High Intensity Violet Light (405 nm) Inactivates Coronaviruses in Phosphate Buffered Saline (PBS) and on Surfaces
Abstract
:1. Introduction
2. Materials and Methods
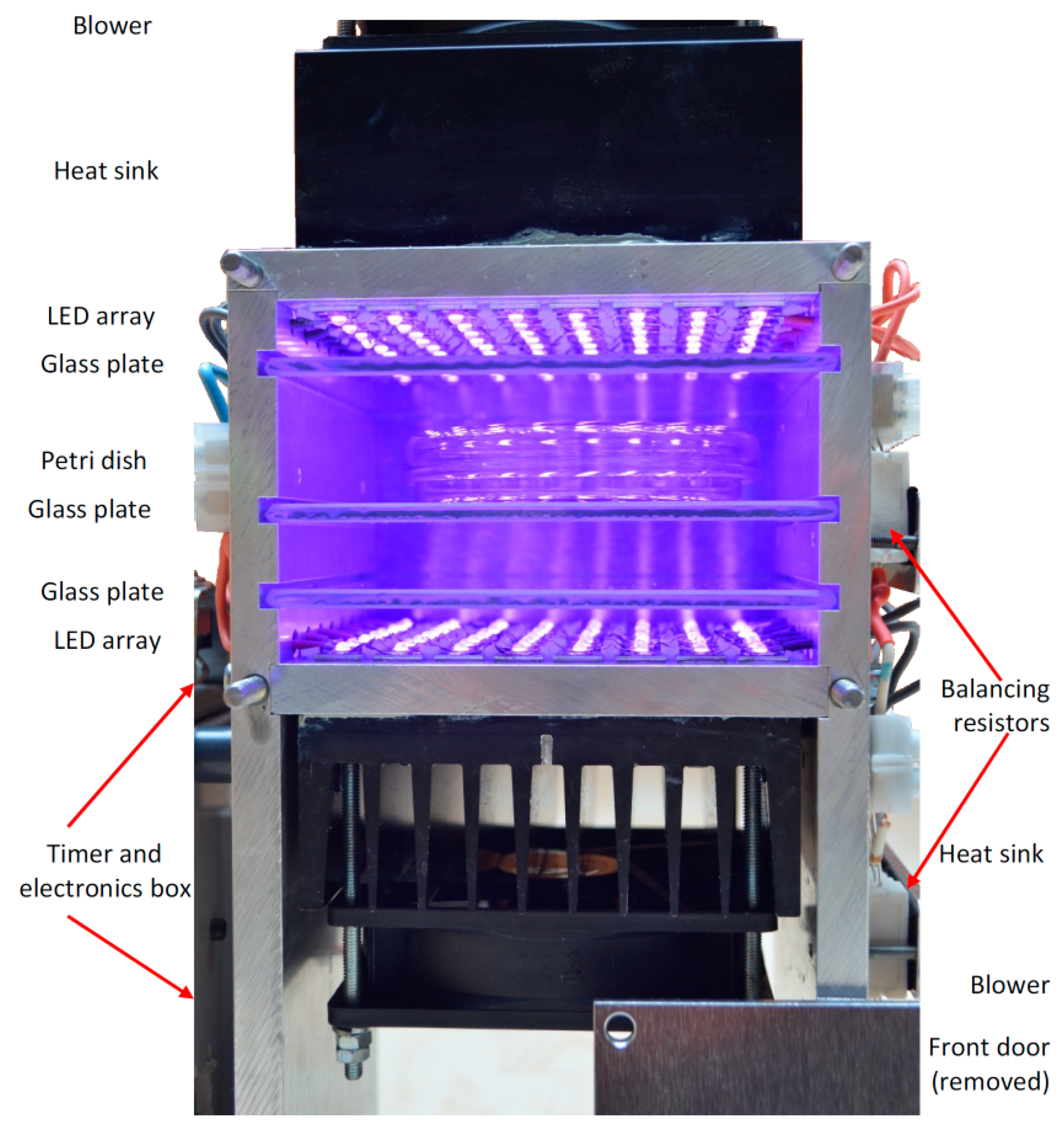
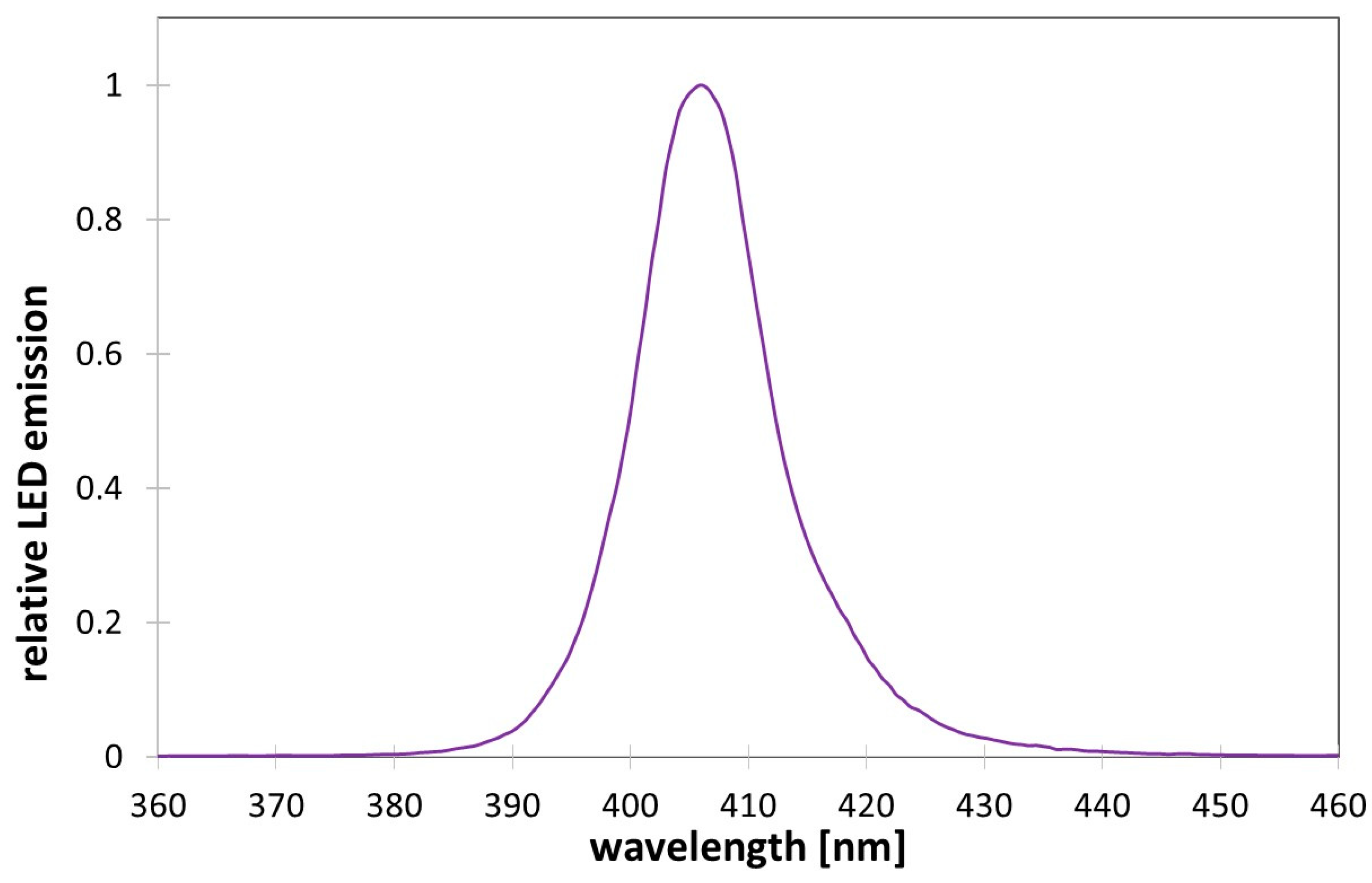
