Contactless Determination of Optimal Chloride Concentration for Power Conversion Efficiency in CH3NH3Pb(Cl,I)3 Using Photoluminescence Spectroscopy
Abstract
:1. Introduction
2. Experimental and Calculation Procedures
- Mesoporous oxide and absolute ethanol were mixed in a weight ratio of 2:7.
- We applied them using spin coating.
- We fixed the substrate in a spin coat.
- We drip-cast 0.2 mL of mesoporous oxide solution to spread throughout.
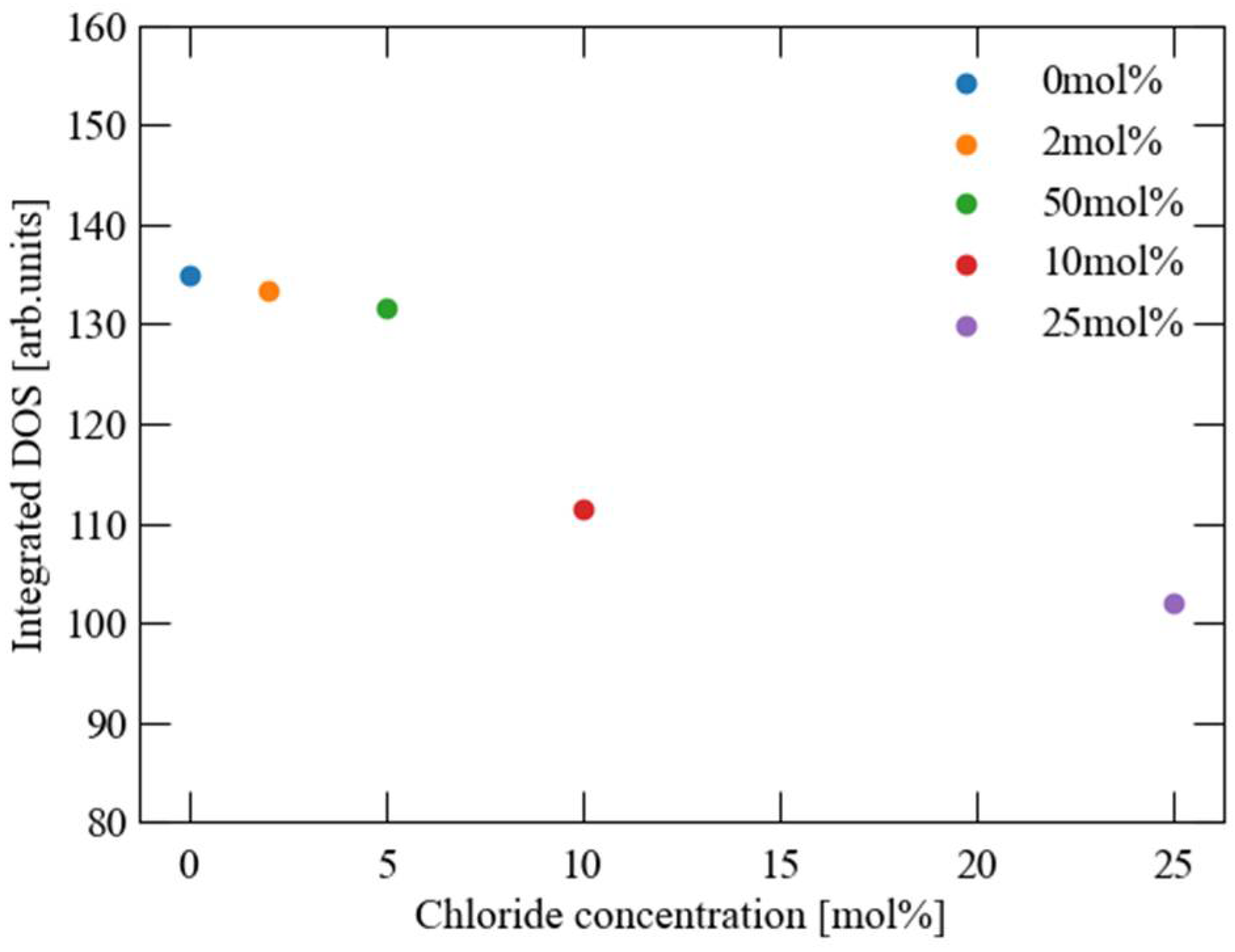
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Ball, J.M.; Lee, M.M.; Hey, A.; Snaith, H.J. Low-Temperature Processed Meso-Superstructure to Thin-Film Perovskite Solar Cells. Energy Environ. Sci. 2013, 6, 1739. [Google Scholar] [CrossRef]
- Mosconi, E.; Amat, A.; Nazeeruddin, M.D.K.; Graetzel, M.; De Angelis, F. First-Principles Modeling of Mixed Halide Organometal Perovskites for Photovoltaic Applications. J. Phys. Chem. C 2013, 117, 13902–13913. [Google Scholar] [CrossRef]
- Ball, J.M.; Stranks, S.D.; Hörantner, M.T.; Hüttner, S.; Zhang, W.; Crossland, E.J.W.; Ramirez, I.; Riede, M.; Johnston, M.B.; Friend, R.H.; et al. Optical Properties and Limiting Photocurrent of Thin-Film Perovskite Solar Cells. Energy Environ. Sci. 2015, 8, 602–609. [Google Scholar] [CrossRef]
- Stranks, S.D.; Snaith, H.J. Metal-Halide Perovskites for Photovoltaic and Light-Emitting Devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhi, L.; Jia, Y.; Li, Y.; Zhao, K.; Cui, X.; Ci, L.; Ding, K.; Wei, J. Enhanced Efficiency of Perovskite Solar Cells by Introducing Controlled Chloride Incorporation into MAPbI3 Perovskite Films. Electrochim. Acta 2018, 275, 1–7. [Google Scholar] [CrossRef]
- Jamshaid, A.; Guo, Z.; Hieulle, J.; Stecker, C.; Ohmann, R.; Ono, L.K.; Qiu, L.; Tong, G.; Yin, W.; Qi, Y. Atomic-Scale Insight into the Enhanced Surface Stability of Methylammonium Lead Iodide Perovskite by Controlled Deposition of Lead Chloride. Energy Environ. Sci. 2021. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-Halide Anion Engineering for α-FAPbI3 Perovskite Solar Cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Beal, R.E.; Slotcavage, D.J.; Leijtens, T.; Bowring, A.R.; Belisle, R.A.; Nguyen, W.H.; Burkhard, G.F.; Hoke, E.T.; McGehee, M.D. Cesium Lead Halide Perovskites with Improved Stability for Tandem Solar Cells. J. Phys. Chem. Lett. 2016, 7, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.J.; Eperon, G.E.; Miranda, L.; Parrott, E.S.; Kamino, B.A.; Patel, J.B.; Hörantner, M.T.; Johnston, M.B.; Haghighirad, A.A.; Moore, D.T.; et al. Bandgap-Tunable Cesium Lead Halide Perovskites with High Thermal Stability for Efficient Solar Cells. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar] [CrossRef]
