Highly Sensitive Biosensor Based on Partially Immobilized Silver Nanopillars in the Terahertz Band
Abstract
:1. Introduction
2. Structural Design and Materials
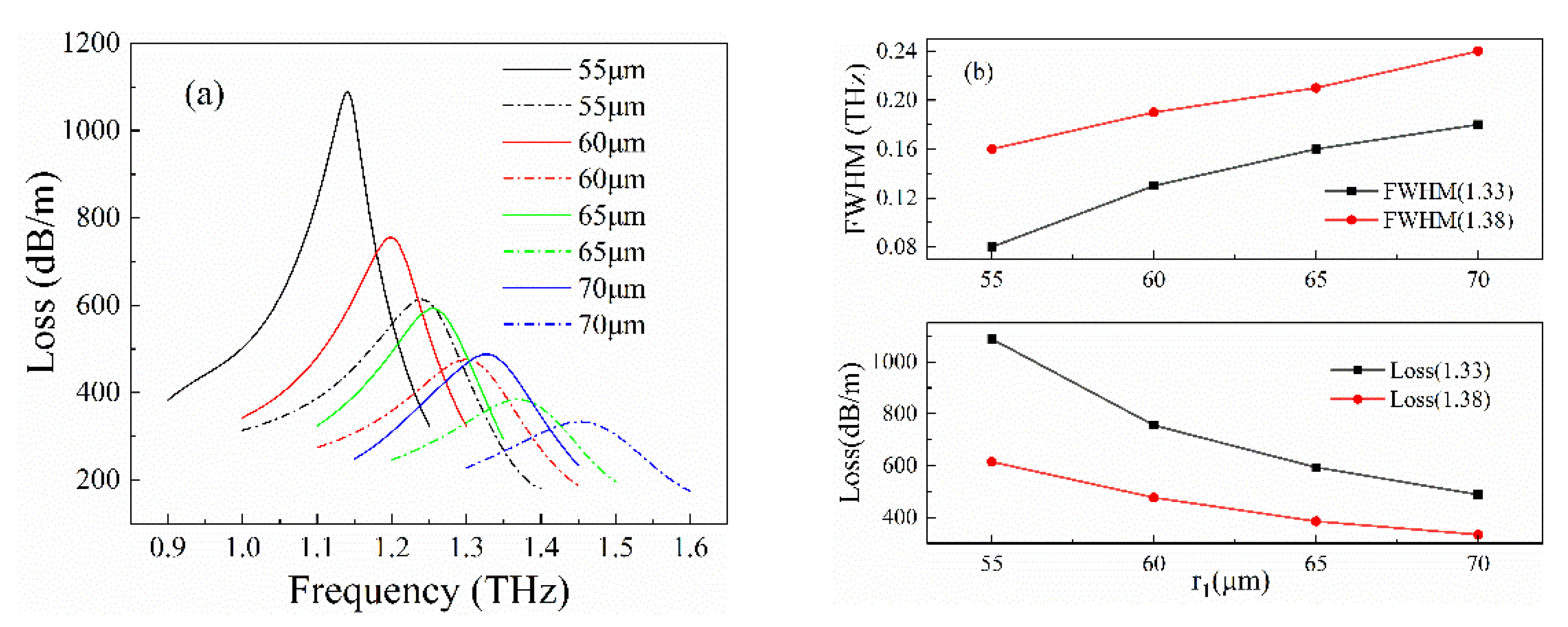
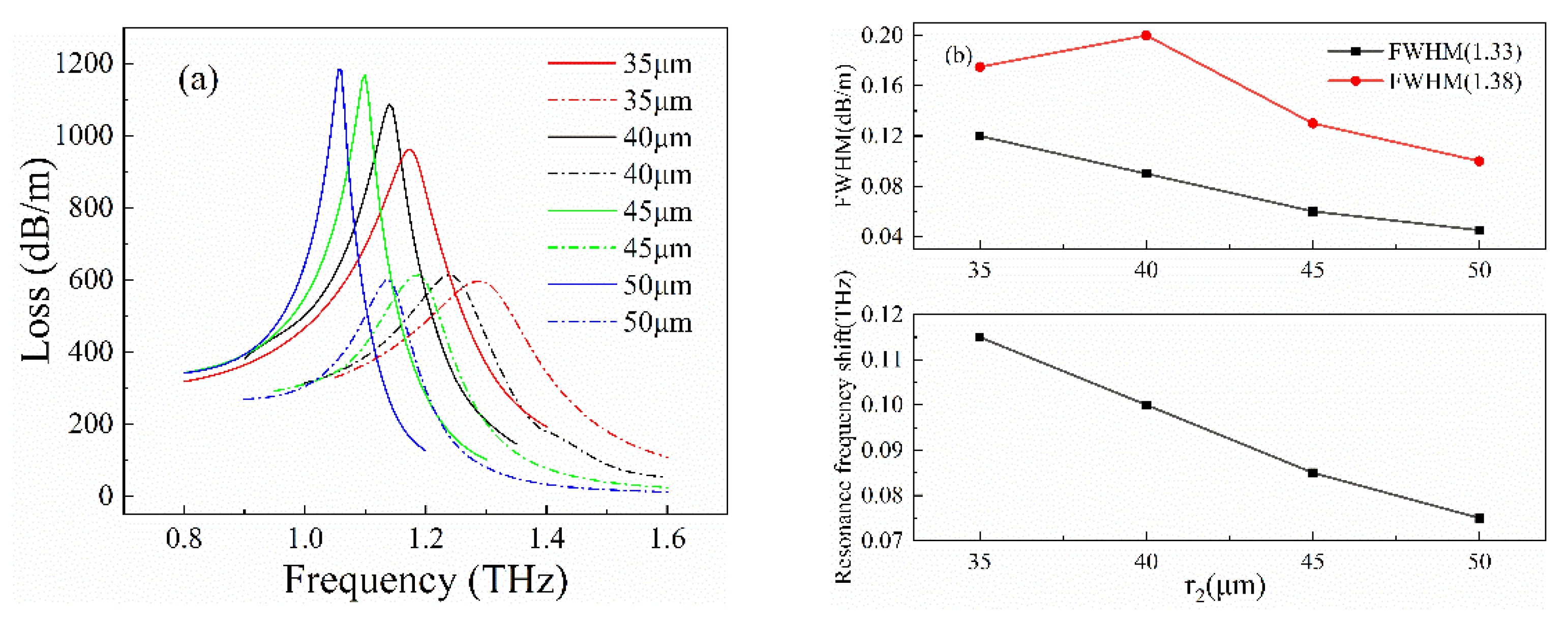
3. Optimization Process
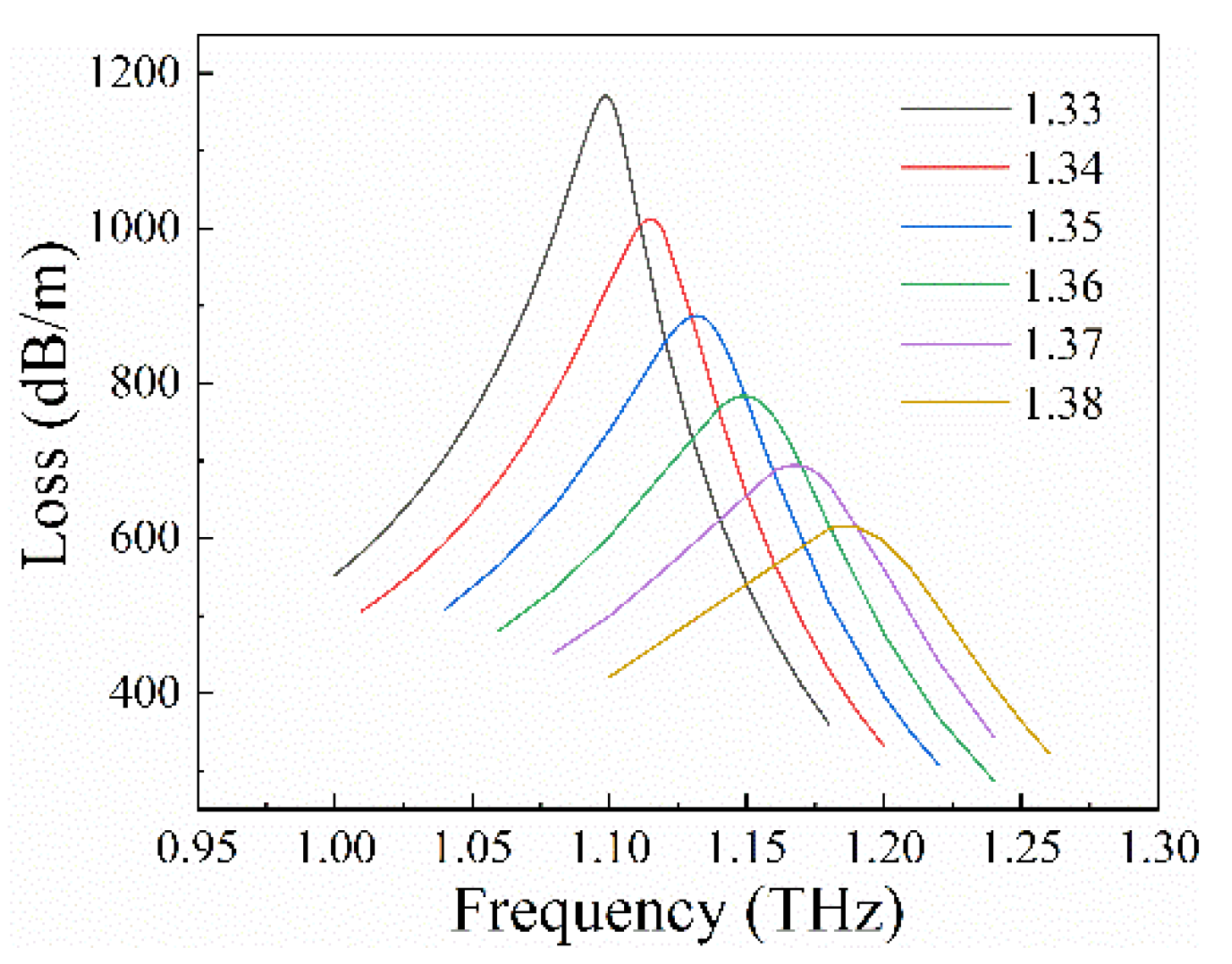
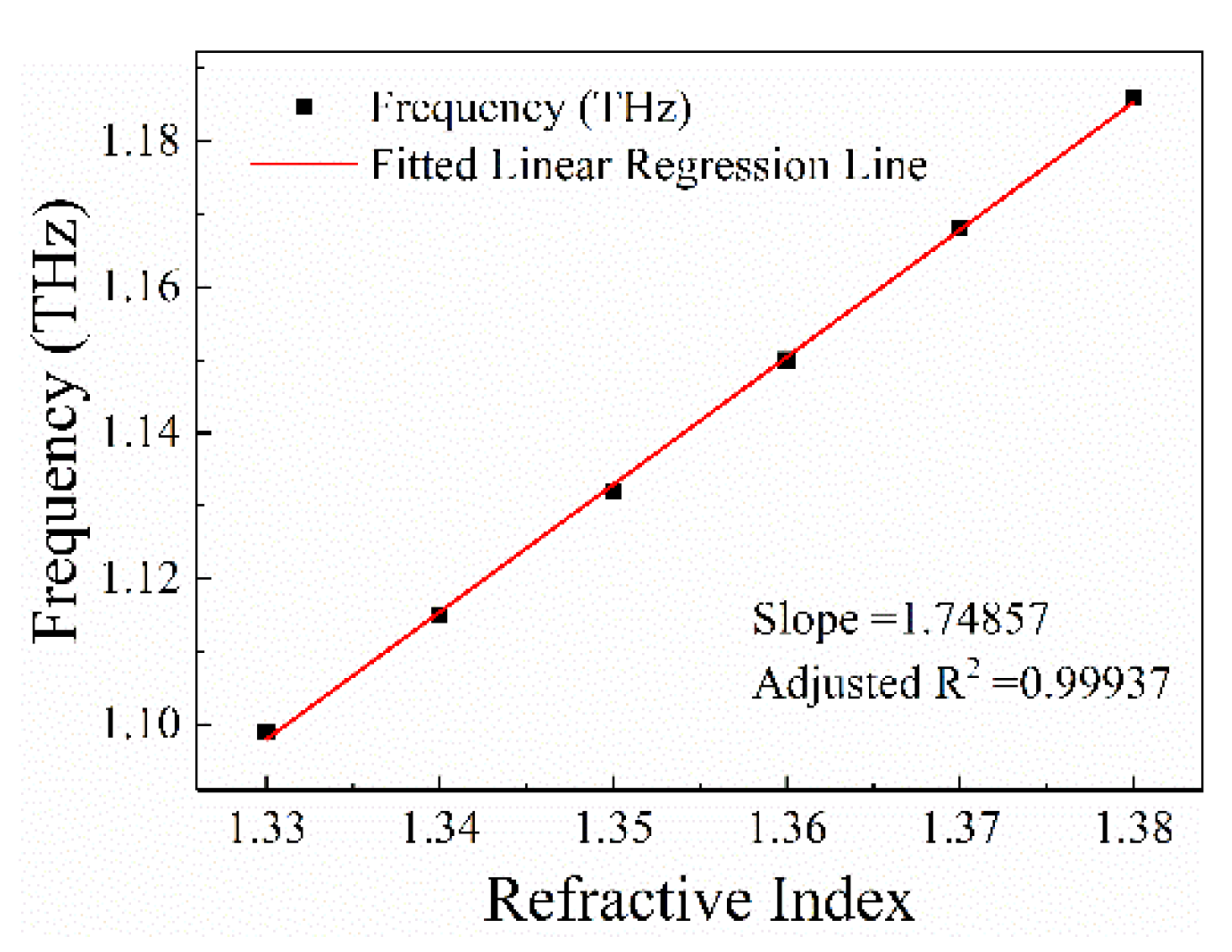
4. Sensing Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lyu, C.; Liu, Z.; Huo, Z.; Ge, C.; Cheng, X.; Tam, H.-Y. High-sensitivity, high-spatial-resolution distributed strain sensing based on a poly(methyl methacrylate) chirped fiber Bragg grating. Photon. Res. 2020, 8, 1134–1139. [Google Scholar] [CrossRef]
- Shen, L.; Wu, H.; Zhao, C.; Shen, L.; Zhang, R.; Tong, W.; Fu, S.; Research, M.T.J.P. Distributed curvature sensing based on a bending loss-resistant ring-core fiber. Photon. Res. 2020, 8, 165. [Google Scholar] [CrossRef]
- Sun, C.T.; Wang, R.; Jin, X.R.; Wang, Z.M.; Liu, W.L.; Zhang, S.; Ma, Y.W.; Lin, J.Y.; Li, Y.; Geng, T.; et al. A new phase-shifted long-period fiber grating for simultaneous measurement of torsion and temperature. Chin. Opt. Lett. 2020, 18, 021203. [Google Scholar] [CrossRef]
- Cao, L.; Yu, Y.; Xiao, M.; Yang, J.B.; Zhang, X.L.; Meng, Z. High sensitivity conductivity-temperature-depth sensing based on an optical microfiber coupler combined fiber loop. Chin. Opt. Lett. 2020, 18, 011202. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, Q.; Wang, C.; Qian, Y.; Liu, G.; Wang, Y.; Fu, L. Surface plasmon resonance (SPR) biosensors for food allergen detection in food matrices. Biosens. Bioelectron. 2019, 142, 111449. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.; Lukaszewski, Z.; Gorodkiewicz, E. Potential of surface plasmon resonance biosensors in cancer detection. J. Pharm. Biomed. Anal. 2021, 194, 113802. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.A.; Zaaba, S.K.; Zakaria, A.; Kamarudin, L.M.; Wan, K.; Shariman, A.B. Quartz crystal microbalance for bacteria application review. In Proceedings of the 2014 2nd International Conference on Electronic Design (ICED), Penang, Malaysia, 19–21 August 2014; pp. 455–460. [Google Scholar]