2.1. High Intensity Light Source
2.2. Virus Preparation
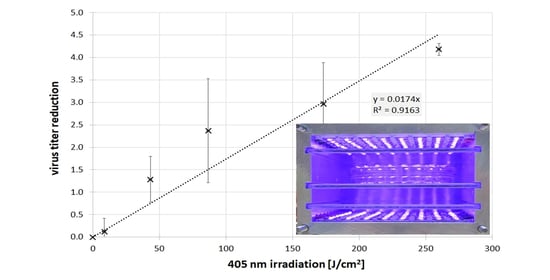
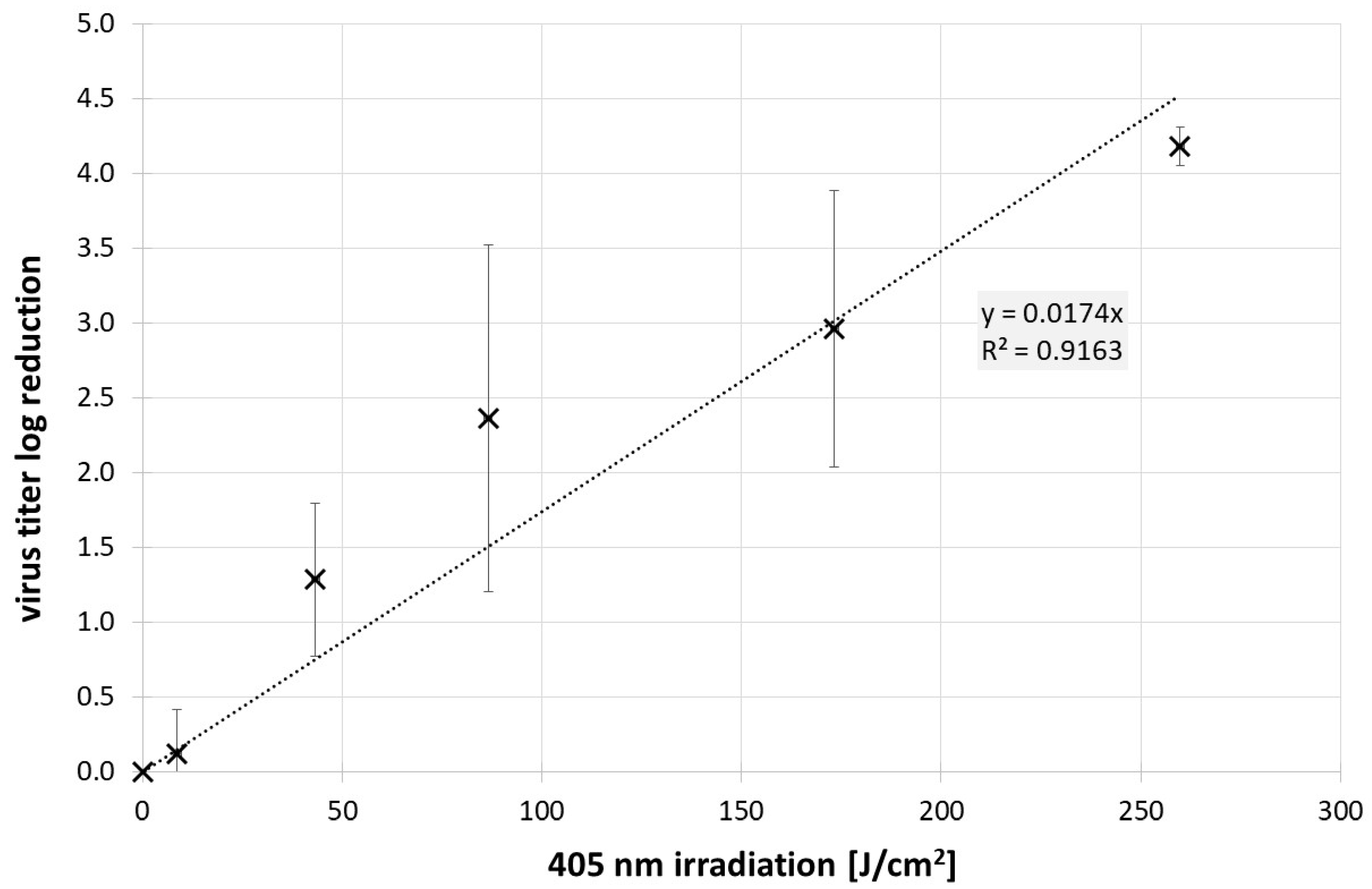
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Wang, Y.; Ye, D.; Liu, Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents 2020, 55, 105948. [Google Scholar] [CrossRef]
- Coronavirus Resource Center. COVID-19 Dashboard: (Global Map). 2021. Available online: https://coronavirus.jhu.edu/map.html (accessed on 29 August 2021).
