Diffuse Optical Spectroscopy Monitoring of Experimental Tumor Oxygenation after Red and Blue Light Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. PDT Protocols in Animal Study
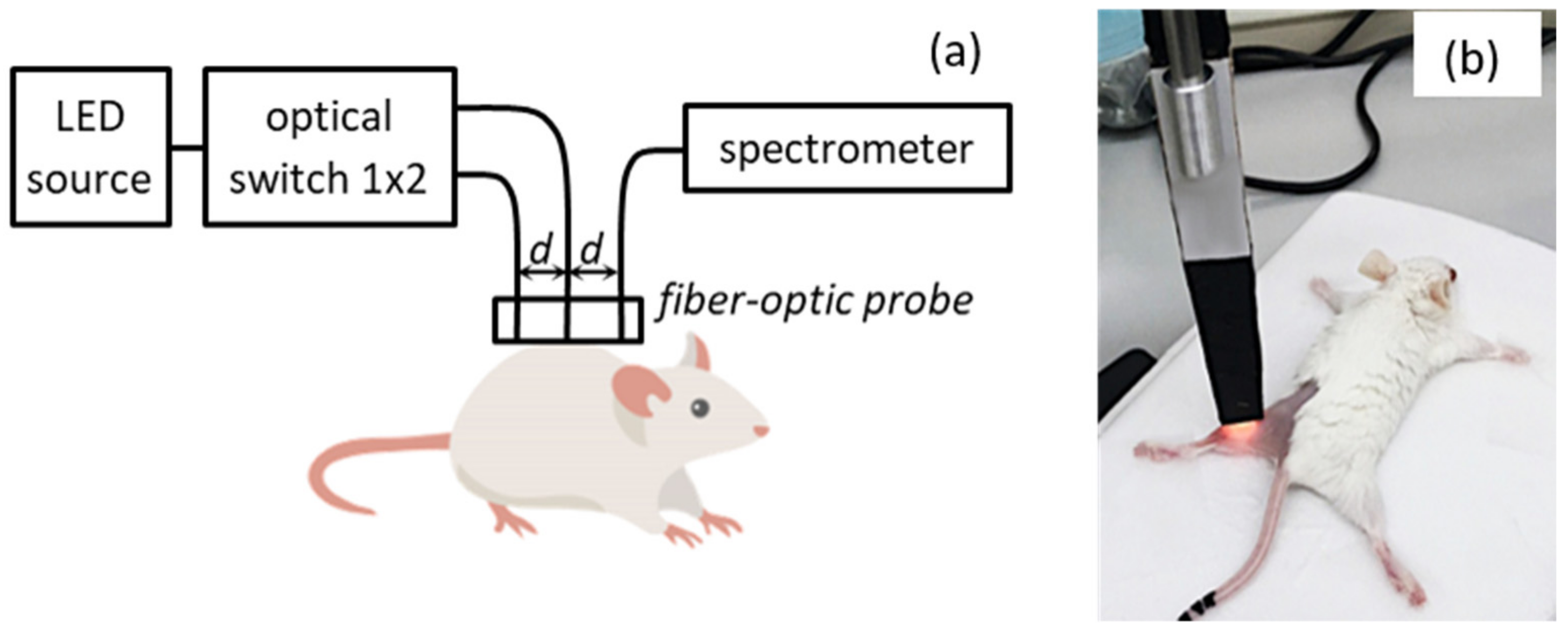
2.2. DOS Measurements
