Liver Status Assessment by Spectrally and Time Resolved IR Detection of Drug Induced Breath Gas Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Handling of Breath Gas
2.2. Spectral Tuning
2.3. Spectral Readjustment
2.4. Setup
2.5. Operation Planning
3. Results
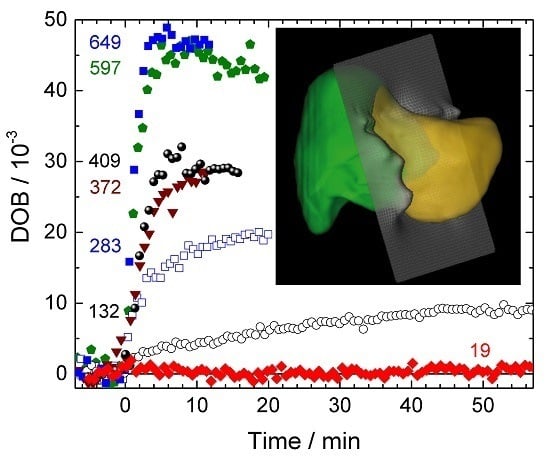
3.1. DOB Kinetics
3.2. Operation Planning
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DOB | Delta over baseline |
| LiMAx | Liver maximal capacity |
| QCL | Quantum cascade laser |
| MCT | Mercury Cadmium Telluride |
References
- Beauchamp, J. Inhaled today, not gone tomorrow: Pharmacokinetics and environmental exposure of volatiles in exhaled breath. J. Breath Res. 2011, 5, 037103. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Pajares, J.M. Review article: 13C-urea breath test in the diagnosis of helicobacter pylori infection—A critical review. Aliment. Pharmacol. Ther. 2004, 20, 1001–1017. [Google Scholar] [CrossRef] [PubMed]
- Haisch, M.; Hering, P.; Fuss, W.; Fabinski, W. A sensitive isotope selective nondispersive infrared spectrometer for 13CO2 and 12CO2 concentration measurements in breath samples. Isot. Isot. Environ. Health Stud. 1994, 30, 247–251. [Google Scholar] [CrossRef]
- Stockmann, M.; Lock, J.F.; Riecke, B.; Heyne, K.; Martus, P.; Fricke, M.; Lehmann, S.; Niehues, S.M.; Schwabe, M.; Lemke, A.J.; et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann. Surg. 2009, 250, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, D.A.; Luke, A.H. Rapid 18O analysis of CO2 samples by continuous-flow isotope ratio mass spectrometry. J. Mass Spectrom. 1997, 32, 1332–1336. [Google Scholar] [CrossRef]
- Braden, B.; Haisch, M.; Duan, L.; Lembcke, B.; Caspary, W.; Hering, P. Clinically feasible stable isotope technique at a reasonable price: Analysis of 13CO2/12CO2-abundance in breath samples with a new isotope selective-nondispersive infrared spectrometer. Z. Gastroenterol. 1994, 32, 675–678. [Google Scholar] [PubMed]
- Haycock, G.B.; Schwartz, G.J.; Wisotsky, D.H. Geometric method for measuring body surface area: A height-weight formula validated in infants, children, and adults. J. Pediatr. 1978, 93, 62–66. [Google Scholar] [CrossRef]
- Stockmann, M.; Lock, J.F.; Malinowski, M.; Niehues, S.M.; Seehofer, D.; Neuhaus, P. The limax test: A new liver function test for predicting postoperative outcome in liver surgery. HPB 2010, 12, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Reese, T.; Malinowski, M.; Valle, E.; Seehofer, D.; Puhl, G.; Neuhaus, P.; Pratschke, J.; Stockmann, M. Reductions in post-hepatectomy liver failure and related mortality after implementation of the limax algorithm in preoperative work-up: A single centre analysis of 1170 hepatectomies of one or more segments. HPB 2015, 17, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Rubin, T.; von Haimberger, T.; Helmke, A.; Heyne, K. Quantitative determination of metabolization dynamics by a real-time 13CO2 breath test. J. Breath Res. 2011, 5, 027102. [Google Scholar] [CrossRef] [PubMed]
- Rubin, T. Konzeption und Entwicklung eines Infrarot-Spektrometers zur Bestimmung der Konzentration von 13CO2 und 12CO2 im Gasfluss. Available online: http://edocs.fu-berlin.de/docs/receive/FUDOCS_document_000000024491 (accessed on 13 May 2016).