- Hessling, M.; Hönes, K.; Vatter, P.; Lingenfelder, C. Ultraviolet irradiation doses for coronavirus inactivation—review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control 2020, 15. [Google Scholar] [CrossRef]
- Chiappa, F.; Frascella, B.; Vigezzi, G.P.; Moro, M.; Diamanti, L.; Gentile, L.; Lago, P.; Clementi, N.; Signorelli, C.; Mancini, N.; et al. The efficacy of ultraviolet light-emitting technology against coronaviruses: A systematic review. J. Hosp. Infect. 2021, 114, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Matsuda, J.; Iwasaki, T.; Hayasaka, D. Efficacy of 265-nm ultraviolet light in inactivating infectious SARS-CoV-2. J. Photochem. Photobiol. 2021, 7, 100050. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Saito, A.; Kaneko, C.; Sugiyama, H.; Okabayashi, T.; Fujimoto, S. Rapid Inactivation of SARS-CoV-2 Variants by Continuous and Intermittent Irradiation with a Deep-Ultraviolet Light-Emitting Diode (DUV-LED) Device. Pathogens 2021, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Linden, Y.S.; Gundy, P.M.; Gerba, C.P.; Sobsey, M.D.; Linden, K.G. Inactivation of Coronaviruses and Phage Phi6 from Irradiation across UVC Wavelengths. Environ. Sci. Technol. Lett. 2021, 8, 425–430. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; You, Y.-H.; Besaratinia, A. Mutations induced by ultraviolet light. Mutat. Res. 2005, 571, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Delic, N.C.; Lyons, J.G.; Di Girolamo, N.; Halliday, G.M. Damaging Effects of Ultraviolet Radiation on the Cornea. Photochem. Photobiol. 2017, 93, 920–929. [Google Scholar] [CrossRef] [Green Version]
- Scientific Committee on Health, Environmental and Emerging Risks. Opinion on biological effects of UV-C radiation relevant to health with particular reference to UV-C lamps. In Proceedings of the Luxembourg: European Commission, Luxembourg, 3 February 2017. [Google Scholar]
- Hessling, M.; Haag, R.; Sieber, N.; Vatter, P. The impact of far-UVC radiation (200–230 nm) on pathogens, cells, skin, and eyes—A collection and analysis of a hundred years of data. GMS Hyg. Infect. Control 2021, 16. [Google Scholar] [CrossRef]
- International Ultraviolet Association. Far UV-C Radiation: Current State-of Knowledge (White Paper); Ational Ultraviolet Association: Chevy Chase, MD, USA, 2021. [Google Scholar]
- Hessling, M.; Spellerberg, B.; Hoenes, K. Photoinactivation of bacteria by endogenous photosensitizers and exposure to visible light of different wavelengths—A review on existing data. FEMS Microbiol. Lett. 2016, 364, fnw270. [Google Scholar] [CrossRef]
- Tomb, R.M.; White, T.A.; Coia, J.E.; Anderson, J.G.; MacGregor, S.J.; Maclean, M. Review of the Comparative Susceptibility of Microbial Species to Photoinactivation Using 380-480 nm Violet-Blue Light. Photochem. Photobiol. 2018, 94, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Hoenes, K.; Bauer, R.; Meurle, T.; Spellerberg, B.; Hessling, M. Inactivation Effect of Violet and Blue Light on ESKAPE Pathogens and Closely Related Non-pathogenic Bacterial Species—A Promising Tool Against Antibiotic-Sensitive and Antibiotic-Resistant Microorganisms. Front. Microbiol. 2020, 11, 612367. