Experimental Investigation on the Sputtering Process for Tantalum Oxynitride Thin Films
Abstract
:1. Introduction
2. Experiment
2.1. Substrate Preparation
2.2. Film Deposition
2.3. Characterization
2.3.1. Thickness and Surface Morphology of Films
2.3.2. Microstructure of Films
2.3.3. Elemental Chemical Composition
2.3.4. Electrical Measurement
2.3.5. Optical Measurement for Films
2.3.6. Photodegradation Test
3. Results
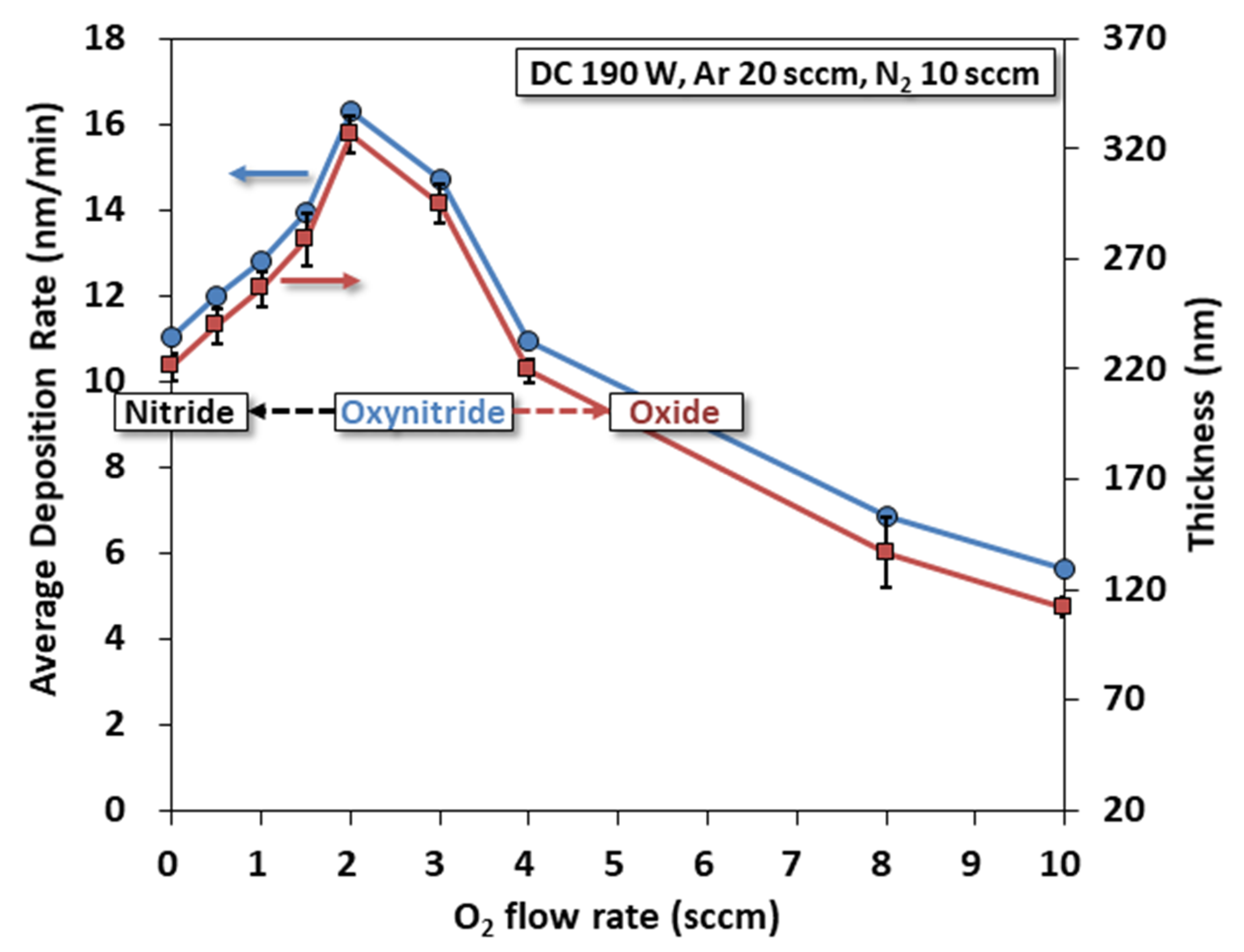
3.1. Average Deposition Rate
3.2. XRD
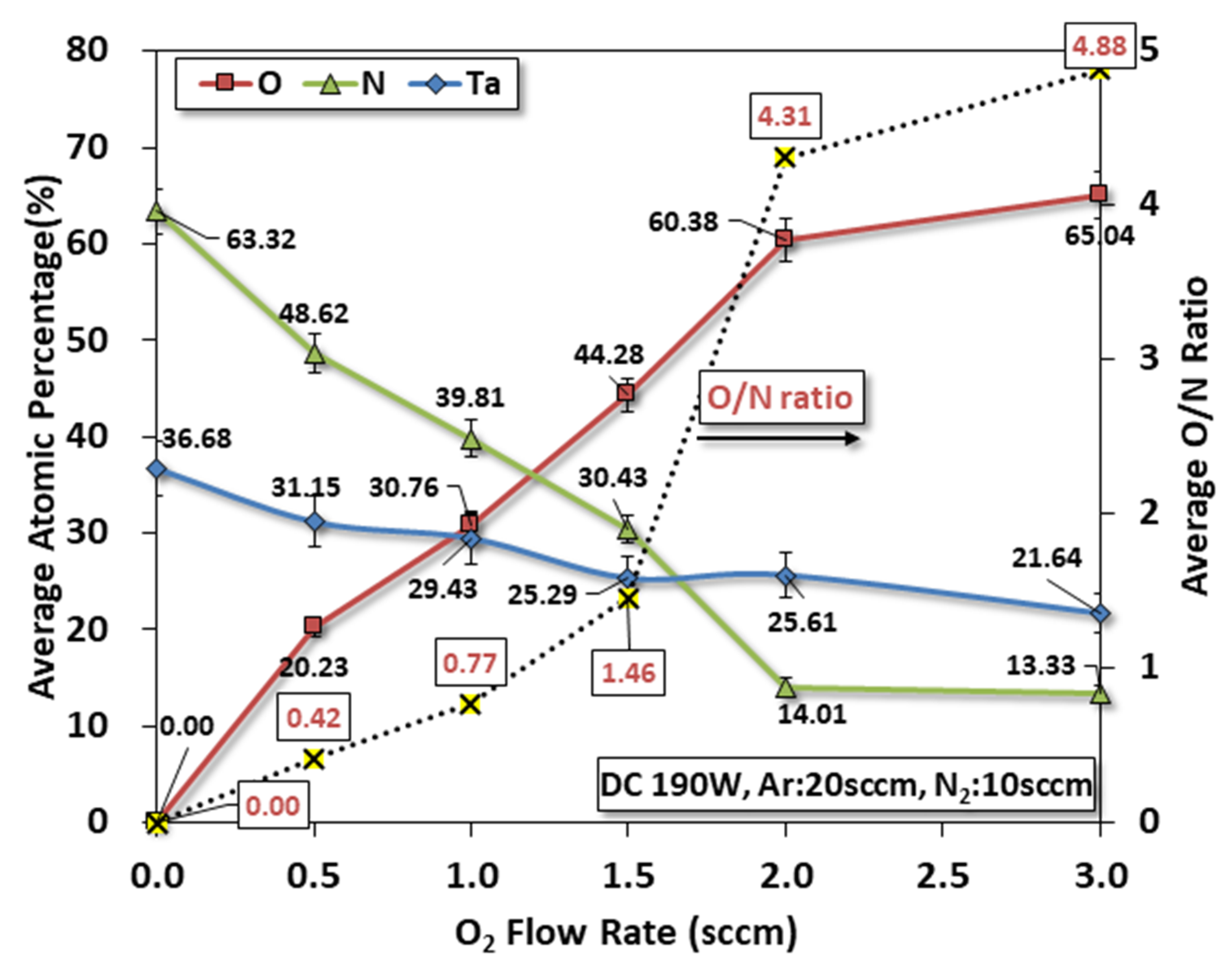
3.3. EDS
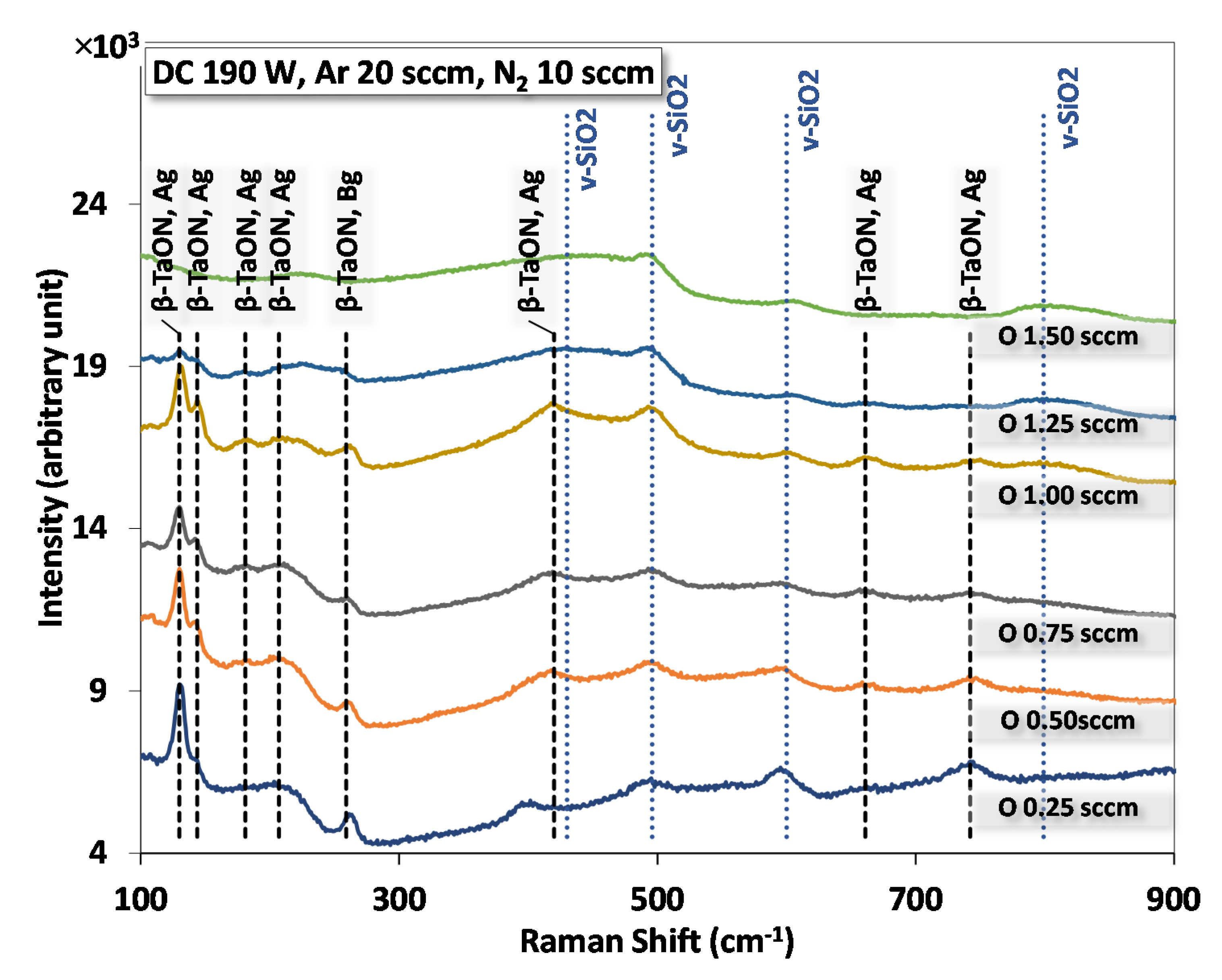
3.4. Raman Spectra
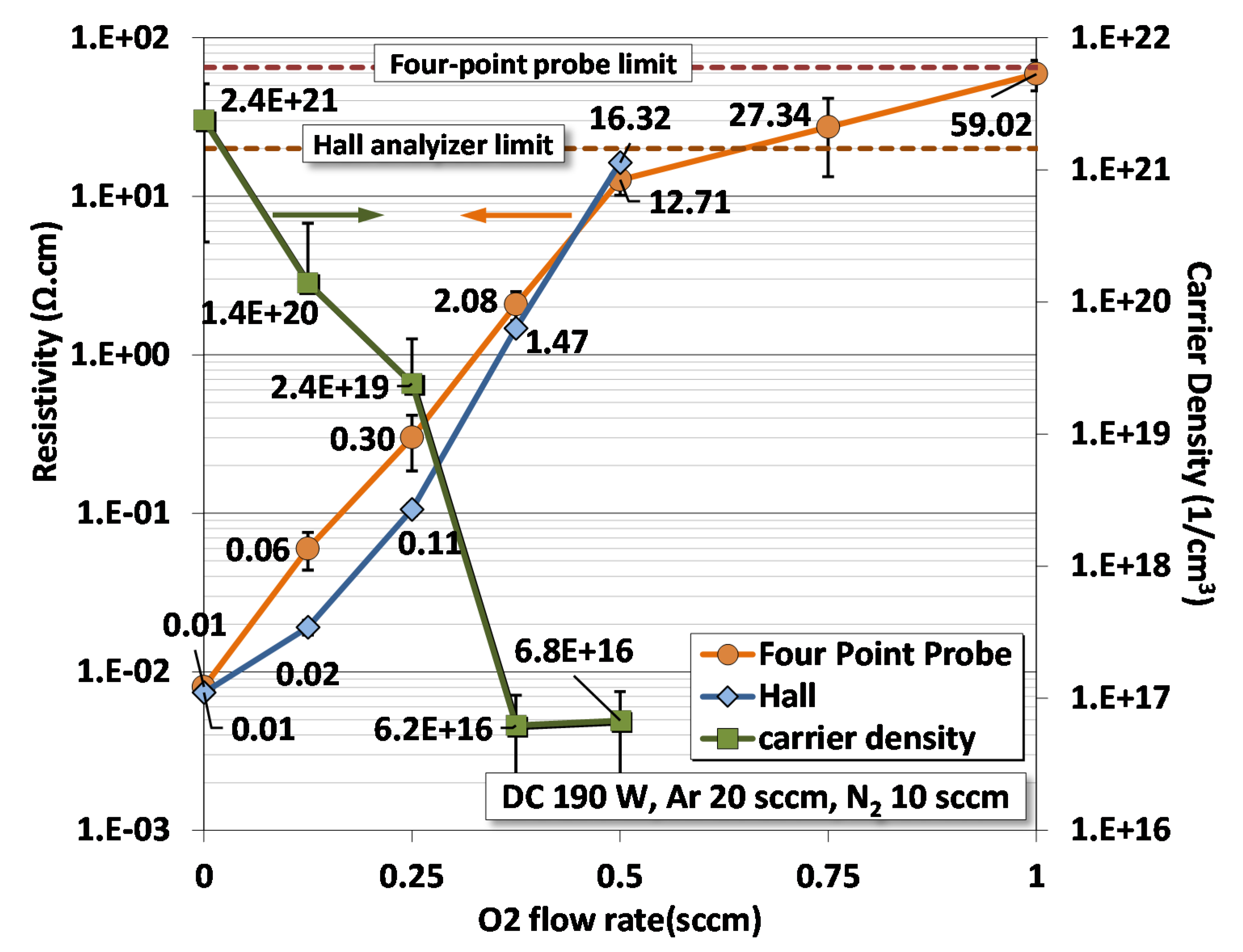
3.5. Electrical Resistivity
3.6. UV-Vis-NIR Spectra
- For zero oxygen supply, the tantalum nitride film is almost opaque with very low transmittance. The optical reflectance is between 30 and 40%, and the absorbance reaches 60–90%.
- As the oxygen supply increases from 0.5 sccm to 2 sccm, the transmittance also increases in the range of visible light. This result implies a gradually increased transparency of the films. Associated with this increase, there are “shoulders” in the variation of transmittance when light changes from the UV to visible light. Just like doping in semi-conductors, these shoulders indicate a possible existence of intra-bands in the films.
