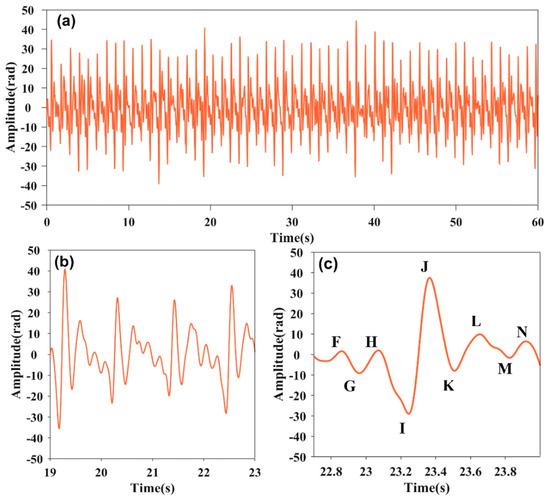
Figure 3.
(a) Heartbeat signal image at 60 s. (b) Heartbeat signal image from 19 to 23 s. (c) Heartbeat signal waveform within a single cycle.
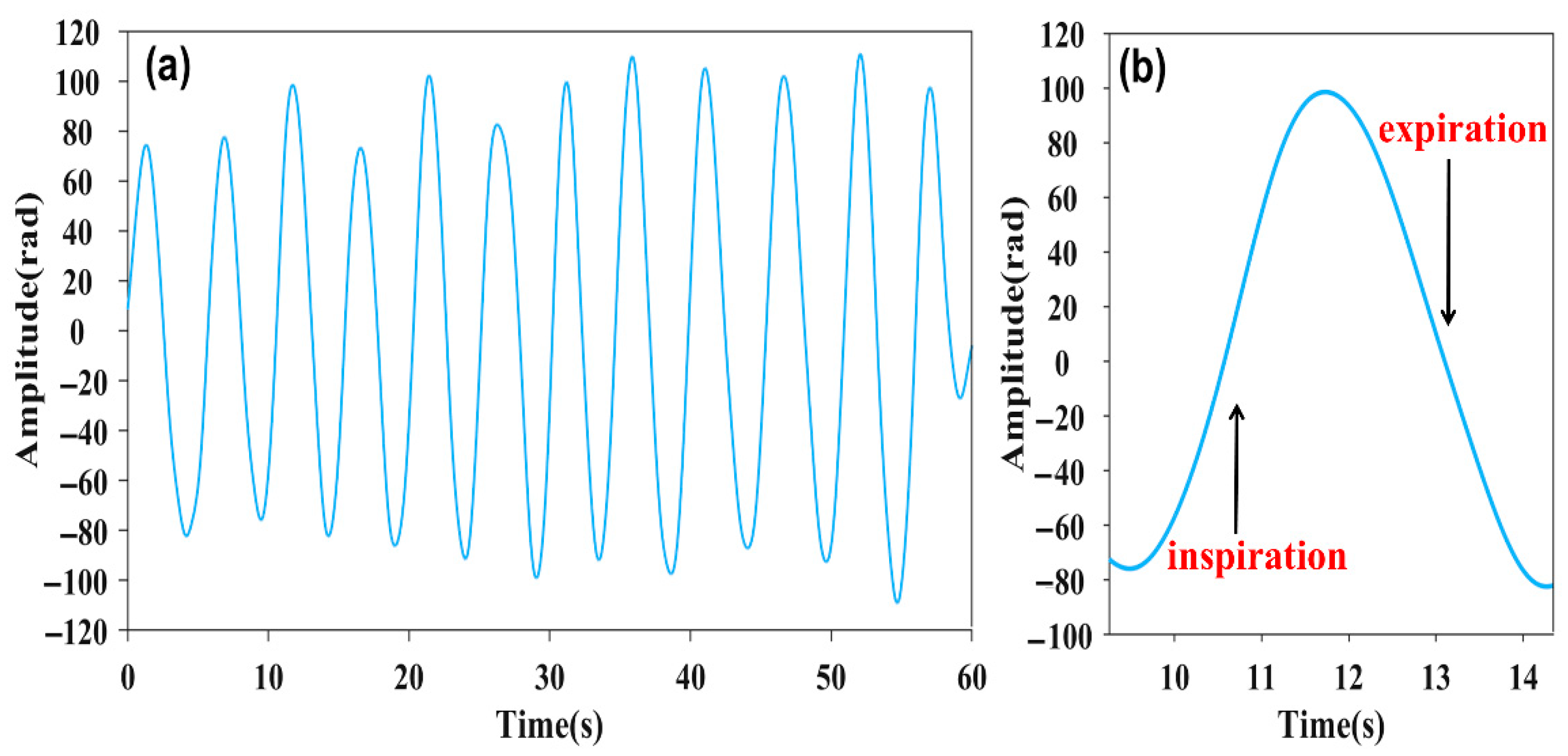
The 60 s respiration signal is shown in Figure 4a, whose cycle is approximately 4 s, corresponding to the first frequency spike in Figure 2b. The respiration waveform exhibits resemblance to the cosine function. And the amplitude fluctuation of the respiration signal can be attributed to the nonuniform signal of the respiration [27]. As is demonstrated in Figure 4b, the waveform reflects the process of inspiration and expiration, respectively. The amplitude of the respiration signal increase corresponds to the inhalation process, while the amplitude decrease corresponds to the expiration process.
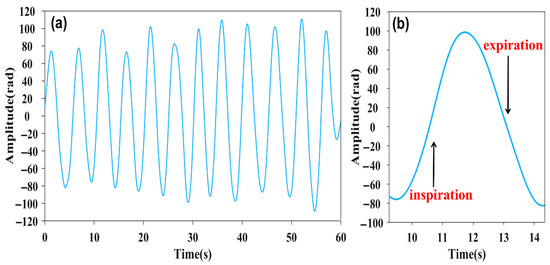