- Ito, S.; Kanaya, S.; Nishino, H.; Umeyama, T.; Imahori, H. Material Exchange Property of Organo Lead Halide Perovskite with Hole-Transporting Materials. Photonics 2015, 2, 1043–1053. [Google Scholar] [CrossRef] [Green Version]
- Parrott, E.S.; Milot, R.L.; Stergiopoulos, T.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Effect of Structural Phase Transition on Charge-Carrier Lifetimes and Defects in CH3NH3SnI3 Perovskite. J. Phys. Chem. Lett. 2016, 7, 1321–1326. [Google Scholar] [CrossRef]
- Rao, H.; Ye, S.; Gu, F.; Zhao, Z.; Liu, Z.; Bian, Z.; Huang, C. Morphology Controlling of All-Inorganic Perovskite at Low Temperature for Efficient Rigid and Flexible Solar Cells. Adv. Energy Mater. 2018, 8, 1800758. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [Green Version]
- Quarti, C.; Grancini, G.; Mosconi, E.; Bruno, P.; Ball, J.M.; Lee, M.M.; Snaith, H.J.; Petrozza, A.; Angelis, F.D. The Raman Spectrum of the CH3NH3PbI3 Hybrid Perovskite: Interplay of Theory and Experiment. J. Phys. Chem. Lett. 2013, 5, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pedro, V.; Juarez-Perez, E.J.; Arsyad, W.-S.; Barea, E.M.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J. General Working Principles of CH3NH3PbX3 Perovskite Solar Cells. Nano Lett. 2014, 14, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Spalla, M.; Perrin, L.; Planès, E.; Matheron, M.; Berson, S.; Flandin, L. Influence of Chloride/Iodide Ratio in MAPbICl Perovskite Solar Devices: Case of Low-Temperature Processable AZO Sub-Layer. Energies 2020, 13, 1927. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Liu, X.; Chai, N.; Huang, F.; Peng, Y.; Zhong, J.; Zhang, Q.; Ku, Z.; Cheng, Y. Alleviate the J-V Hysteresis of Carbon-Based Perovskite Solar Cells via Introducing Additional Methylammonium Chloride into MAPbI3 Precursor. RSC Adv. 2018, 8, 35157–35161. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.D.; Verdi, C.; Milot, R.L.; Eperon, G.E.; Pérez-Osorio, M.A.; Snaith, H.J.; Giustino, F.; Johnston, M.B.; Herz, L.M. Electron-Phonon Coupling in Hybrid Lead Halide Perovskites. Nat. Commun. 2016, 7, 11755. [Google Scholar] [CrossRef]