- Atashbar, M.Z.; Bejcek, B.; Vijh, A.; Singamaneni, S. QCM biosensor with ultra thin polymer film. Sens. Actuators B Chem. 2005, 107, 945–951. [Google Scholar] [CrossRef]
- Arwin, H. Spectroscopic ellipsometry and biology: Recent developments and challenges. Thin Solid Film. 1998, 313–314, 764–774. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.Z.; Liu, C.; Liu, Z.H.; Zhang, Y.X.; Yang, X.H.; Zhang, J.Z.; Yang, J.; Yuan, L.B. Dual-channel microfluidic sensor based on side-hole fiber with two long-period fiber gratings. Chin. Opt. Lett. 2020, 18. [Google Scholar] [CrossRef]
- Yin, M.-J.; Huang, B.; Gao, S.; Zhang, A.P.; Ye, X. Optical fiber LPG biosensor integrated microfluidic chip for ultrasensitive glucose detection. Biomed. Opt. Express 2016, 7, 2067–2077. [Google Scholar] [CrossRef] [Green Version]
- Estevez, M.C.; Alvarez, M.; Lechuga, L.M. Integrated optical devices for lab-on-a-chip biosensing applications. Laser Photonics Rev. 2012, 6, 463–487. [Google Scholar] [CrossRef] [Green Version]
- Zinoviev, K.E.; Gonzalez-Guerrero, A.B.; Dominguez, C.; Lechuga, L.M. Integrated bimodal waveguide interferometric biosensor for label-free analysis. J. Lightwave Technol. 2011, 29, 1926–1930. [Google Scholar] [CrossRef] [Green Version]
- Conteduca, D.; Brunetti, G.; Dell’Olio, F.; Armenise, M.N.; Krauss, T.F.; Ciminelli, C. Monitoring of individual bacteria using electro-photonic traps. Biomed. Opt. Express 2019, 10, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Algorri, J.F.; Zografopoulos, D.C.; Ferraro, A.; García-Cámara, B.; Beccherelli, R.; Sánchez-Pena, J.M. Ultrahigh-quality factor resonant dielectric metasurfaces based on hollow nanocuboids. Opt. Express 2019, 27, 6320–6330. [Google Scholar] [CrossRef]