Figure 4.
(a) Respiration signal image at 60 s. (b) Respiration signal waveform within a single cycle.
The results above confirm the feasibility of the proposed sensor in monitoring respiration and heartbeat signals. The proposed sensor not only can achieve the HR and RR counting, but also can accurately record the detailed waveforms with low distortion.
3.2. Body Movement Monitoring
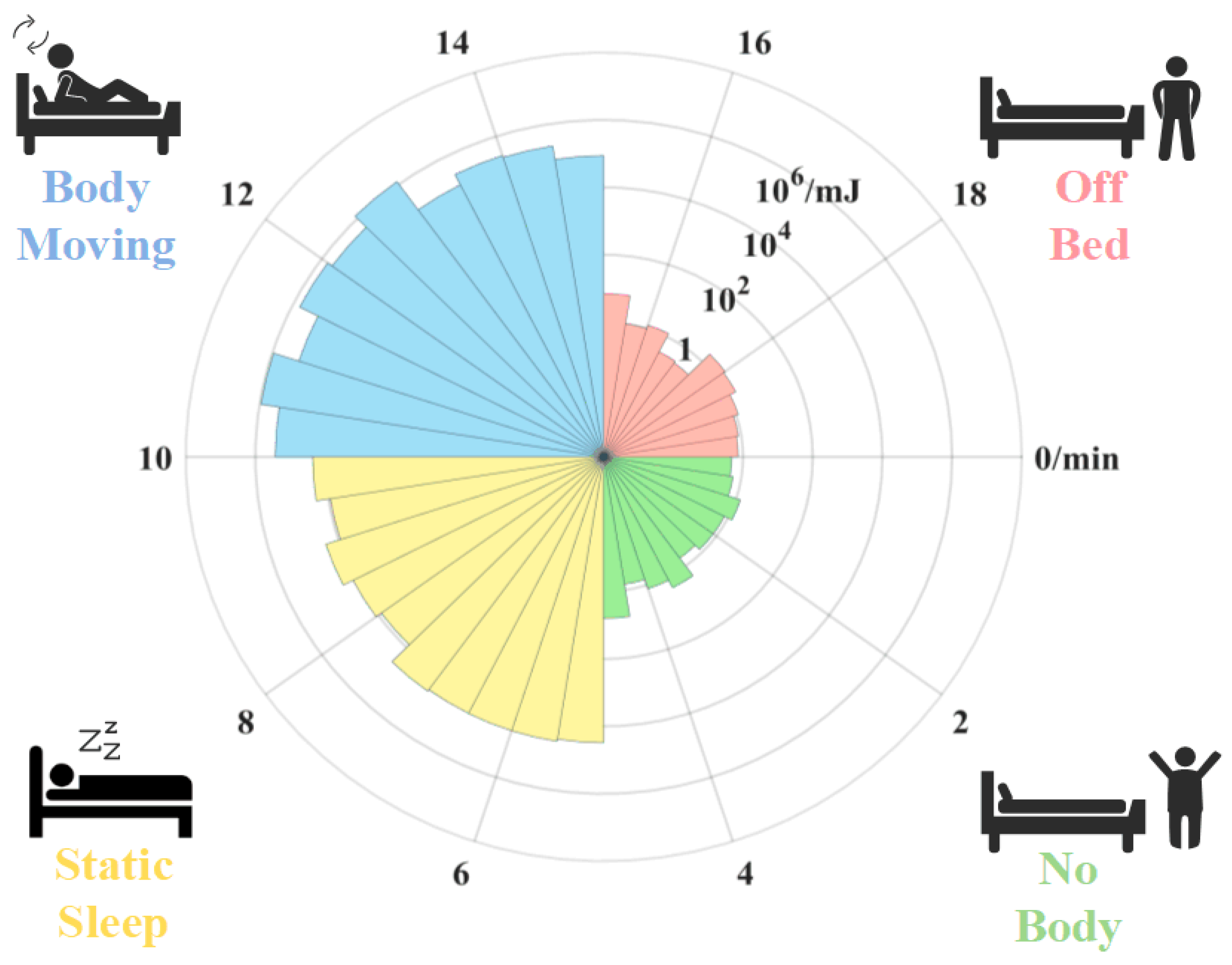
Body movement monitoring is a significant component of sleep monitoring [28]. An experiment is conducted to test whether the proposed sensor can distinguish the body movement state. In the experiment, the body movement states are classified into four categories: no body, static sleep, body moving, and off bed [29]. Specifically, the state “no body” means that the participator has not lain on the bed. The state “off bed” means that the participator has left the bed. The spectral energy of the signals in the four states is calculated by Equation (8).
