Imaging of Chemical Reactions Using a Terahertz Chemical Microscope
Abstract
1. Introduction
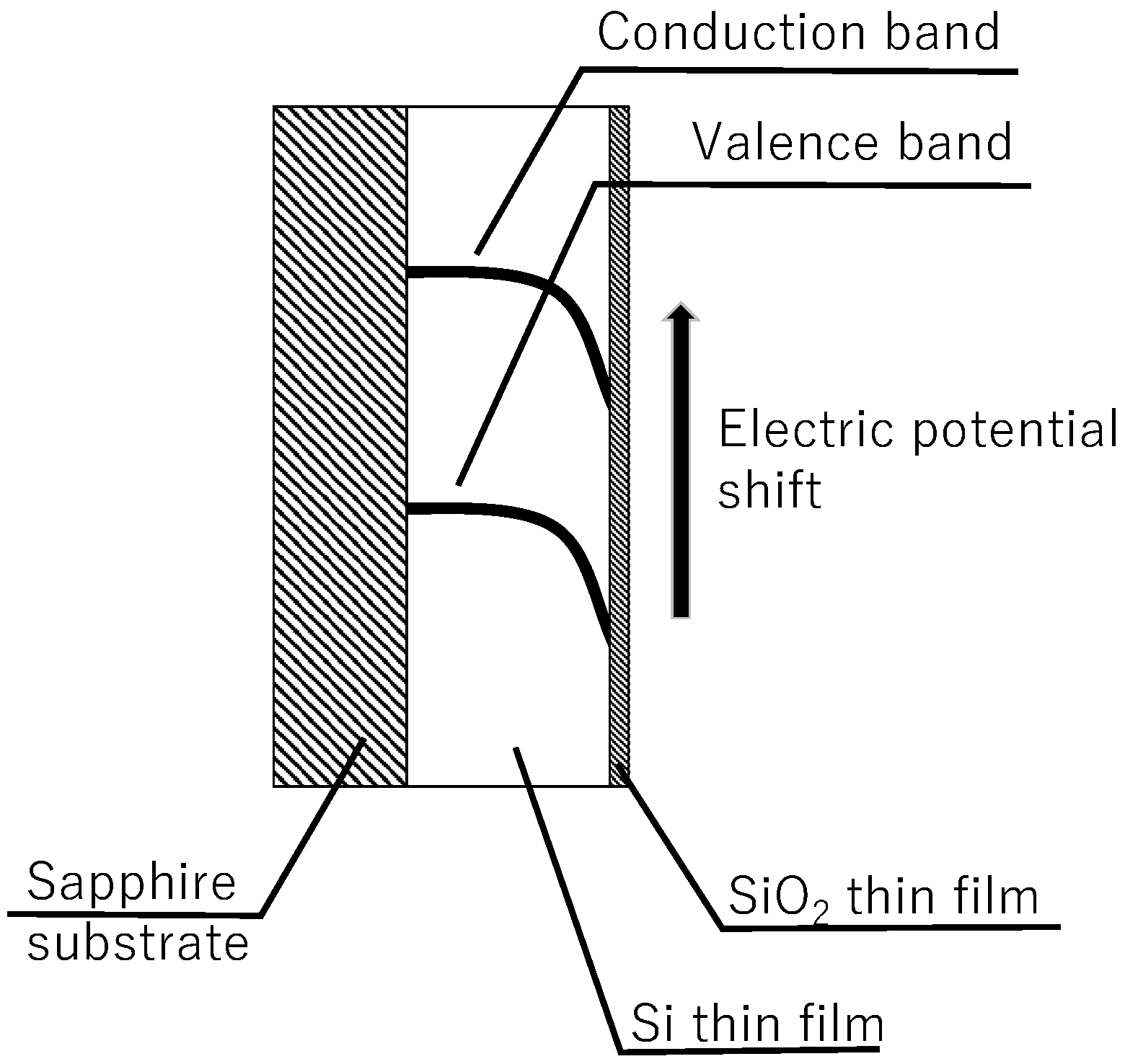
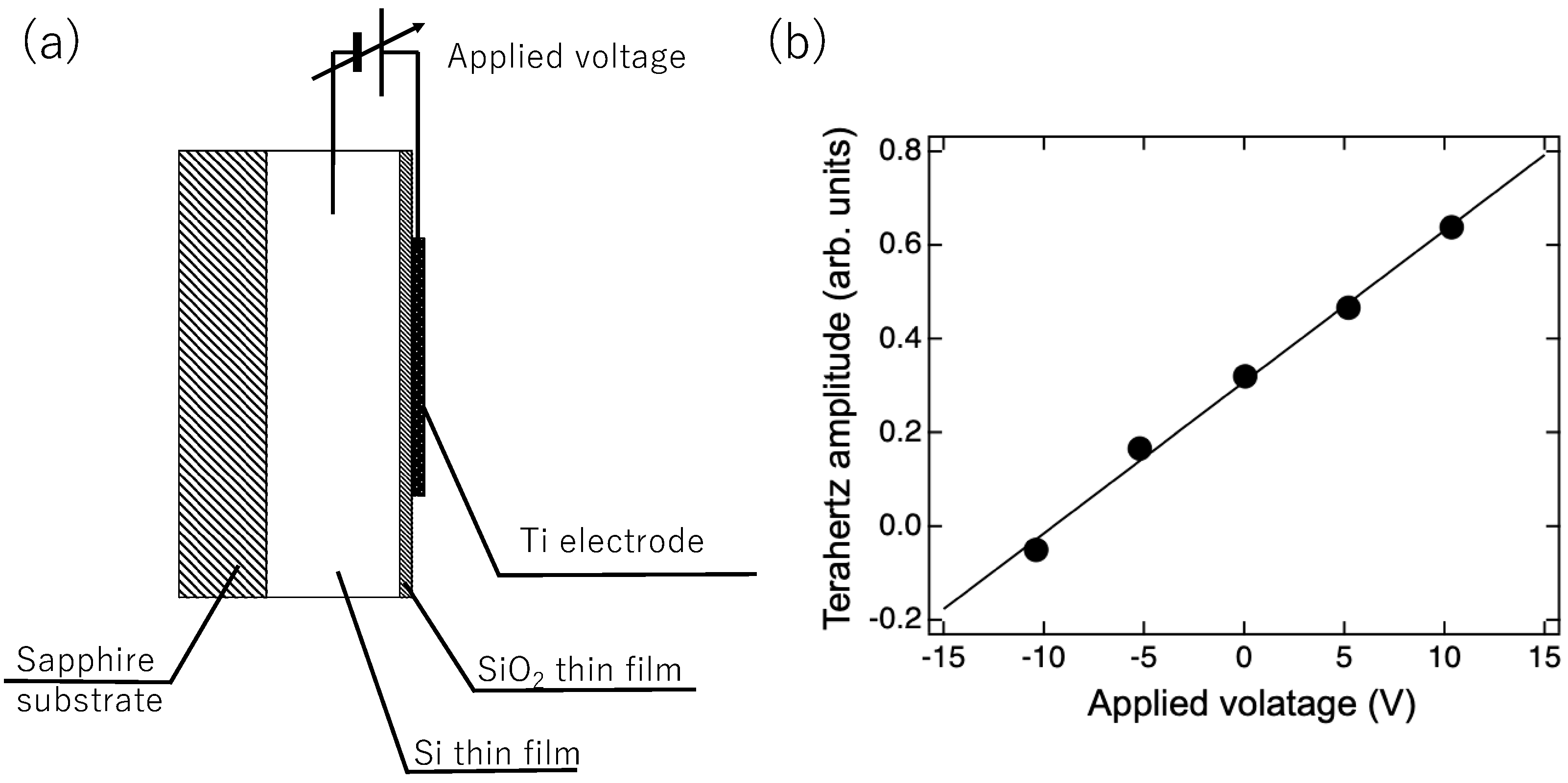
2. Measurement Principle
3. Optical Setup
4. Imaging of Chemical Reactions
4.1. pH Measurements
4.2. Ion Measurements
- Polyvinyl chloride (PVC), 30.9 wt.% (Fujifilm Wako Pure Chemicals, Tokyo, Japan)
- DOA Standard, 67.0 wt.% (99.0 +% (Capillary GC), Fujifilm Wako Pure Chemicals, Tokyo, Japan)
- ETH 2120, 1.8 wt.% (≥98.0%(HPLC), Sigma–Aldrich, Saint-Louis, CA, USA)
- Sodium tetraphenylborate, 0.3 wt.% (≥99.5%, Sigma–Aldrich, Saint-Louis, CA, USA)
- Tetrahydrofuran (THF), 1.0 mL (99.5 +%(mass/mass) (GC), Fujifilm Wako Pure Chemical, Tokyo, Japan)
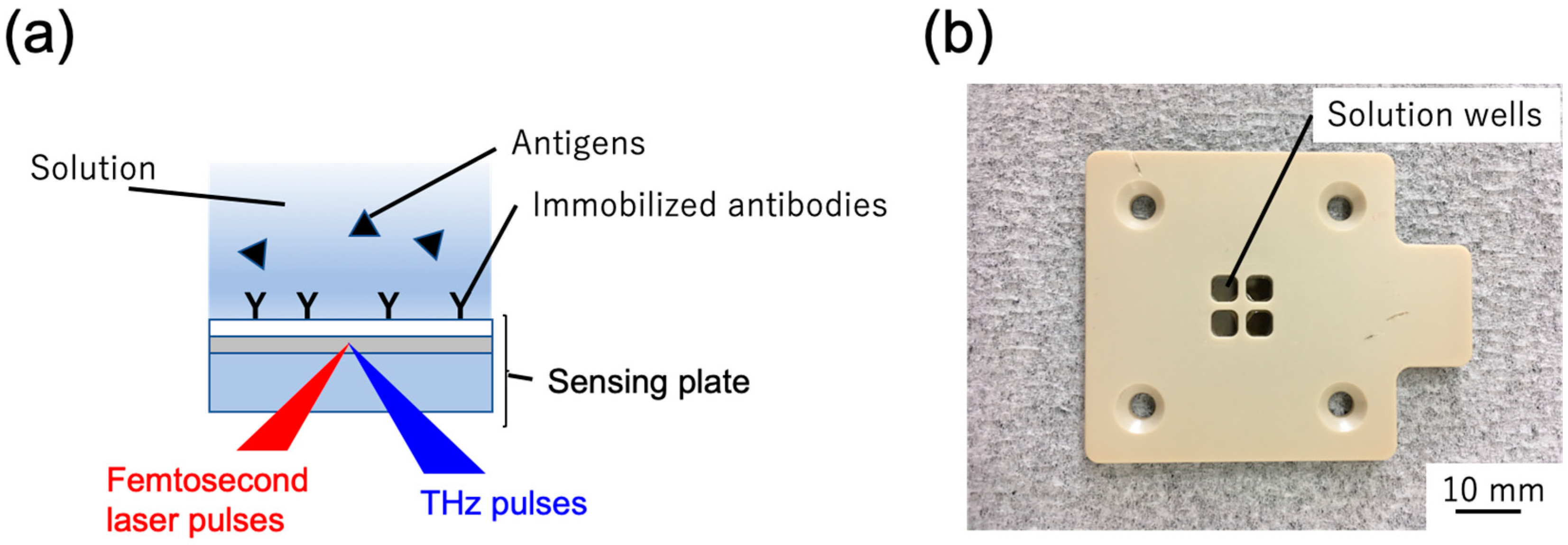
4.3. Measurement of Immune Reactions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Auston, D.H.; Glass, A.M. Optical generation of intense picosecond electrical pulses. Appl. Phys. Lett. 1972, 20, 398–399. [Google Scholar] [CrossRef]
- Auston, D.H. Picosecond optoelectronic switching and gating in silicon. Appl. Phys. Lett. 1975, 26, 101–103. [Google Scholar] [CrossRef]
- Tonouchi, M. Cutting-edge terahertz technology. Nat. Photonics 2007, 1, 97–105. [Google Scholar] [CrossRef]
- Exter, M.; Fattinger, C.; Grischkowsky, D. Terahertz time-domain spectroscopy of water vapor. Opt. Lett. 1989, 14, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.U.; Cooke, D.G.; Koch, M. Terahertz spectroscopy and imaging—Modern techniques and applications. Laser Photonics Rev. 2011, 5, 124–166. [Google Scholar] [CrossRef]