- When the oxygen supply exceeds 2 sccm, the transmittance reaches above 80% in the range of visible light, while the absorbance reduces simultaneously to very low values. The switch between high and low transmittance and absorbance occurs around 300 nm. In other words, films become transparent in visible light. This also shows that these high-oxygen films are effective filters for UV light.
- The color change of films is discussed in the section on XRD, and this change is in accordance with the variation of transmittance as shown in the inset of the photo.
- Note that certain degrees of transparency with sufficient optical absorbance are a necessity for oxynitride films to achieve photocatalysis.
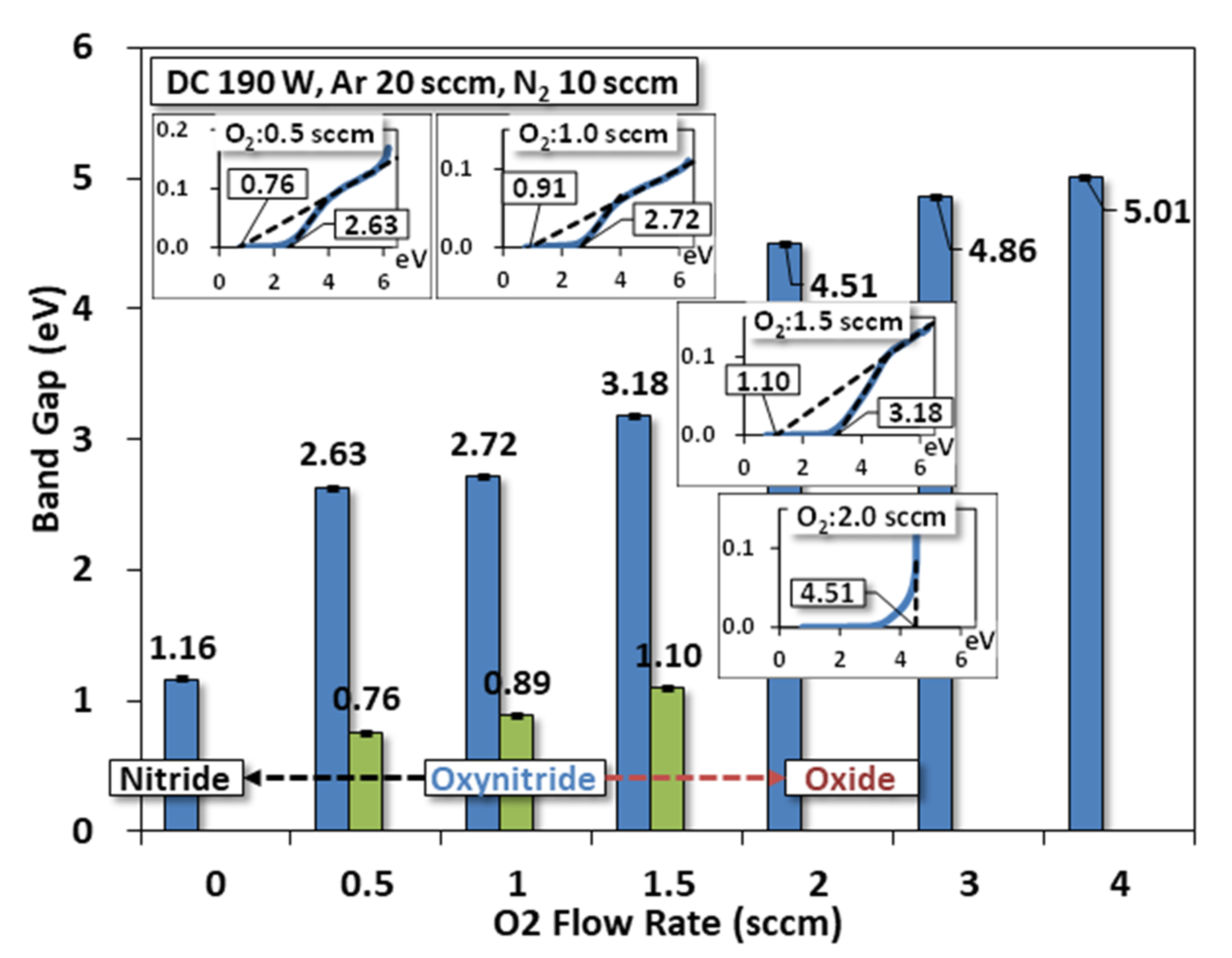
3.7. Optical Band Gap
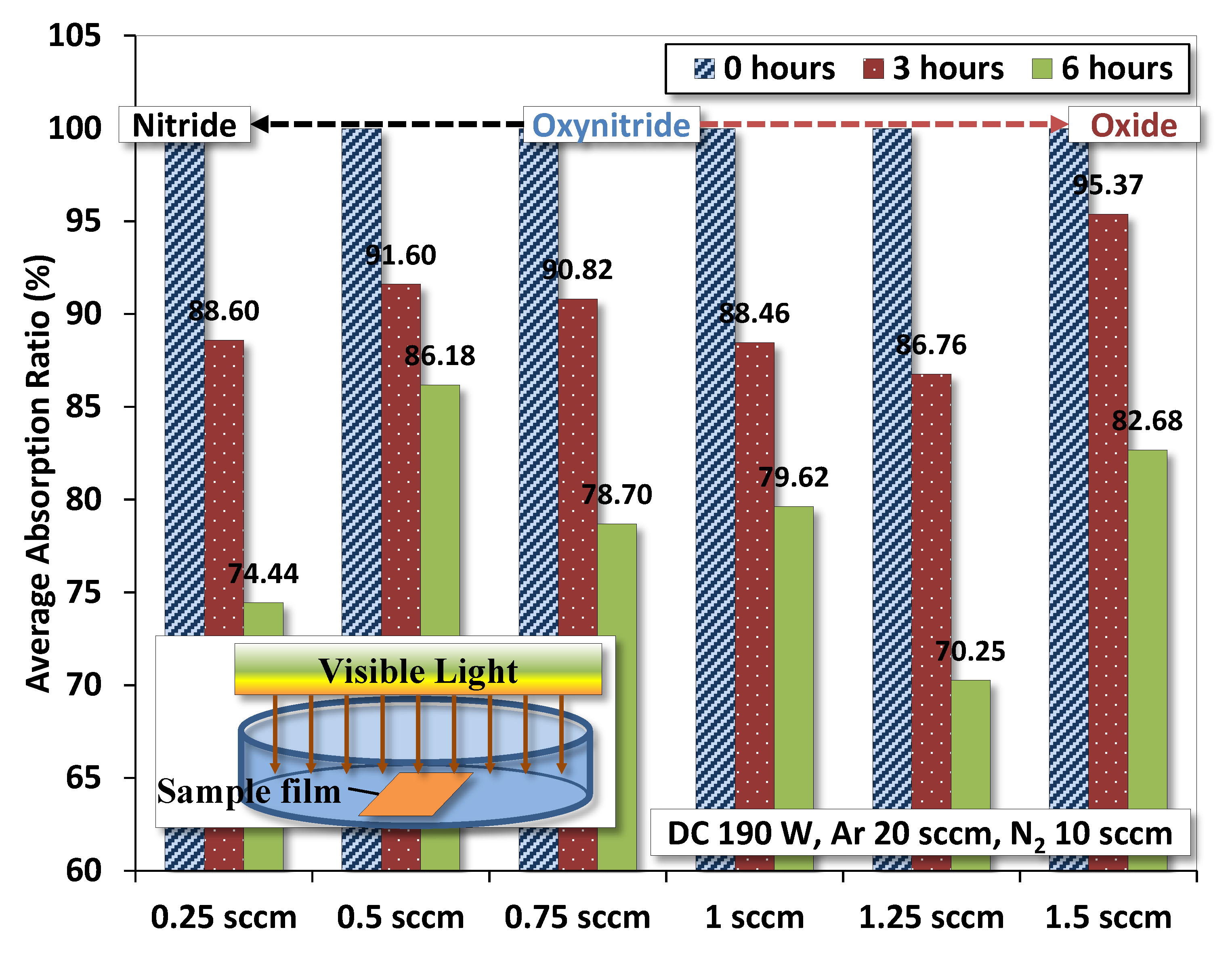
3.8. Photodegradation Test
4. Discussion
4.1. Optimal Choice for Photocatalysis
- Crystalline phases: Both XRD and Raman detects the formation in films for O2 flow between ~0.25 and ~1.25 sccm;
- Limited oxygen: EDS roughly indicates that the content in films can be maintained for O2 flow less than 1.5 sccm;
- Semi-conductivity: Four-point probe and Hall effect analyzer predict finite electrical resistivity for O2 flow between ~0.25 and ~1.0 sccm;
- Semi-transparent with sufficient optical absorption: UV-Vis-NIR observes such for O2 flow between ~0.5 –~1.5 sccm;
- Intra-bands: Tauc plot estimates the presence of dual optical bandgaps for O2 flow between ~0.5 and ~1.5 sccm;
- Efficiency: Photodegradation of methyl orange estimates higher efficiency for films deposited by ~0.25 and 1.25 sccm O2 flow.
4.2. Electrical and Optical Characters Comparison
5. Conclusions
Author Contributions
Conflicts of Interest
Appendix A. Estimated Crystal Size
| O2 Flow Rate (sccm) | 2-Theta | Est. Crystal Size (Å) | (h,k,l) |
|---|---|---|---|
| 0 sccm, TaN | 36.28 | 83.60 | (1,2,1) |
| 42.08 | 88.85 | (0,0,2) | |
| 27.99 | 70.58 | (2,0,0) | |
| 22.99 | 94.82 | (1,0,1) | |
| 60.98 | 67.84 | (3,1,2) | |
| 0.25 sccm, TaON | 31.82 | 173.18 | unknown |
| 36.76 | 147.64 | (2,0,0) | |
| 29.14 | 96.23 | (−1,1,1) | |
| 53.08 | 167.86 | (−2,2,1) | |
| 28.44 | 116.25 | (1,11,0) | |
| 0.5 sccm, TaON | 29.19 | 151.18 | (−1,1,1) |
| 32.79 | 132.71 | (1,1,1) | |
| 31.72 | 155.24 | unknown | |
| 36.74 | 169.45 | (2,0,0) | |
| 52.24 | 169.50 | (−1,2,2) | |
| 35.26 | 87.84 | (0,0,2) | |
| 0.75 sccm, TaON | 29.18 | 137.28 | (−1,1,1) |
| 32.81 | 144.53 | (1,1,1) | |
| 36.76 | 143.84 | (2,0,0) | |
| 35.27 | 77.98 | (0,0,2) | |
| 52.20 | 171.11 | (2,2,0) | |
| 1.0 sccm, TaON | 29.21 | 148.72 | (−1,1,1) |
| 32.83 | 156.26 | (1,1,1) | |
| 36.79 | 185.22 | (2,0,0) | |
| 35.80 | 186.35 | (0,2,0) | |
| 52.24 | 205.29 | (−1,2,2) | |
| 1.25 sccm, TaON | 29.01 | 90.97 | (2,1,1) |
| 36.78 | 151.39 | (2,0,0) * | |
| 32.87 | 150.05 | (1,1,1) | |
| 28.56 | 126.51 | (−1,1,1) * | |
| 52.28 | 184.36 | (−1,2,2) | |
| 1.5 sccm, Ta2O5 | 36.92 | 161.37 | (2,0,0) |
| 22.94 | 166.45 | (0,0,1) | |
| 55.75 | 101.32 | (1,11,2) | |
| 50.42 | 95.13 | (3,3,1) | |
| 28.48 | 157.92 | (−1,1,1) | |