- Singh, P. SPR biosensors: Historical perspectives and current challenges. Sens. Actuators B Chem. 2016, 229, 110–130. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, J.; Wang, B. Highly sensitive SPR biosensor based on graphene oxide and staphylococcal protein a Co-Modified TFBG for human IgG detection. IEEE Trans. Instrum. Meas. 2019, 68, 3350–3357. [Google Scholar] [CrossRef]
- Moreno, Y.; Song, Q.; Xing, Z.; Sun, Y.; Letters, Z.Y.J.C.O. Hybrid tilted fiber gratings-based surface plasmon resonance sensor and its application for hemoglobin detection. Chin. Opt. Lett. 2020, 18, 100601. [Google Scholar] [CrossRef]
- Wu, L.; Jia, Y.; Jiang, L.; Guo, J.; Dai, X.; Xiang, Y.; Fan, D. Sensitivity improved SPR biosensor based on the MoS2/graphene–aluminum hybrid structure. J. Lightwave Technol. 2017, 35, 82–87. [Google Scholar] [CrossRef]
- Wang, W.; Mai, Z.; Chen, Y.; Wang, J.; Li, L.; Su, Q.; Li, X.; Hong, X. A label-free fiber optic SPR biosensor for specific detection of C-reactive protein. Sci. Rep. 2017, 7, 16904. [Google Scholar] [CrossRef]
- Aray, A.; Chiavaioli, F.; Arjmand, M.; Trono, C.; Tombelli, S.; Giannetti, A.; Cennamo, N.; Soltanolkotabi, M.; Zeni, L.; Baldini, F. SPR-based plastic optical fibre biosensor for the detection of C-reactive protein in serum. J. Biophotonics 2016, 9, 1077–1084. [Google Scholar] [CrossRef]
- Dash, J.N.; Jha, R. SPR biosensor based on polymer PCF coated with conducting metal oxide. IEEE Photonics Technol. Lett. 2014, 26, 595–598. [Google Scholar] [CrossRef]
- Wu, Q.; Li, N.; Wang, Y.; Liu, Y.; Xu, Y.; Wei, S.; Wu, J.; Jia, G.; Fang, X.; Chen, F.; et al. A 2D transition metal carbide MXene-based SPR biosensor for ultrasensitive carcinoembryonic antigen detection. Biosens. Bioelectron. 2019, 144, 111697. [Google Scholar] [CrossRef]
- Menikh, A.; MacColl, R.; Mannella, C.A.; Zhang, X.-C. Terahertz biosensing technology: Frontiers and progress. ChemPhysChem 2002, 3, 655–658. [Google Scholar] [CrossRef]