- Fischer, B.M.; Walther, M.; Jepsen, P.U. Far-infrared vibration modes of DNA components studied by terahertz time-domain spectroscopy. Phys. Med. Biol. 2002, 47, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, S.E.; Wolpert, D.; Markelz, A.G.; Hillbrecht, J.R.; Galan, J.; Brige, R.R. Protein Flexbility and Conformation State: A Comparison of Collective Vibrational Modes of Wild-Type and D96N Bacteriorhodopsin. Biophys. J. 2003, 85, 1269–1277. [Google Scholar] [CrossRef]
- Tonouchi, M.; Yamashita, M.; Hangyo, M. Vortex penetration in YBCO thin film strip observed by THz radiation imaging. Phys. B 2000, 284–288, 853–854. [Google Scholar] [CrossRef]
- Yamashita, M.; Tonouchi, M.; Hangyo, M. Visualization of supercurrent distribution by THz radiation mapping. Phys. B 2000, 284–288, 2067–2068. [Google Scholar] [CrossRef]
- Sotome, M.; Kida, N.; Horiuchi, S.; Okamoto, H. Terahertz radiation imaging of ferroelectric domain topography in room-temerature hjydorogen-bonded supermolecular ferroelectrics. ACS Photonics 2015, 2, 1373–1383. [Google Scholar] [CrossRef]
- Kiwa, T.; Tonouchi, M.; Yamashita, M.; Kawase, K. Laser terahertz-emission microscope for inspecting electrical faults in integrated circuits. Opt. Lett. 2003, 28, 2058–2060. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Kawase, K.; Otani, C.; Kiwa, T.; Tonouochi, M. Imaging of large-scale integrated circuits using laser terahertz emission microscopy. Opt. Exp. 2005, 13, 115–120. [Google Scholar] [CrossRef]