- Koinuma, H.; Takeuchi, I. Combinatorial Solid-State Chemistry of Inorganic Materials. Nat. Mater. 2004, 3, 429. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.R.; Verdi, C.; Giustino, F. GW Band Structures and Carrier Effective Masses of CH3NH3PbI3 and Hypothetical Perovskites of the Type APbI3: A = NH4, PH4, AsH4, and SbH4. J. Phys. Chem. C 2015, 119, 25209–25219. [Google Scholar] [CrossRef]
- Kawashima, K.; Okamoto, Y.; Annayev, O.; Toyokura, N.; Takahashi, R.; Lippmaa, M.; Itaka, K.; Suzuki, Y.; Matsuki, N.; Koinuma, H. Combinatorial Screening of Halide Perovskite Thin Films and Solar Cells by Mask-Defined IR Laser Molecular Beam Epitaxy. Sci. Technol. Adv. Mater. 2017, 18, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.D.S.; Dey, B.; Rana, M.D.M.; Islam, A.S.M.J.; Park, J.; Makino, T. Temperature-Induced Localized Exciton Dynamics in Mixed Lead-Tin Based CH3NH3PbSnI3 Perovskite Materials. AIP Adv. 2020, 10, 065331. [Google Scholar] [CrossRef]
- Akai, H. Electronic Structure Nipd Alloys Calculated by the Self-Consistent KKR-CPA Method. J. Phys. Soc. Jpn. 1982, 51, 468–474. [Google Scholar] [CrossRef]
- Akai, H. Fast Korringa-Kohn-Rostoker Coherent Potential Approximation and Its Application to FCC Ni-Fe Systems. J. Phys. Condens. Matter 1989, 1, 8045–8064. [Google Scholar] [CrossRef]
- Kobayashi, T.; Shinmura, S.; Ito, S.; Makino, T. A new photoreflectance signal possibly due to midgap interface states in buried F-doped SnO2/TiO2 junctions. Jpn. J. Appl. Phys. 2021, 59, SCCB23. [Google Scholar] [CrossRef]
- Kotani, T.; Akai, H. KKR-ASA Method in Exact Exchange-Potential Band-Structure Calculations. Phys. Rev. B 1996, 54, 16502–16514. [Google Scholar] [CrossRef]
- Akai, H. Ferromagnetism and Its Stability in the Diluted Magnetic Semiconductor (In, Mn)As. Phys. Rev. Lett. 1998, 81, 3002–3005. [Google Scholar] [CrossRef]
- Uribe, J.I.; Ramirez, D.; Osorio-Guillén, J.M.; Osorio, J.; Jaramillo, F. CH3NH3CaI3 Perovskite: Synthesis, Characterization, and First-Principles Studies. J. Phys. Chem. C 2016, 120, 16393–16398. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; Kolodziej, C.; Kuyuldar, S.; Warren, W.S.; Burda, C.; Fischer, M.C. Visualizing the Impact of Chloride Addition on the Microscopic Carrier Dynamics of MAPbI3 Thin Films Using Femtosecond Transient Absorption Microscopy. J. Chem. Phys. 2019, 151, 234710. [Google Scholar] [CrossRef]