- Markelz, A.G. Terahertz dielectric sensitivity to biomolecular structure and function. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 180–190. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, G.; Kim, C.; Jhon, Y.M.; Kim, J.H.; Lee, T.; Son, J.H.; Seo, M. Ultrasensitive detection of residual pesticides using THz near-field enhancement. IEEE Trans. Terahertz Sci. Technol. 2016, 6, 389–395. [Google Scholar] [CrossRef]
- Saadeldin, A.S.; Hameed, M.F.O.; Elkaramany, E.M.A.; Obayya, S.S.A. Highly sensitive terahertz metamaterial sensor. IEEE Sens. J. 2019, 19, 7993–7999. [Google Scholar] [CrossRef]
- Mou, F.A.; Rahman, M.M.; Islam, M.R.; Bhuiyan, M.I.H. Development of a photonic crystal fiber for THz wave guidance and environmental pollutants detection. Sens. Bio-Sens. Res. 2020, 29, 100346. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mou, F.A.; Bhuiyan, M.I.H.; Islam, M.R. Photonic crystal fiber based terahertz sensor for cholesterol detection in human blood and liquid foodstuffs. Sens. Bio-Sens. Res. 2020, 29, 100356. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, L.; Ding, L.; Jin, B.; Hou, Y.; Li, C.; Jiang, L.; Liu, W.; Hu, W.; Lu, Y.; et al. Label-free measurements on cell apoptosis using a terahertz metamaterial-based biosensor. Appl. Phys. Lett. 2016, 108, 241105. [Google Scholar] [CrossRef]
- Sultana, J.; Islam, M.S.; Ahmed, K.; Dinovitser, A.; Ng, B.W.H.; Abbott, D. Terahertz detection of alcohol using a photonic crystal fiber sensor. Appl. Opt. 2018, 57, 2426–2433. [Google Scholar] [CrossRef]
- Biswas, T.; Chattopadhyay, R.; Bhadra, S.K. Plasmonic hollow-core photonic band gap fiber for efficient sensing of biofluids. J. Opt. 2014, 16, 045001. [Google Scholar] [CrossRef]
- Klantsataya, E.; François, A.; Ebendorff-Heidepriem, H.; Hoffmann, P.; Monro, T.M. Surface plasmon scattering in exposed core optical fiber for enhanced resolution refractive index sensing. Sensors 2015, 15, 25090–25102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Cao, S.; Liao, C.; Wang, Y.; Wang, G.; Xu, X.; Fu, C.; Xu, G.; Lian, J.; Wang, Y. Surface plasmon resonance refractive sensor based on silver-coated side-polished fiber. Sens. Actuators B Chem. 2016, 230, 206–211. [Google Scholar] [CrossRef]