- Lourenco, C.; Turner, C. Breath analysis in disease diagnosis: Methodological considerations and applications. Metabolites 2014, 4, 465–498. [Google Scholar] [CrossRef] [PubMed]
- Rubin, T.; Heyne, K. Apparatus for Infrared Absorption Spectroscopy with Pre-Chamber for Homogenising A Test Gas. De. Patent EP 2626128 A1 20130814, 2009. [Google Scholar]
- Nelson, D.D.; McManus, B.; Urbanski, S.; Herndon, S.; Zahniser, M.S. High precision measurements of atmospheric nitrous oxide and methane using thermoelectrically cooled mid-infrared quantum cascade lasers and detectors. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 3325–3335. [Google Scholar] [CrossRef] [PubMed]
- McManus, J.B.; Zahniser, M.S.; Nelson, D.D.; Williams, L.R.; Kolb, C.E. Infrared laser spectrometer with balanced absorption for measurement of isotopic ratios of carbon gases. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2002, 58, 2465–2479. [Google Scholar] [CrossRef]
- Wysocki, G.; McCurdy, M.; So, S.; Weidmann, D.; Roller, C.; Curl, R.F.; Tittel, F.K. Pulsed quantum-cascade laser-based sensor for trace-gas detection of carbonyl sulfide. Appl. Opt. 2004, 43, 6040–6046. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Shorter, J.; McManus, J.; Zahniser, M. Sub-part-per-billion detection of nitric oxide in air using a thermoelectrically cooled mid-infrared quantum cascade laser spectrometer. Appl. Phys. B 2002, 75, 343–350. [Google Scholar] [CrossRef]
- Rothman, L.S.; Gordon, I.E.; Barbe, A.; Benner, D.C.; Bernath, P.F.; Birk, M.; Boudon, V.; Brown, L.R.; Campargue, A.; Champion, J.-P. The hitran 2008 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 533–572. [Google Scholar] [CrossRef]
- Herzberg, G. Infrared and Raman Spectra of Polyatomic Molecules; D. Van Nostrand Company: New York, NY, USA, 1945. [Google Scholar]
- Niehues, S.M.; Unger, J.K.; Malinowski, M.; Neymeyer, J.; Hamm, B.; Stockmann, M. Liver volume measurement: Reason of the difference between in vivo ct-volumetry and intraoperative ex vivo determination and how to cope it. Eur J. Med. Res. 2010, 15, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.F.; Schwabauer, E.; Martus, P.; Videv, N.; Pratschke, J.; Malinowski, M.; Neuhaus, P.; Stockmann, M. Early diagnosis of primary nonfunction and indication for reoperation after liver transplantation. Liver Transplant. 2010, 16, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Kaffarnik, M.F.; Lock, J.F.; Vetter, H.; Ahmadi, N.; Lojewski, C.; Malinowski, M.; Neuhaus, P.; Stockmann, M. Early diagnosis of sepsis-related hepatic dysfunction and its prognostic impact on survival: A prospective study with the limax test. Crit. Care 2013, 17, R259. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.F.; Kotobi, A.N.; Malinowski, M.; Schulz, A.; Jara, M.; Neuhaus, P.; Stockmann, M. Predicting the prognosis in acute liver failure: Results from a retrospective pilot study using the limax test. Ann. Hepatol. 2013, 12, 556–562. [Google Scholar] [PubMed]
- Brinkhaus, G.; Lock, J.F.; Malinowski, M.; Denecke, T.; Neuhaus, P.; Hamm, B.; Gebauer, B.; Stockmann, M. Ct-guided high-dose-rate brachytherapy of liver tumours does not impair hepatic function and shows high overall safety and favourable survival rates. Ann. Surg. Oncol. 2014, 21, 4284–4292. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, S.; von Loeffelholz, C.; Lock, J.F.; Doecke, S.; Sinn, B.V.; Rieger, A.; Malinowski, M.; Pfeiffer, A.F.; Neuhaus, P.; Stockmann, M. Nonalcoholic steatohepatits and liver steatosis modify partial hepatectomy recovery. J. Investig. Surg. 2015, 28, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Malinowski, M.; Lüttgert, K.; Schott, E.; Neuhaus, P.; Stockmann, M. Prognostic value of enzymatic liver function for the estimation of short-term survival of liver transplant candidates: A prospective study with the limax test. Transplant Int. 2015, 28, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, M.; Stary, V.; Lock, J.F.; Schulz, A.; Jara, M.; Seehofer, D.; Gebauer, B.; Denecke, T.; Geisel, D.; Neuhaus, P.; et al. Factors influencing hypertrophy of the left lateral liver lobe after portal vein embolization. Langenbecks Arch. Surg. 2015, 400, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Bednarsch, J.; Malinowski, M.; Pratschke, J.; Stockmann, M. Effects of oxaliplatin-based chemotherapy on liver function-an analysis of impact and functional recovery using the limax test. Langenbecks Arch. Surg. 2016, 401, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Bednarsch, J.; Valle, E.; Lock, J.F.; Malinowski, M.; Schulz, A.; Seehofer, D.; Jung, T.; Stockmann, M. Reliable assessment of liver function using limax. J. Surg. Res. 2015, 193, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, M.; Lock, J.F.; Malinowski, M.; Seehofer, D.; Puhl, G.; Pratschke, J.; Neuhaus, P. How to define initial poor graft function after liver transplantation?