Figure 5 shows the variation in SE acquired by the mattress with time as the subject is at one of the four states in turn. At 0–5 min, the subject is not on the bed and SE is approximately . At 5 to 10 min, the subject lies quietly on the mattress, and the SE increases as about . At 10 to 15 min, the subject moves on the bed. The peak value of the SE reaches . At the 15th minute, the SE decreases to a similar level as the first stage while the subject gets off the bed. Consequently, the SE is related to the body movement of the subject. The results show that the proposed sensor has potential for monitoring body movement during sleep.
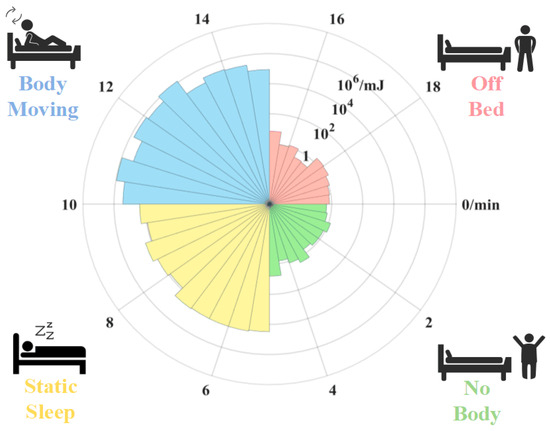
Figure 5.
Signal energy variation over time with time interval of 30 s.