- Abdullah, H.; Ahmed, K.; Mitu, S.A. Ultrahigh sensitivity refractive index biosensor based on gold coated nano-film photonic crystal fiber. Results Phys. 2020, 17, 103151. [Google Scholar] [CrossRef]
- Jeong, H.-H.; Erdene, N.; Park, J.-H.; Jeong, D.-H.; Lee, H.-Y.; Lee, S.-K. Real-time label-free immunoassay of interferon-gamma and prostate-specific antigen using a Fiber-Optic Localized Surface Plasmon Resonance sensor. Biosens. Bioelectron. 2013, 39, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized surface plasmon resonance biosensing: Current challenges and approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Semwal, V.; Gupta, B.D. Highly sensitive and selective localized surface plasmon resonance biosensor for detecting glutamate realized on optical fiber substrate using gold nanoparticles. Photonics Nanostruct.—Fundam. Appl. 2019, 37, 100730. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, B.K.; Singh, R.; Chen, N.-K.; Yang, Q.S.; Zhang, X.; Wang, W.; Zhang, B. LSPR-based cholesterol biosensor using a tapered optical fiber structure. Biomed. Opt. Express 2019, 10, 2150–2160. [Google Scholar] [CrossRef]
- Jin, Y.; Wong, K.H.; Granville, A.M. Developing localized surface plasmon resonance biosensor chips and fiber optics via direct surface modification of PMMA optical waveguides. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 100–109. [Google Scholar] [CrossRef]
- Amirjani, A.; Haghshenas, D.F. Ag nanostructures as the surface plasmon resonance (SPR)-based sensors: A mechanistic study with an emphasis on heavy metallic ions detection. Sens. Actuators B: Chem. 2018, 273, 1768–1779. [Google Scholar] [CrossRef]