- Ripolles, T.S.; Nishinaka, K.; Ogomi, Y.; Miyata, Y.; Hayase, S. Efficiency Enhancement by Changing Perovskite Crystal Phase and Adding a Charge Extraction Interlayer in Organic Amine Free-Perovskite Solar Cells Based on Cesium. Sol. Energy Mater. Sol. Cells 2016, 144, 532–536. [Google Scholar] [CrossRef]
- Leblebici, S.Y.; Leppert, L.; Li, Y.; Reyes-Lillo, S.E.; Wickenburg, S.; Wong, E.; Lee, J.; Melli, M.; Ziegler, D.; Angell, D.K.; et al. Facet-Dependent Photovoltaic Efficiency Variations in Single Grains of Hybrid Halide-Perovskite. Nat. Energy 2016, 1, 93. [Google Scholar] [CrossRef]
- Tombe, S.; Adam, G.; Heilbrunner, H.; Apaydin, D.H.; Ulbricht, C.; Sariciftci, N.S.; Arendse, C.J.; Iwuoha, E.; Scharber, M.C. Optical and Electronic Properties of Mixed Halide (X = I, Cl, Br) Methylammonium Lead Perovskite Solar Cells. J. Mater. Chem. C 2017, 5, 1714–1723. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Xia, W.; Zhou, S.; Sun, L.; Cheng, J.; Xu, C.; Lu, Y. Solvent Engineering for Ambient-Air-Processed, Phase-Stable CsPbI3 in Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 3603–3608. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Takahashi, J.; Goto, T. Exciton State in Two-Dimensional Perovskite Semiconductor (C10H21NH3)2PbI4. Solid State Commun. 1989, 69, 933–936. [Google Scholar] [CrossRef]
- Hirasawa, M.; Ishihara, T.; Goto, T. Exciton Features in 0-, 2-, and 3-Dimensional Networks of [PbI6]4-Octahedra. J. Phys. Soc. Jpn. 1994, 63, 3870–3879. [Google Scholar] [CrossRef]
- Galkowski, K.; Mitioglu, A.A.; Surrente, A.; Yang, Z.; Maude, D.K.; Kossacki, P.; Eperon, G.E.; Wang, J.T.-W.; Snaith, H.J.; Plochocka, P.; et al. Spatially Resolved Studies of the Phases and Morphology of Methylammonium and Formamidinium Lead Tri-Halide Perovskites. Nanoscale 2017, 9, 3222–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Cheng, L.; Ge, R.; Zhang, S.; Miao, Y.; Zou, W.; Yi, C.; Sun, Y.; Cao, Y.; Yang, R.; et al. Perovskite Light-Emitting Diodes Based on Solution-Processed Self-Organized Multiple Quantum Wells. Nat. Photonics 2016, 10, 699–704. [Google Scholar] [CrossRef]
- Ouedraogo, N.A.N.; Chen, Y.; Xiao, Y.Y.; Meng, Q.; Han, C.B.; Yan, H.; Zhang, Y. Stability of All-Inorganic Perovskite Solar Cells. Nano Energy 2019, 67, 104249. [Google Scholar] [CrossRef]