3.3. All-Night Sleep Monitoring
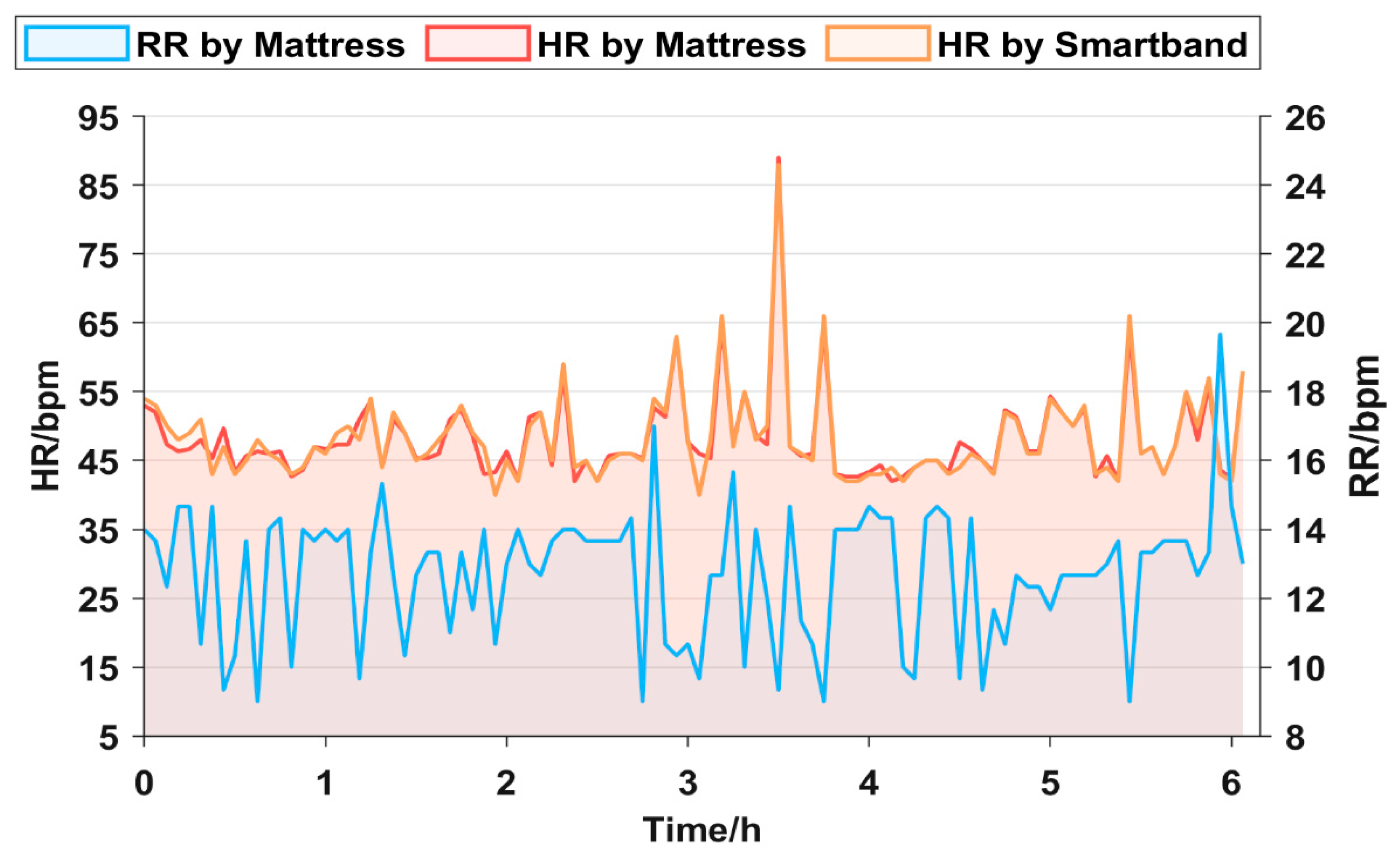
An experiment is conducted to verify the effectiveness of the mattress in monitoring sleep overnight. The subject lies on the proposed mattress. For comparison, the subject wears the smart band based on the PPG principle to monitor HR [30]. The HR curves obtained by the proposed mattress (red line) and smart band (orange line) are presented in Figure 6. Both the two HR curves are highly consistent, with a maximum error of 6 bpm (beat per minute). During the all-night sleep process, HR remains less than 60 bpm most of the time. It is noted that there is an extremely high value exceeding 80 bpm from 3 to 4 h. The abnormal value may be related to the subject suddenly waking up or having nightmares.