- Agrawal, N.; Zhang, B.; Saha, C.; Kumar, C.; Kaushik, B.K.; Kumar, S. Development of dopamine sensor using silver nanoparticles and PEG-functionalized tapered optical fiber structure. IEEE Trans. Biomed. Eng. 2020, 67, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hou, T.; Lin, H.; Lv, W.; Li, H.; Li, F. In situ template generation of silver nanoparticles as amplification tags for ultrasensitive surface plasmon resonance biosensing of microRNA. Biosens. Bioelectron. 2019, 137, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Piesiewicz, R.; Jansen, C.; Wietzke, S.; Mittleman, D.; Koch, M.; Kürner, T.J.I.J.o.I.; Waves, M. Properties of building and plastic materials in the THz range. Int. J. Infrared Millim. Waves 2007, 28, 363–371. [Google Scholar] [CrossRef]
- Hosseininejad, S.E.; Komjani, N. Waveguide-fed tunable terahertz antenna based on hybrid graphene-metal structure. IEEE Trans. Antennas Propag. 2016, 64, 3787–3793. [Google Scholar] [CrossRef]
- Fasano, A.; Woyessa, G.; Stajanca, P.; Markos, C.; Stefani, A.; Nielsen, K.; Rasmussen, H.K.; Krebber, K.; Bang, O. Fabrication and characterization of polycarbonate microstructured polymer optical fibers for high-temperature-resistant fiber Bragg grating strain sensors. Opt. Mater. Express 2016, 6, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Dadabayev, R.; Malka, D. A visible light RGB wavelength demultiplexer based on polycarbonate multicore polymer optical fiber. Opt. Laser Technol. 2019, 116, 239–245. [Google Scholar] [CrossRef]
- Sultangazin, A.; Kusmangaliyev, J.; Aitkulov, A.; Akilbekova, D.; Olivero, M.; Tosi, D. Design of a smartphone plastic optical fiber chemical sensor for hydrogen sulfide detection. IEEE Sens. J. 2017, 17, 6935–6940. [Google Scholar] [CrossRef] [Green Version]
- Grassini, S.; Ishtaiwi, M.; Parvis, M.; Vallan, A. Design and deployment of low-cost plastic optical fiber sensors for gas monitoring. Sensors 2015, 15, 485–498. [Google Scholar] [CrossRef]
- Shobin, L.R.; Sastikumar, D.; Manivannan, S. Glycerol mediated synthesis of silver nanowires for room temperature ammonia vapor sensing. Sens. Actuators A Phys. 2014, 214, 74–80. [Google Scholar] [CrossRef]