—A new functional definition by the limax test. Transpl. Int. 2010, 23, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Kaffarnik, M.F.; Lock, J.F.; Seehofer, D.; Stockmann, M.; Neuhaus, P. Leberresektionen—Was ist perioperativ zu beachten? Viszeralmedizin 2011, 27, 65–73. [Google Scholar] [CrossRef]
- Fan, S.-T.; Lo, C.-M.; Liu, C.-L.; Yong, B.-H.; Chan, J.K.-F.; Ng, I.O.-L. Safety of donors in live donor liver transplantation using right lobe grafts. Arch. Surg. 2000, 135, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.; Viganò, L.; Polastri, R.; Muratore, A.; Eminefendic, H.; Regge, D.; Capussotti, L. Postoperative liver dysfunction and future remnant liver: Where is the limit? World J. Surg. 2007, 31, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, A.; Ruzzenente, A.; Conci, S.; Valdegamberi, A.; Iacono, C. How much remnant is enough in liver resection? Dig. Surg. 2012, 29, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Truant, S.; Oberlin, O.; Sergent, G.; Lebuffe, G.; Gambiez, L.; Ernst, O.; Pruvot, F.-R. Remnant liver volume to body weight ratio ≥0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J. Am. Coll. Surg. 2007, 204, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broek, M.A.; Olde Damink, S.W.; Dejong, C.H.; Lang, H.; Malagó, M.; Jalan, R.; Saner, F.H. Liver failure after partial hepatic resection: Definition, pathophysiology, risk factors and treatment. Liver Int. 2008, 28, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Kasyutich, V.L.; Martin, P.A. (CO2)-C-13/(CO2)-C-12 isotopic ratio measurements with a continuous-wave quantum cascade laser in exhaled breath. Infrared Phys. Technol. 2012, 55, 60–66. [Google Scholar] [CrossRef]
- Lock, J.F.; Malinowski, M.; Seehofer, D.; Hoppe, S.; Röhl, R.I.; Niehues, S.M.; Neuhaus, P.; Stockmann, M. Function and volume recovery after partial hepatectomy: Influence of preoperative liver function, residual liver volume, and obesity. Langenbecks Arch. Surg. 2012, 397, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, M.; Jara, M.; Lüttgert, K.; Orr, J.; Lock, J.F.; Schott, E.; Stockmann, M. Enzymatic liver function capacity correlates with disease severity of patients with liver cirrhosis: A study with the limax test. Dig. Dis. Sci. 2014, 59, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Alizai, P.H.; Wendl, J.; Roeth, A.A.; Klink, C.D.; Luedde, T.; Steinhoff, I.; Neumann, U.P.; Schmeding, M.; Ulmer, F. Functional liver recovery after bariatric surgery—A prospective cohort study with the limax test. Obes. Surg. 2015, 25, 2047–2053. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubin, T.; Von Haimberger, T.; Helmke, A.; Lock, J.; Stockmann, M.; Heyne, K. Liver Status Assessment by Spectrally and Time Resolved IR Detection of Drug Induced Breath Gas Changes. Photonics 2016, 3, 31. https://doi.org/10.3390/photonics3020031
Rubin T, Von Haimberger T, Helmke A, Lock J, Stockmann M, Heyne K. Liver Status Assessment by Spectrally and Time Resolved IR Detection of Drug Induced Breath Gas Changes. Photonics. 2016; 3(2):31. https://doi.org/10.3390/photonics3020031
Chicago/Turabian StyleRubin, Tom, Theodore Von Haimberger, Alexander Helmke, Johan Lock, Martin Stockmann, and Karsten Heyne. 2016. "Liver Status Assessment by Spectrally and Time Resolved IR Detection of Drug Induced Breath Gas Changes" Photonics 3, no. 2: 31. https://doi.org/10.3390/photonics3020031
APA StyleRubin, T., Von Haimberger, T., Helmke, A., Lock, J., Stockmann, M., & Heyne, K. (2016). Liver Status Assessment by Spectrally and Time Resolved IR Detection of Drug Induced Breath Gas Changes. Photonics, 3(2), 31. https://doi.org/10.3390/photonics3020031