- Kiwa, T.; Tsukada, K.; Suzuki, M.; Tonouchi, M.; Migitaka, S.; Yokosawa, K. Laser terahertz emission system to investigate hydrogen gas sensors. Appl. Phys. Lett. 2005, 86, 261102. [Google Scholar] [CrossRef]
- Murakami, H.; Uchida, N.; Inoue, R.; Kim, S.; Kiwa, T.; Tonouchi, M. Laser terahertz emission microscope. Proc. IEEE 2007, 95, 1646–1657. [Google Scholar] [CrossRef]
- Klarskov, P.; Kim, H.; Colvin, V.L.; Mittleman, D.M. Nanoscale Laser Terahertz Emission Microsocpy. ACS Photonics 2017, 4, 2675–2680. [Google Scholar] [CrossRef]
- Kiwa, T.; Oka, S.; Kondo, J.; Kawayama, I.; Yamada, H.; Tonouchi, M.; Tsuakda, K. A Terahertz Chemical Microscope to Visualize Chemical Concentrations in Microfluidic Chips. Jpn. J. Appl. Phys. 2007, 46, 41–44. [Google Scholar] [CrossRef]
- Kiwa, T.; Kondo, J.; Oka, S.; Kawayama, I.; Yamada, H.; Tonouchi, M.; Tsukada, K. Chemical sensing plate with a laser-terahertz monitorins system. Appl. Opt. 2008, 37, 3324–3327. [Google Scholar] [CrossRef]
- Kiwa, T.; Kamiya, T.; Morimoto, T.; Sakai, K.; Tsukada, K. pH measurments in 16-nL-volume solutions using terahetz chemical microscopy. Opt. Exp. 2018, 26, 8232–8238. [Google Scholar] [CrossRef]