- Yaşlı, A.; Ademgil, H. Modeling the photonic crystal fiber based surface plazmon resonance biosensor to detect blood components. In Proceedings of the 2021 29th Signal Processing and Communications Applications Conference (SIU), Istanbul, Turkey, 9–11 June 2021; pp. 1–4. [Google Scholar]
- Zolghadri, S.; Saboury, A.A.; Golestani, A.; Divsalar, A.; Rezaei-Zarchi, S.; Moosavi-Movahedi, A.A. Interaction between silver nanoparticle and bovine hemoglobin at different temperatures. J. Nanoparticle Res. 2008, 11, 1751. [Google Scholar] [CrossRef]
- Liu, S.; Ma, R.; Li, Y.; Zhao, L.; Xia, Y.; Dong, X.; Pang, Y. D-shaped surface plasmon resonance biosensor based on MoS2 in terahertz band. Opt. Fiber Technol. 2021, 66, 102631. [Google Scholar] [CrossRef]
- Arunkumar, R.; Suaganya, T.; Robinson, S.J.P.S. Design and analysis of 2D photonic crystal based biosensor to detect different blood components. Photonic Sens. 2019, 9, 69–77. [Google Scholar] [CrossRef]
- Sridevi, S.; Vasu, K.S.; Sampath, S.; Asokan, S.; Sood, A.K. Optical detection of glucose and glycated hemoglobin using etched fiber Bragg gratings coated with functionalized reduced graphene oxide. J. Biophotonics 2016, 9, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.R.; Akter, S.; Rifat, A.A.; Rana, S.; Ali, S. A highly sensitive gold-coated photonic crystal fiber biosensor based on surface plasmon resonance. Photonics 2017, 4, 18. [Google Scholar] [CrossRef]
- Tong, K.; Wang, F.; Wang, M.; Dang, P.; Wang, Y. Three-core photonic crystal fiber surface plasmon resonance sensor. Opt. Fiber Technol. 2018, 46, 306–310. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Q. Sensitivity-enhanced optical fiber biosensor based on coupling effect between SPR and LSPR. IEEE Sens. J. 2018, 18, 8303–8310. [Google Scholar] [CrossRef]
- Shengxi, J.; Sanfeng, G.; Hanrui, Y.; Hairui, F. Research on dual-core photonic crystal fiber based on local surface plasmon resonance sensor with silver nanowires. J. Nanophotonics 2018, 12, 1–15. [Google Scholar] [CrossRef]