| 46.74 | 189.37 | (3,7,0) | |
| 2.0 sccm, Ta2O5 | 28.59 | 181.37 | (−1,1,1) |
| 36.98 | 181.71 | (2,0,0) | |
| 22.99 | 199.19 | (0,0,1) | |
| 55.84 | 99.90 | (1,11,2) | |
| 50.60 | 90.96 | (3,3,1) | |
| 46.86 | 202.29 | (3,7,0) | |
| 4.0 sccm, Ta2O5 | 23.01 | 227.09 | (0,0,1) |
| 28.65 | 192.92 | (−1,1,1) | |
| 37.03 | 214.82 | (2,0,0) | |
| 55.83 | 121.33 | (1,11,2) | |
| 46.91 | 240.55 | (3,7,0) | |
| 50.68 | 115.81 | (3,3,1) |
Appendix B. SEM


References
- Ahmed, M.; Guo, X. A review of metal oxynitrides for photocatalysis. Inorg. Chem. Front. 2016, 3, 578–590. [Google Scholar] [CrossRef]
- Cubillos, G.I.; Bethencourt, M.; Olaya, J.J. Corrosion resistance of zirconium oxynitride coatings deposited via DC unbal-anced magnetron sputtering and spray pyrolysis-nitriding. Appl. Surf. Sci. 2015, 327, 288–295. [Google Scholar] [CrossRef]
- Maeda, K.; Kazunari, D. Oxynitride materials for solar water splitting. MRS Bull. 2011, 36, 25–31. [Google Scholar] [CrossRef]
- Cao, G.Z.; Metselaar, R. α’-Sialon Ceramics: A Review. Chem. Mater. 1991, 3, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Qu, G.; Zhang, Y.; Cui, L.; Lu, A. Transparent oxynitride glasses: Synthesis, microstructure, optical transmittance and ballistic resistance. J. Non-Cryst. Solids 2013, 378, 45–49. [Google Scholar] [CrossRef]
- Lei, P.; Guo, S.; Zhu, J.; Dai, B.; Liu, G.; Wang, Y.; Han, J. Enhanced mechanical properties of HfO2film by nitrogen doping. Surf. Eng. 2016, 32, 585–588. [Google Scholar] [CrossRef]
- Gibson, J.S.K.-L.; Rezaei, S.; Rueß, H.; Hans, M.; Music, D.; Wulfinghoff, S.; Schneider, J.M.; Reese, S.; Korte-Kerzel, S. From quantum to continuum mechanics: Studying the fracture toughness of transition metal nitrides and oxynitrides. Mater. Res. Lett. 2018, 6, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Ebbinghaus, S.G.; Abicht, H.-P.; Dronskowski, R.; Müller, T.; Reller, A.; Weidenkaff, A. Perovskite-related oxynitrides—Recent developments in synthesis, characterization and investigations of physical properties. Prog. Solid State Chem. 2009, 37, 173–205. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Khan, I.; Yamani, Z.H.; Qurashi, A. Sonochemical Assisted Solvothermal Synthesis of Gallium Oxynitride Nanosheets and their Solar-Driven Photoelectrochemical Water-Splitting Applications. Sci. Rep. 2016, 6, 32319. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, B.S.; Hafez, A.M.; Murali, B.; Kirmani, A.R.; Amassian, A.; Mohammed, O.F.; Allam, N.K. 10-fold enhancement in light-driven water splitting using niobium oxynitride microcone array films. Sol. Energy Mater. Sol. Cells 2016, 151, 149–153. [Google Scholar] [CrossRef]
- Black, A.P.; Suzuki, H.; Higashi, M.; Frontera, C.; Ritter, C.; De, C.; Sundaresan, A.; Abe, R.; Fuertes, A. New rare earth hafnium oxynitride perovskites with photocatalytic activity in water oxidation and reduction. Chem. Commun. 2018, 54, 1525–1528. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.-C.; Yang, T.-S.; Wong, M.-S. Nitrogen-doped titanium oxide films as visible light photocatalyst by vapor deposition. Thin Solid Films 2004, 469, 1–5. [Google Scholar] [CrossRef]