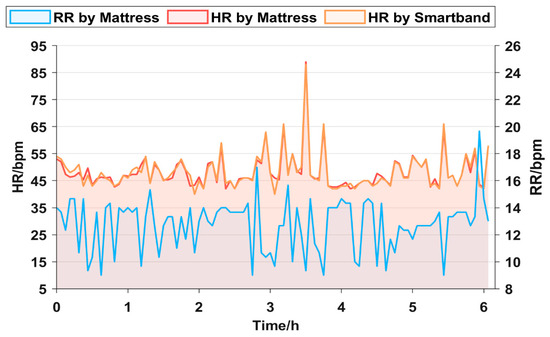
Figure 6.
All-night HR curves monitored by two devices and all-night RR curves monitored by the mattress.
The overnight RR is monitored by the mattress. As shown in Figure 6 (blue line), the overnight RR curve of the subject remains stable, ranging from 10 to 14 bpm and exhibiting a smooth trend. In general, the experiment results above show that the mattress possesses commendable stability and high sensitivity in monitoring respiration and heartbeat signals overnight. Its accuracy attains a comparable level with the commercial smart band.
To enhance the robustness of the conclusions and verify the stability of the mattress, repeated monitoring lasts for 4 days. The HR error in each day is calculated. Meanwhile, the maximum error, average error, and standard deviation of the error in each day are also calculated. The results of the error analysis are shown in Table 1:

Table 1.
Error analysis between HR extracted by the mattress and the smart band in 4 days.
It is obvious that the maximum error of the mattress remains about 6 bpm and the average errors are all less than 3 bpm. Moreover, the standard deviation of the error remains stable, which fluctuates around 1.5. It verifies the stability of the mattress in all-night sleep monitoring.
4. Conclusions
A non-intrusive sleep monitoring mattress based on an optical-fiber Michelson interferometer is proposed. The sensing arm of the optical-fiber Michelson interferometer is designed as a circular winding structure to enhance the sensitivity. The polarization fading is suppressed by the Faraday rotation mirrors, which improves the anti-interference capability. The phase of the interferometer is obtained by the PGC demodulation method. The proposed sensor not only can achieve the HR and RR counting, but also can accurately record the detailed waveforms with low distortion. The sensor can also distinguish the body movement state by the SE. An all-night sleep monitoring experiment was conducted. The results show good agreement between the proposed mattress and the commercial smart band. In conclusion, the proposed mattress shows good performance in sleep monitoring. The mattress has great potential for daily sleep monitoring and sleep disorder diagnosis in the future.