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, H.; Malik, Z.; Harth, Y.; Nitzan, Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 2003, 35, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guffey, J.S.; Wilborn, J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed. Laser Surg. 2006, 24, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiol. Lett. 2008, 285, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerstein, O.; Ginsburg, I.; Dayan, E.; Veler, D.; Weiss, E.I. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem. Photobiol. 2005, 81, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plavskii, V.Y.; Mikulich, A.V.; Tretyakova, A.I.; Leusenka, I.A.; Plavskaya, L.G.; Kazyuchits, O.A.; Dobysh, I.I.; Krasnenkova, T. Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J. Photochem. Photobiol. B 2018, 183, 172–183. [Google Scholar] [CrossRef]
- Cieplik, F.; Spath, A.; Leibl, C.; Gollmer, A.; Regensburger, J.; Tabenski, L.; Hiller, K.; Maisch, T.; Schmalz, G. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin. Oral Investig. 2014, 18, 1763–1769. [Google Scholar] [CrossRef]
- Kingsley, D.; Kuis, R.; Perez, R.; Basaldua, I.; Burkins, P.; Marcano, A.; Johnson, A. Oxygen-dependent laser inactivation of murine norovirus using visible light lasers. Virol. J. 2018, 15, 117. [Google Scholar] [CrossRef]
- Vatter, P.; Hoenes, K.; Hessling, M. Photoinactivation of the Coronavirus Surrogate phi6 by Visible Light. Photochem. Photobiol. 2021, 97, 122–125. [Google Scholar] [CrossRef]
- Vatter, P.; Hoenes, K.; Hessling, M. Blue light inactivation of the enveloped RNA virus Phi6. BMC Res. Notes 2021, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Santis, R.; de Luca, V.; Faggioni, G.; Fillo, S.; Stefanelli, P.; Rezza, G.; Lista, F. Rapid inactivation of SARS-CoV-2 with LED irradiation of visible spectrum wavelenghts. MedRxiv 2020. [Google Scholar] [CrossRef]
- Rathnasinghe, R.; Jangra, S.; Miorin, L.; Schotsasert, M.; Yahnke, C.; Garcίa-Sastre, A. Lighting a better future: The virucidal effects of 405 nm visible light on SARS-CoV-2 and influenza A virus. BioRxiv 2021. [Google Scholar] [CrossRef]
- Zwinkels, J.C.; Noël, M.; Dodd, C.X. Procedures and standards for accurate spectrophotometric measurements of specular reflectance. Appl. Opt. 1994, 33, 7933–7944. [Google Scholar] [CrossRef]
- Lim, W.; Ng, K.-C.; Tsang, D.N.C. Laboratory containment of SARS virus. Ann. Acad. Med. Singap. 2006, 35, 354–360. [Google Scholar] [PubMed]
- Saif, L.J.; Jung, K. Comparative Pathogenesis of Bovine and Porcine Respiratory Coronaviruses in the Animal Host Species and SARS-CoV-2 in Humans. J. Clin. Microbiol. 2020. [Google Scholar] [CrossRef]
- Llanes, A.; Restrepo, C.M.; Caballero, Z.; Rajeev, S.; Kennedy, M.A.; Lleonart, R. Betacoronavirus Genomes: How Genomic Information has been Used to Deal with Past Outbreaks and the COVID-19 Pandemic. IJMS 2020, 21, 4546. [Google Scholar] [CrossRef]
- Ghosh, S.; Malik, Y.S. Drawing Comparisons between SARS-CoV-2 and the Animal Coronaviruses. Microorganisms 2020, 8, 1840. [Google Scholar] [CrossRef]
- Alluwaimi, A.M.; Alshubaith, I.H.; Al-Ali, A.M.; Abohelaika, S. The Coronaviruses of Animals and Birds: Their Zoonosis, Vaccines, and Models for SARS-CoV and SARS-CoV2. Front. Vet. Sci. 2020, 7, 582287. [Google Scholar] [CrossRef]
- Vijgen, L.; Keyaerts, E.; Lemey, P.; Maes, P.; van Reeth, K.; Nauwynck, H.; Pensaert, M.; van Ranst, M. Evolutionary history of the closely related group 2 coronaviruses: Porcine hemagglutinating encephalomyelitis virus, bovine coronavirus, and human coronavirus OC43. J. Virol. 2006, 80, 7270–7274. [Google Scholar] [CrossRef] [Green Version]