- Kitano, M.; Matsuoka, M.; Ueshima, M.; Anpo, M. Recent developments in titanium oxide-based photocatalysts. Appl. Catal. A Gen. 2007, 325, 1–14. [Google Scholar] [CrossRef]
- Lai, K.; Huang, J.Y.; Zhang, H.F.; Subramaniam, V.P.; Tang, Y.X.; Gong, D.G.; Sundar, L.; Sun, L.; Chen, Z.; Lin, C.J. Nitrogen-doped TiO2 nanotube array films with enhanced photocatalytic activity under various light sources. J. Hazard Mater. 2010, 184, 855–863. [Google Scholar] [CrossRef]
- Triantis, T.M.; Fotiou, T.; Kaloudis, T.; Kontos, A.G.; Falaras, P.; Dionysiou, D.D.; Pelaez, M.; Hiskia, A. Photocatalytic deg-radation and mineralization of microcystin-LR under UV-A, solar and visible light using nanostructured nitrogen doped TiO2. J. Hazard Mater. 2012, 211, 196–202. [Google Scholar] [CrossRef]
- Fuertes, A. Synthetic approaches in oxynitride chemistry. Prog. Solid State Chem. 2018, 51, 63–70. [Google Scholar] [CrossRef]
- Watanabe, T.; Tajima, K.; Li, J.; Matsushita, N.; Yoshimura, M. Low-temperature Ammonothermal Synthesis of LaTaON2. Chem. Lett. 2011, 40, 1101–1102. [Google Scholar] [CrossRef]
- Higashi, M.; Domen, K.; Abe, R. Fabrication of an Efficient BaTaO2N Photoanode Harvesting a Wide Range of Visible Light for Water Splitting. J. Am. Chem. Soc. 2013, 135, 10238–10241. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Si, W.; Woodward, P.M.; Sutter, E.; Park, S.; Vogt, T. Epitaxial Thin-Film Deposition and Dielectric Properties of the Perovskite Oxynitride BaTaO2N. Chem. Mater. 2007, 19, 618–623. [Google Scholar] [CrossRef]
- Khodaei, M.; Yaghobizadeh, O.; Alhosseini, S.H.N.; Esmaeeli, S.; Mousavi, S.R. The effect of oxide, carbide, nitride and boride additives on properties of pressureless sintered SiC: A review. J. Eur. Ceram. Soc. 2019, 39, 2215–2231. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lu, F.-H. Oxidation behavior of titanium nitride films. J. Vac. Sci. Technol. A 2005, 23, 1006–1009. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.S.; Lim, W.; Norton, D.P.; Pearton, S.J.; Ren, F.; Johnson, J.L.; Ural, A. Nitride and oxide semiconductor nanostruc-tured hydrogen gas sensors. Semicond. Sci. Technol. 2010, 25, 024002. [Google Scholar] [CrossRef]
- Ghosh, S.; Jeong, S.M.; Polaki, S.R. A review on metal nitrides/oxynitrides as an emerging supercapacitor electrode beyond oxide. Korean J. Chem. Eng. 2018, 35, 1389–1408. [Google Scholar] [CrossRef]
- Bahari, A.; Morgen, P.; Pedersen, K.; Li, Z.S. Growth of a stacked silicon nitride/silicon oxide dielectric on Si (100). J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2006, 24, 2119. [Google Scholar] [CrossRef]
- Leitao, A.B.V.; Lima, C.L.; Viana, B.C.; Neto, B.; Costa, J.A.P.; Costa, T.H.C.; De Souza, I.A.; Naeem, M.; Sousa, R.R.M. Novel synthesis of molybdenum nitride/oxide on AISI-316 steel assisted with active screen plasma treatment. Mater. Res. Express 2019, 6, 116501. [Google Scholar] [CrossRef]