Author Contributions
Conceptualization, Y.Z. and X.H.; methodology, Y.Z.; software, S.L.; validation, M.H.; resources, Y.L. (Yang Lu); data curation, Y.L. (Yiao Liu); writing—original draft preparation, Y.Z.; writing—review and editing, X.H.; visualization, Y.Z.; supervision, K.Z.; project administration, X.H.; funding acquisition, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Student Innovation Training Project of Hunan Province, China (S202490002491) and the National Natural Science Foundation of China under Grant 12204542.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
Data are unavailable due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PGC | Phase-Generated Carrier |
| HR | Heart Rate |
| RR | Respiration Rate |
| bpm | Beat Per Minute |
| PSG | Polysomnography |
| PPG | Photoplethysmography |
| SE | Signal Energy |
| BCG | Ballistocardiogram |
| FFT | Fast Fourier Transformation |
References
- Rundo, J.V.; Downey, R., III. Polysomnography Handb. Clin. Neurol. 2019, 160, 381–392. [Google Scholar] [CrossRef]
- Dhingra, L.S.; Aminorroaya, A.; Oikonomou, E.K.; Nargesi, A.A.; Wilson, F.P.; Krumholz, H.M.; Khera, R. Use of wearable devices in individuals with or at risk for cardiovascular disease in the US, 2019 to 2020. JAMA Netw. Open 2023, 6, e2316634. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Gong, S.; Jiang, N.; Dai, Y.; Yang, J.; Jiang, L.; Tong, J. Mattress-based non-influencing sleep apnea monitoring system. Sensors 2023, 23, 3675. [Google Scholar] [CrossRef]
- Adnane, M.; Jiang, Z.; Choi, S.; Jang, H. Detecting specific health-related events using an integrated sensor system for vital sign monitoring. Sensors 2009, 9, 6897–6912. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H. Research on flexible sensors for wearable devices: A review. Nanomaterials 2025, 15, 520. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Mishra, P.; Kumar, S. Advancements in optical fiber-based wearable sensors for smart health monitoring. Biosens. Bioelectron. 2024, 254, 116232. [Google Scholar] [CrossRef]
- Wo, J.; Wang, H.; Sun, Q.; Shum, P.P.; Liu, D. Noninvasive respiration movement sensor based on distributed Bragg reflector fiber laser with beat frequency interrogation. J. Biomed. Opt. 2014, 19, 17003. [Google Scholar] [CrossRef]
- Sadek, I.; Biswas, J.; Fook, V.F.S.; Mokhtari, M. Automatic heart rate detection from FBG sensors using sensor fusion and enhanced empirical mode decomposition. In Proceedings of the 2015 IEEE International Symposium on Signal Processing and Information Technology (ISSPIT), Abu Dhabi, United Arab Emirates, 7–10 December 2015; pp. 349–353. [Google Scholar]
- Han, P.; Li, L.; Zhang, H.; Guan, L.; Marques, C.; Savović, S.; Ortega, B.; Min, R.; Li, X. Low-cost plastic optical fiber sensor embedded in mattress for sleep performance monitoring. Opt. Fiber Technol. 2021, 64, 102541. [Google Scholar] [CrossRef]
- Tan, F.; Chen, S.; Lyu, W.; Liu, Z.; Yu, C.; Lu, C.; Tam, H.-Y. Non-invasive human vital signs monitoring based on twin-core optical fiber sensors. Biomed. Opt. Express 2019, 10, 5940–5952. [Google Scholar] [CrossRef]
- Fook, V.F.S.; Jayachandran, M.; Jiliang, E.P.; Yongwei, Z.; Jianzhong, E.H. Fiber Bragg Grating-Based Monitoring and Alert System for Care of Residents in Nursing Homes. In Proceedings of the 2018 IEEE 4th World Forum on Internet of Things (WF-IoT), Singapore, 5–8 February 2018; pp. 195–200. [Google Scholar] [CrossRef]
- Zhou, F.; Luo, B.; Zou, X.; Zou, C.; Wu, D.; Wang, Z.; Bai, Y.; Zhao, M. A wearable sandwich heterostructure multimode fiber optic microbend sensor for vital signal monitoring. Sensors 2024, 24, 2209. [Google Scholar] [CrossRef]