- Handa, T.; Aharen, T.; Wakamiya, A.; Kanemitsu, Y. Radiative Recombination and Electron-Phonon Coupling in Lead-Free CH3NH3SnI3 Perovskite Thin Films. Phys. Rev. Mater. 2018, 2, 075402. [Google Scholar] [CrossRef]
- Goetz, K.P.; Taylor, A.D.; Paulus, F.; Vaynzof, Y. Shining Light on the Photoluminescence Properties of Metal Halide Perovskites. Adv. Funct. Mater. 2020, 30, 1910004. [Google Scholar] [CrossRef]
- Yamada, T.; Handa, T.; Yamada, Y.; Kanemitsu, Y. Light Emission from Halide Perovskite Semiconductors: Bulk Crystals, Thin Films, and Nanocrystals. J. Phys. D Appl. Phys. 2021, 54, 383001. [Google Scholar] [CrossRef]
- Qin, C.; Matsushima, T.; Potscavage, W.J.; Sandanayaka, A.S.D.; Leyden, M.R.; Bencheikh, F.; Goushi, K.; Mathevet, F.; Heinrich, B.; Yumoto, G.; et al. Triplet Management for Efficient Perovskite Light-Emitting Diodes. Nat. Photonics 2019, 14, 70–75. [Google Scholar] [CrossRef]
- Ito, R.; Yoshida, K.; Yamazaki, Y.; Makino, T. Time-Resolved Dynamics in CH3NH3Pb(I,Cl)3 Alloy Systems Using Pump-Probe Method in a Weak Excitation Regime. In Proceedings of the 78th Japan Society of Applied Physics Autumn Meeting, Fukuoka, Japan, 5–8 September 2017. 8p-A414-5. [Google Scholar]
- Lin, J.; Lai, M.; Dou, L.; Kley, C.S.; Chen, H.; Peng, F.; Sun, J.; Lu, D.; Hawks, S.A.; Xie, C.; et al. Thermochromic Halide Perovskite Solar Cells. Nat. Mater. 2018, 17, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Concentration | Jsc | Voc | FF | PCE |
|---|---|---|---|---|
| mol% | mA/cm2 | V | % | |
| 0 | 6.8 | 0.81 | 0.56 | 7.7 |
| 2 | 8.3 | 0.87 | 0.60 | 9.7 |
| 10 | 20.7 | 0.92 | 0.62 | 12.0 |
| 25 | 9.6 | 0.90 | 0.60 | 10.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asai, T.; Ito, S.; Makino, T. Contactless Determination of Optimal Chloride Concentration for Power Conversion Efficiency in CH3NH3Pb(Cl,I)3 Using Photoluminescence Spectroscopy. Photonics 2021, 8, 412. https://doi.org/10.3390/photonics8100412
Asai T, Ito S, Makino T. Contactless Determination of Optimal Chloride Concentration for Power Conversion Efficiency in CH3NH3Pb(Cl,I)3 Using Photoluminescence Spectroscopy. Photonics. 2021; 8(10):412. https://doi.org/10.3390/photonics8100412
Chicago/Turabian StyleAsai, Takaho, Seigo Ito, and Takayuki Makino. 2021. "Contactless Determination of Optimal Chloride Concentration for Power Conversion Efficiency in CH3NH3Pb(Cl,I)3 Using Photoluminescence Spectroscopy" Photonics 8, no. 10: 412. https://doi.org/10.3390/photonics8100412