- Akimune, K.; Okawa, Y.; Sakai, K.; Kiwa, T.; Tsukada, K. Multi-ion sensing of buffer solutions using terahertz chemical microscopy. APEX 2014, 7, 122401. [Google Scholar] [CrossRef]
- Kiwa, T.; Kondo, Y.; Minami, Y.; Kawayama, I.; Tonouchi, M.; Tsukada, K. Terahertz chemical microscope for label-free detection of protein complex. Appl. Phys. Lett. 2010, 96, 211114. [Google Scholar] [CrossRef]
- Kiwa, T.; Tenma, A.; Takahashi, S.; Sakai, K.; Tsukada, K. Label free immune assay using terahertz chemical microscope. Sens. Actuators B 2013, 187, 8–11. [Google Scholar] [CrossRef]
- Kiwa, T.; Sakai, K.; Tsuakada, K. Stabilization method for signal drifts in terahertz chemical microscopy. Opt. Exp. 2014, 22, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, T.; Ogawa, M.; Sakai, K.; Kiwa, T.; Tsukada, K. Label-free detection of low-molecular-weight samples using a terahertz chemical microscope. APEX 2016, 9, 042401. [Google Scholar] [CrossRef]
- Kiwa, T.; Hagiwara, T.; Shinomiya, M.; Sakai, K.; Tsukada, K. Wrok function shifts of catalytic metals under hydrogen gas visualized by terahertz chemical microscopy. Opt. Exp. 2012, 20, 11637–11642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-C.; Hu, B.B.; Darrow, J.T.; Auston, D.H. Generation of femtosecond electromagnetic pulses from semiconductor surfaces. Appl. Phys. Lett. 1990, 56, 1011. [Google Scholar] [CrossRef]
- Tsukada, K.; Sebata, M.; Miyahara, Y.; Miyagi, H. Long-life multiple-ISFETS with polymeric gates. Sens. Actuators 1989, 18, 329–336. [Google Scholar] [CrossRef]