- Wang, S.; Ni, X.; Li, L.; Wang, J.; Liu, Q.; Yan, Z.; Zhang, L.; Sun, Q. Noninvasive monitoring of vital signs based on highly sensitive fiber optic mattress. IEEE Sens. J. 2020, 20, 6182–6190. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Z.; Tang, M. Advances in multicore fiber interferometric sensors. Sensors 2023, 23, 3436. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Z.; Tang, M.; Fu, S.; Wang, L.; Guo, N.; Jin, C.; Tam, H.Y.; Lu, C. Robust in-fiber spatial interferometer using multicore fiber for vibration detection. Opt. Express 2018, 26, 29629–29637. [Google Scholar] [CrossRef]
- Stolárik, M.; Kepák, S.; Pinka, M.; Uubík, J.; Nedoma, J. Comparative in situ study of dynamic load generated by gravel piles measured by a fiber-optic interferometer. Sensors 2022, 22, 5579. [Google Scholar] [CrossRef]
- Martinez-Ramirez, L.G.; Hernández-Romano, I.; Guzmán-Cano, C.; Marrujo-García, S.; Fernandez-Jaramillo, A.A.; Estudillo-Ayala, J.M.; Rojas-Laguna, R.; Sierra-Hernandez, J.M. Experimental demonstration to enhance the curvature sensitivity of a fiber Mach–Zehnder interferometer based on a waist-enlarged technique using polymer. Photonics 2024, 11, 262. [Google Scholar] [CrossRef]
- Shao, M.; Yuan, Y.; Liu, Y.; Fu, H.; Qiao, X. All-Fiber Michelson interferometer for heart rate and breath monitoring. IEEE Sens. J. 2024, 24, 23909–23917. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Q.; Wang, J.; Chen, M.; Zhang, Y.; Lu, Y.; Meng, Z. Fiber Optic Sensing and Demodulation Method and Device Based on Electro-Optic Phase Modulator. Chinese Patent CN115235519A, 25 October 2022. [Google Scholar]
- Chylinski, D.; Rudzik, F.; Coppieters ‘t Wallant, D.; Grignard, M.; Vandeleene, N.; Van Egroo, M.; Thiesse, L.; Solbach, S.; Maquet, P.; Phillips, C.; et al. Validation of an automatic arousal detection algorithm for whole-night sleep EEG recordings. Clocks Sleep 2020, 2, 258–272. [Google Scholar] [CrossRef]
- T/CPAM 002-2020; Machinery Analysis and Interpretation Specifications for the Polysomnograms. China International Exchange and Promotive Association for Medical and Health Care: Beijing, China, 2020.
- Zhao, L.; Peng, M.; Yang, X.; Zhou, Q. Detection Method of Sleep Information Based on Piezoceramic. Chin. J. Sci. Instrum. 2018, 39, 246–252. [Google Scholar] [CrossRef]
- Bouni, A.; Arsac, L.M.; Chevalerias, O.; Deschodt-Arsac, V. From accelerometer to cognition: Hand motion can reflect effects of cardiac coherence on cognitive flexibility. Sensors 2025, 25, 2942. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.S.; Zhu, W.W.; Yang, T. Research progress of ballistocardiogram signal and its application in medicine. China Med. Devices 2021, 36, 168–172. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, H.; Chen, L.; Chen, W.; Jiang, C.; Pan, Y. Intelligent analysis and heartbeat saliency map representation of postoperative atrial fibrillation recurrence based on mobile single-lead electrocardiogram. IEEE Trans. Instrum. Meas. 2024, 73, 2520610. [Google Scholar] [CrossRef]
- Ernst, G. Hidden signals—The history and methods of heart rate variability. Front. Public Health 2017, 5, 265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qi, D.; Liu, J.; Guo, S. Unconstrained Monitoring of Sleep Respiration Based on Detection of Pressure Fluctuations on Mattress. J. Med. Biomech. 2021, 36, 116. [Google Scholar] [CrossRef]
- Jiang, Z.; Lee, Y.S.; Wang, Y.; John, H.; Fang, L.; Tang, Y. Advancements in flexible sensors for monitoring body movements during sleep: A review. Sensors 2024, 24, 5091. [Google Scholar] [CrossRef] [PubMed]
- Recmanik, M.; Martinek, R.; Nedoma, J.; Jaros, R.; Pelc, M.; Hajovsky, R.; Velicka, J.; Pies, M.; Sevcakova, M.; Kawala-Sterniuk, A. A review of patient bed sensors for monitoring of vital signs. Sensors 2024, 24, 4767. [Google Scholar] [CrossRef]
- Hong, H.; Dai, L.; Zheng, X. Advances in wearable sensors for learning analytics: Trends, challenges, and prospects. Sensors 2025, 25, 2714. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).