- Dietl, T. A ten-year perspective on dilute magnetic semiconductors and oxides. Nat. Mater. 2010, 9, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Gerstenberg, D.; Calbick, C.J. Effects of nitrogen, methane, and oxygen on structure and electrical properties of thin tantalum films. J. Appl. Phys. 1964, 35, 402–407. [Google Scholar] [CrossRef]
- Cardonne, S.; Kumar, P.; Michaluk, C.; Schwartz, H. Tantalum and its alloys. Int. J. Refract. Met. Hard Mater. 1995, 13, 187–194. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Beer, A. Bestimmung der absorption des rothen lichts in farbigen flussigkeiten. Ann. Phys. 1852, 162, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Krishna, K.; Soga, T.; Jimbo, T.; Umeno, M. Optical properties and X-ray photoelectron spectroscopic study of pure and Pb-doped TiO2 thin films. J. Phys. Chem. Solids 1999, 60, 201–210. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjinès, R.; Schmid, P.E.; Lévy, F. Electrical and optical properties of TiO2anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Sharma, P.; Vashistha, M.; Jain, I.P. Optical properties of Ge20Se80-xBix thin films. J. Optoelectron. Adv. Mater. 2005, 7, 2647–2654. [Google Scholar]
- Li, C.; Hsieh, J.H.; Hung, M.T.; Huang, B.Q. The deposition and microstructure of amorphous tungsten oxide films by sputtering. Vacuum 2015, 118, 125–132. [Google Scholar] [CrossRef]
- Li, C.; Hsieh, J.H.; Su, T.Y.; Wu, P.L. Experimental Study on Property and Electrochromic Function of Stacked WO3/Ta2O5/NiO Films by Sputtering. Thin Solid Films 2018, 66, 373–379. [Google Scholar] [CrossRef]
- Salamon, K.; Mičetić, M.; Sancho-Parramon, J.; Radović, I.B.; Siketić, Z.; Šarić, I.; Petravić, M.; Bernstorff, S. β-TaON thin films: Production by reactive magnetron sputtering and the question of non-stoichiometry. J. Phys. D Appl. Phys. 2019, 52, 305304. [Google Scholar] [CrossRef]
- Čapek, J.; Batková, Š.; Haviar, S.; Houška, J.; Čerstvý, R.; Zeman, P. Effect of annealing on structure and properties of Ta–O–N films prepared by high power impulse magnetron sputtering. Ceram. Int. 2019, 45, 9454–9461. [Google Scholar] [CrossRef]
- Nurlaela, E.; Harb, M.; Del Gobbo, S.; Vashishta, M.; Takanabe, K. Combined experimental and theoretical assessments of the lattice dynamics and optoelectronics of TaON and Ta3N5. J. Solid State Chem. 2015, 229, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Fan, W.; Li, Y.; Sun, H.; Cheng, X.; Zhao, X.; Jiang, M. First-Principles Study of the Electronic, Optical Properties and Lattice Dynamics of Tantalum Oxynitride. Inorg. Chem. 2010, 49, 6917–6924. [Google Scholar] [CrossRef] [PubMed]
- Ivanda, M.; Clasen, R.; Hornfeck, M.; Kiefer, W. Raman spectroscopy on SiO2 glasses sintered from nanosized particles. J. Non-Cryst. Solids 2003, 322, 46–52. [Google Scholar] [CrossRef]
- Chung, C.; Chen, T.; Chang, N. Effect of reactive gases flow ratios on the microstructure and electrical resistivity of Ta–N–O thin films by reactive co-sputtering. Thin Solid Films 2011, 519, 5099–5102. [Google Scholar] [CrossRef]