- Franke, G.; Knobling, B.; Brill, F.H.; Becker, B.; Klupp, E.M.; Belmar Campos, C.B.; Pfefferle, S.; Lütgehetmann, M.; Knobloch, J.K. An automated room disinfection system using ozone is highly active against surrogates for SARS-CoV-2. J. Hosp. Infect. 2021, 112, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.A.; Zahran, E.M.; Abdel Fadeel, M.R.; Albohy, A.; Safwat, M.A. New Acaciin-Loaded Self-Assembled Nanofibers as MPro Inhibitors Against BCV as a Surrogate Model for SARS-CoV-2. Int. J. Nanomed. 2021, 16, 1789–1804. [Google Scholar] [CrossRef] [PubMed]
- Kenney, S.P.; Wang, Q.; Vlasova, A.; Jung, K.; Saif, L. Naturally Occurring Animal Coronaviruses as Models for Studying Highly Pathogenic Human Coronaviral Disease. Vet. Pathol. 2021, 58, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Salvo, M.; Moller, A.; Alvareda, E.; Gamazo, P.; Colina, R.; Victoria, M. Evaluation of low-cost viral concentration methods in wastewaters: Implications for SARS-CoV-2 pandemic surveillances. J. Virol. Methods 2021, 297, 114249. [Google Scholar] [CrossRef]
- Barrios, M.E.; Díaz, S.M.; Torres, C.; Costamagna, D.M.; Blanco Fernández, M.D.; Mbayed, V.A. Dynamics of SARS-CoV-2 in wastewater in three districts of the Buenos Aires metropolitan region, Argentina, throughout nine months of surveillance: A pilot study. Sci. Total Environ. 2021, 800, 149578. [Google Scholar] [CrossRef]
- Todt, D.; Meister, T.L.; Tamele, B.; Howes, J.; Paulmann, D.; Becker, B.; Brill, F.H.; Wind, M.; Schijven, J.; Heinen, N.; et al. A realistic transfer method reveals low risk of SARS-CoV-2 transmission via contaminated euro coins and banknotes. IScience 2021, 24, 102908. [Google Scholar] [CrossRef]
- Yoshizawa, N.; Ishihara, R.; Omiya, D.; Ishitsuka, M.; Hirano, S.; Suzuki, T. Application of a Photocatalyst as an Inactivator of Bovine Coronavirus. Viruses 2020, 12, 1372. [Google Scholar] [CrossRef] [PubMed]
- Wensman, J.J.; Stokstad, M. Could Naturally Occurring Coronaviral Diseases in Animals Serve as Models for COVID-19? A Review Focusing on the Bovine Model. Pathogens 2020, 9, 991. [Google Scholar] [CrossRef]
- Lin, C.-H.; Yang, C.-Y.; Ou, S.-C.; Wang, M.; Lo, C.-Y.; Tsai, T.-L.; Wu, H.-Y. The Impacts of Antivirals on the Coronavirus Genome Structure and Subsequent Pathogenicity, Virus Fitness and Antiviral Design. Biomedicines 2020, 8, 376. [Google Scholar] [CrossRef]
- Ma, C.; Gong, C. ACE2 models of frequently contacted animals provide clues of their SARS-CoV-2 S protein affinity and viral susceptibility. J. Med. Virol. 2021, 93, 4469–4479. [Google Scholar] [CrossRef]
- Mullis, L.; Saif, L.J.; Zhang, Y.; Zhang, X.; Azevedo, M.S.P. Stability of bovine coronavirus on lettuce surfaces under household refrigeration conditions. Food Microbiol. 2012, 30, 180–186. [Google Scholar] [CrossRef]
- Kapil, S.; Oberst, R.; Bieker, J.; Tucker, M.; Souza, C.; Williams, C. Rapid Inactivation of SARS-Like Coronaviruses; Sandia National Laboratories: Albuquerque, NM, USA, 2004. [Google Scholar]