- Tero, R.; Misawa, N.; Watanabe, H.; Yamamura, S.; Nambu, S.; Nonogaki, Y.; Urisu, T. Fabrication of avidin single molecular layer on silicon oxide surfaces and formation of tethered lipid bilayer membranes. e-J. Surf. Sci. Nanotechnol. 2005, 3, 237–243. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiwa, T.; Kamiya, T.; Morimoto, T.; Fujiwara, K.; Maeno, Y.; Akiwa, Y.; Iida, M.; Kuroda, T.; Sakai, K.; Nose, H.; et al. Imaging of Chemical Reactions Using a Terahertz Chemical Microscope. Photonics 2019, 6, 10. https://doi.org/10.3390/photonics6010010
Kiwa T, Kamiya T, Morimoto T, Fujiwara K, Maeno Y, Akiwa Y, Iida M, Kuroda T, Sakai K, Nose H, et al. Imaging of Chemical Reactions Using a Terahertz Chemical Microscope. Photonics. 2019; 6(1):10. https://doi.org/10.3390/photonics6010010
Chicago/Turabian StyleKiwa, Toshihiko, Tatsuki Kamiya, Taiga Morimoto, Kentaro Fujiwara, Yuki Maeno, Yuki Akiwa, Masahiro Iida, Taihei Kuroda, Kenji Sakai, Hidetoshi Nose, and et al. 2019. "Imaging of Chemical Reactions Using a Terahertz Chemical Microscope" Photonics 6, no. 1: 10. https://doi.org/10.3390/photonics6010010
APA StyleKiwa, T., Kamiya, T., Morimoto, T., Fujiwara, K., Maeno, Y., Akiwa, Y., Iida, M., Kuroda, T., Sakai, K., Nose, H., Kobayashi, M., & Tsukada, K. (2019). Imaging of Chemical Reactions Using a Terahertz Chemical Microscope. Photonics, 6(1), 10. https://doi.org/10.3390/photonics6010010