- Suzuki, A.; Hirose, Y.; Oka, D.; Nakao, S.; Fukumura, T.; Ishii, S.; Sasa, K.; Matsuzaki, H.; Hasegawa, T. High-Mobility Electron Conduction in Oxynitride: Anatase TaON. Chem. Mater. 2014, 26, 976–981. [Google Scholar] [CrossRef]
- Ayerdi, I.; Castaño, E.; García-Alonso, A.; Gracia, F.J. Characterization of tantalum oxynitride thin films as high-temperature strain gauges. Sens. Actuator A Phys. 1995, 46, 218–221. [Google Scholar] [CrossRef]
- Lee, P.-Y.; Lin, C.-X.; Lee, Y.-C. Microstructure and electrical property of tantalum oxynitride thin films prepared using high-power impulse reactive magnetron sputtering. Jpn. J. Appl. Phys. 2020, 59, 116502. [Google Scholar] [CrossRef]
- Cristea, D.; Cunha, L.; Gabor, C.; Ghiuta, I.; Croitoru, C.; Marin, A.; Velicu, L.; Besleaga, A.; Vasile, B. Tantalum Oxynitride Thin Films: Assessment of the Photocatalytic Efficiency and Antimicrobial Capacity. Nanomater 2019, 9, 476. [Google Scholar] [CrossRef] [Green Version]
- Zhen, C.; Chen, R.; Wang, L.; Liu, G.; Cheng, H.-M. Tantalum (oxy)nitride based photoanodes for solar driven water oxidation. J. Mater. Chem. A 2016, 4, 2783–2800. [Google Scholar] [CrossRef] [Green Version]




| Sputtering | |
|---|---|
| Parameter | Set Value |
| Target power (W) | DC 190 |
| Target composition | Ta (99.9999 wt.%) |
| Background pressure (Pa) | 6.4 × 10−4 |
| Working pressure (Pa) | 0.87–0.93 approx. |
| Substrate temperature (°C) | Room temperature |
| Target-substrate distance (cm) | 10 |
| Substrate bias (V) | 0 |
| O2 flow rate (sccm) | 0, 0.13, 0.25, 0.38, 0.5, 0.75, 1, 1.5, 2, 4 |
| Ar flow rate (sccm) | 20 |
| N2 flow rate (sccm) | 10 |
| Annealing | |
| Parameter | Set Value |
| Temperature (°C) | 800 |
| Time (min) | 5 |
| Ar flow rate (sccm) | 5000 |
| Wavenumber (cm−1) | Mode | Description | Reference |
|---|---|---|---|
| 131.5 | Ag | β-TaON vibration | [39,40,41] |
| 143.8 | Ag | β-TaON vibration | [39,40,41] |
| 183.8 | Ag | β-TaON vibration | [39] |
| 207.0 | Ag | β-TaON vibration | [40,41] |
| 257.6 | Bg | β-TaON vibration | [39,40] |
| 419.7 | Ag | β-TaON vibration | [39] |
| 350~450 | v-SiO2 Bond bending | [41] | |
| 495 | v-SiO2 D1 | [41] | |
| 606 | v-SiO2 D2 | [41] | |
| 652.9 | Ag | β-TaON vibrations | [39] |
| 735.6 | Bg | β-TaON vibrations | [39,40] |
| 780~850 | v-SiO2 Tetrahedral | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Hsieh, J.-H.; Chuang, Y.R. Experimental Investigation on the Sputtering Process for Tantalum Oxynitride Thin Films. Photonics 2021, 8, 53. https://doi.org/10.3390/photonics8020053
Li C, Hsieh J-H, Chuang YR. Experimental Investigation on the Sputtering Process for Tantalum Oxynitride Thin Films. Photonics. 2021; 8(2):53. https://doi.org/10.3390/photonics8020053
Chicago/Turabian StyleLi, Chuan, Jang-Hsing Hsieh, and Y. R. Chuang. 2021. "Experimental Investigation on the Sputtering Process for Tantalum Oxynitride Thin Films" Photonics 8, no. 2: 53. https://doi.org/10.3390/photonics8020053
APA StyleLi, C., Hsieh, J.-H., & Chuang, Y. R. (2021). Experimental Investigation on the Sputtering Process for Tantalum Oxynitride Thin Films. Photonics, 8(2), 53. https://doi.org/10.3390/photonics8020053