- Steinmann, J. Surrogate viruses for testing virucidal efficacy of chemical disinfectants. J. Hosp. Infection. 2004, 56, S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Kratzel, A.; Todt, D.; V′kovski, P.; Steiner, S.; Gultom, M.; Thao, T.T.N.; Ebert, N.; Holwerda, M.; Steinmann, J.; Niemeyer, D.; et al. Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 by WHO-Recommended Hand Rub Formulations and Alcohols. Emerg. Infect. Dis. 2020, 26, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Karthigeyan, K.P.; Flanigan, C.; Machado, D.J.; Kiziltas, A.A.; Janies, D.A.; Chen, J.; Cooke, D.; Lee, M.V.; Saif, L.J.; Henegar, S.; et al. Heat efficiently inactivates coronaviruses inside vehicles. BioRxiv 2021. [Google Scholar] [CrossRef]
- Graham, K.E.; Loeb, S.K.; Wolfe, M.K.; Catoe, D.; Sinnott-Armstrong, N.; Kim, S.; Yamahara, K.M.; Sassoubre, L.M.; Grijalva, L.M.M.; Roldan-Hernandez, L.; et al. SARS-CoV-2 RNA in Wastewater Settled Solids Is Associated with COVID-19 Cases in a Large Urban Sewershed. Env. Sci. Technol. 2021, 55, 488–498. [Google Scholar] [CrossRef]
- Gonzalez, R.; Curtis, K.; Bivins, A.; Bibby, K.; Weir, M.H.; Yetka, K.; Thompson, H.; Keeling, D.; Mitchell, J.; Gonzalez, D. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 2020, 186, 116296. [Google Scholar] [CrossRef] [PubMed]
- Kwiek, J.J.; Pickett, C.R.; Flanigan, C.A.; Lee, M.V.; Saif, L.J.; Jahnes, J.; Blonder, G. A practical PPE decontamination method using warm air and ambient humidity. BioRxiv 2020. [Google Scholar] [CrossRef]
- Lucassen, R.; Weide, M.; Bockmühl, D. Virucidal Efficacy of Household Dishwashers. Microbiol. Res. 2021, 12, 395–402. [Google Scholar] [CrossRef]
- Gardner, A.; Ghosh, S.; Dunowska, M.; Brightwell, G. Virucidal Efficacy of Blue LED and Far-UVC Light Disinfection against Feline Infectious Peritonitis Virus as a Model for SARS-CoV-2. Viruses 2021, 13, 1436. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Bumah, V.V.; Mokili, J.L. Pulsed blue light inactivates two strains of human coronavirus. J. Photochem. Photobiol. B 2021, 222, 112282. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; Macgregor, S.J.; Anderson, J.G.; Woolsey, G.A.; Coia, J.E.; Hamilton, K.; Taggart, I.; Watson, S.B.; Thakker, B.; Gettinby, G. Environmental decontamination of a hospital isolation room using high-intensity narrow-spectrum light. J. Hosp. Infect. 2010, 76, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Buehler, J.; Sommerfeld, F.; Meurle, T.; Hoenes, K.; Hessling, M. Disinfection Properties of Conventional White LED Illumination and Their Potential Increase by Violet LEDs for Applications in Medical and Domestic Environments. Adv. Sci. Technol. Res. J. 2021, 15, 169–175. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, B.; Becher, D.; Hessling, M. High Intensity Violet Light (405 nm) Inactivates Coronaviruses in Phosphate Buffered Saline (PBS) and on Surfaces. Photonics 2021, 8, 414. https://doi.org/10.3390/photonics8100414
Lau B, Becher D, Hessling M. High Intensity Violet Light (405 nm) Inactivates Coronaviruses in Phosphate Buffered Saline (PBS) and on Surfaces. Photonics. 2021; 8(10):414. https://doi.org/10.3390/photonics8100414
Chicago/Turabian StyleLau, Bernhard, Dietmar Becher, and Martin Hessling. 2021. "High Intensity Violet Light (405 nm) Inactivates Coronaviruses in Phosphate Buffered Saline (PBS) and on Surfaces" Photonics 8, no. 10: 414. https://doi.org/10.3390/photonics8100414
APA StyleLau, B., Becher, D., & Hessling, M. (2021). High Intensity Violet Light (405 nm) Inactivates Coronaviruses in Phosphate Buffered Saline (PBS) and on Surfaces. Photonics, 8(10), 414. https://doi.org/10.3390/photonics8100414