- Samusenko, A.; Gandolfi, D.; Pucker, G.; Chalyan, T.; Guider, R.; Ghulinyan, M.; Pavesi, L. A SiON microring resonator-based platform for biosensing at 850 nm. J. Lightwave Technol. 2016, 34, 969–977. [Google Scholar] [CrossRef]
- Tsai, M.-Z.; Hsiung, C.-T.; Chen, Y.; Huang, C.-S.; Hsu, H.-Y.; Hsieh, P.-Y. Real-time CRP detection from whole blood using micropost-embedded microfluidic chip incorporated with label-free biosensor. Analyst 2018, 143, 503–510. [Google Scholar] [CrossRef] [PubMed]


| Sensor Configuration/Materials Coating | Type of Excitation | Sensitivity | Working Band | Ref |
|---|---|---|---|---|
| PCF/gold film | SPR | 2200 nm/RIU | 0.6–0.652 μm | [56] |
| PCF/gold film | SPR | 3435 nm/RIU | 575–815 nm | [57] |
| PCF/gold nanoparticles | LSPR | 3915 nm/RIU | 656–813 nm | [58] |
| SP-SMF/silver film | SPR | 4365.5 nm/RIU | 508–731 nm | [34] |
| PCF/silver nanowires | LSPR | 9000 nm/RIU | 599–768 nm | [59] |
| Microring resonator | / | 80 nm/RIU | ~850 nm | [60] |
| GMR sensor | / | 186 nm /RIU | 841–863 nm | [61] |
| This paper | LSPR | 230,164 nm/RIU | 253–273 μm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Li, L.; Bai, Z. Highly Sensitive Biosensor Based on Partially Immobilized Silver Nanopillars in the Terahertz Band. Photonics 2021, 8, 438. https://doi.org/10.3390/photonics8100438
Liu S, Li L, Bai Z. Highly Sensitive Biosensor Based on Partially Immobilized Silver Nanopillars in the Terahertz Band. Photonics. 2021; 8(10):438. https://doi.org/10.3390/photonics8100438
Chicago/Turabian StyleLiu, Shuo, Lin Li, and Zhenxu Bai. 2021. "Highly Sensitive Biosensor Based on Partially Immobilized Silver Nanopillars in the Terahertz Band" Photonics 8, no. 10: 438. https://doi.org/10.3390/photonics8100438
APA StyleLiu, S., Li, L., & Bai, Z. (2021). Highly Sensitive Biosensor Based on Partially Immobilized Silver Nanopillars in the Terahertz Band. Photonics, 8(10), 438. https://doi.org/10.3390/photonics8100438