2.3. Evaluation of Blood Oxygenation Characteristics from DOS Measurements
2.4. Histology Studies
2.5. Statistical Analysis
3. Results
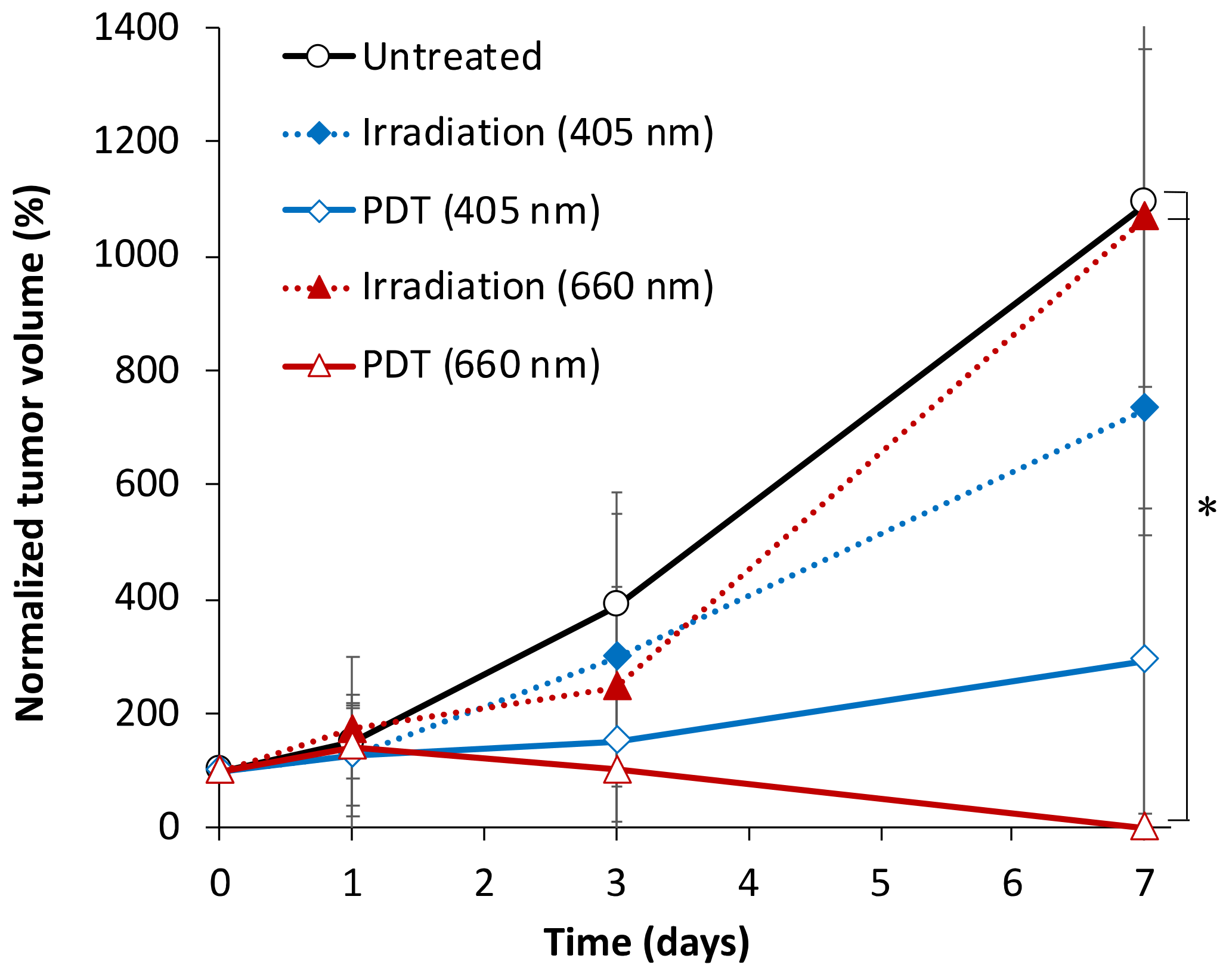
3.1. Tumor Growth Dynamics
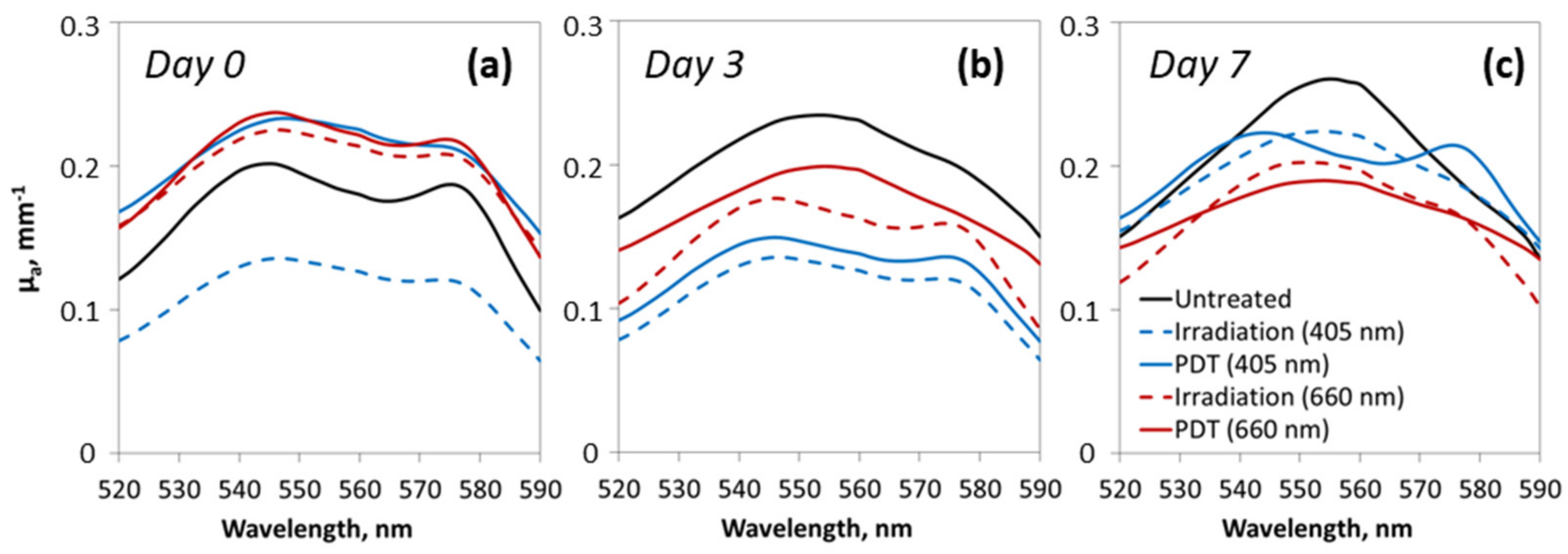
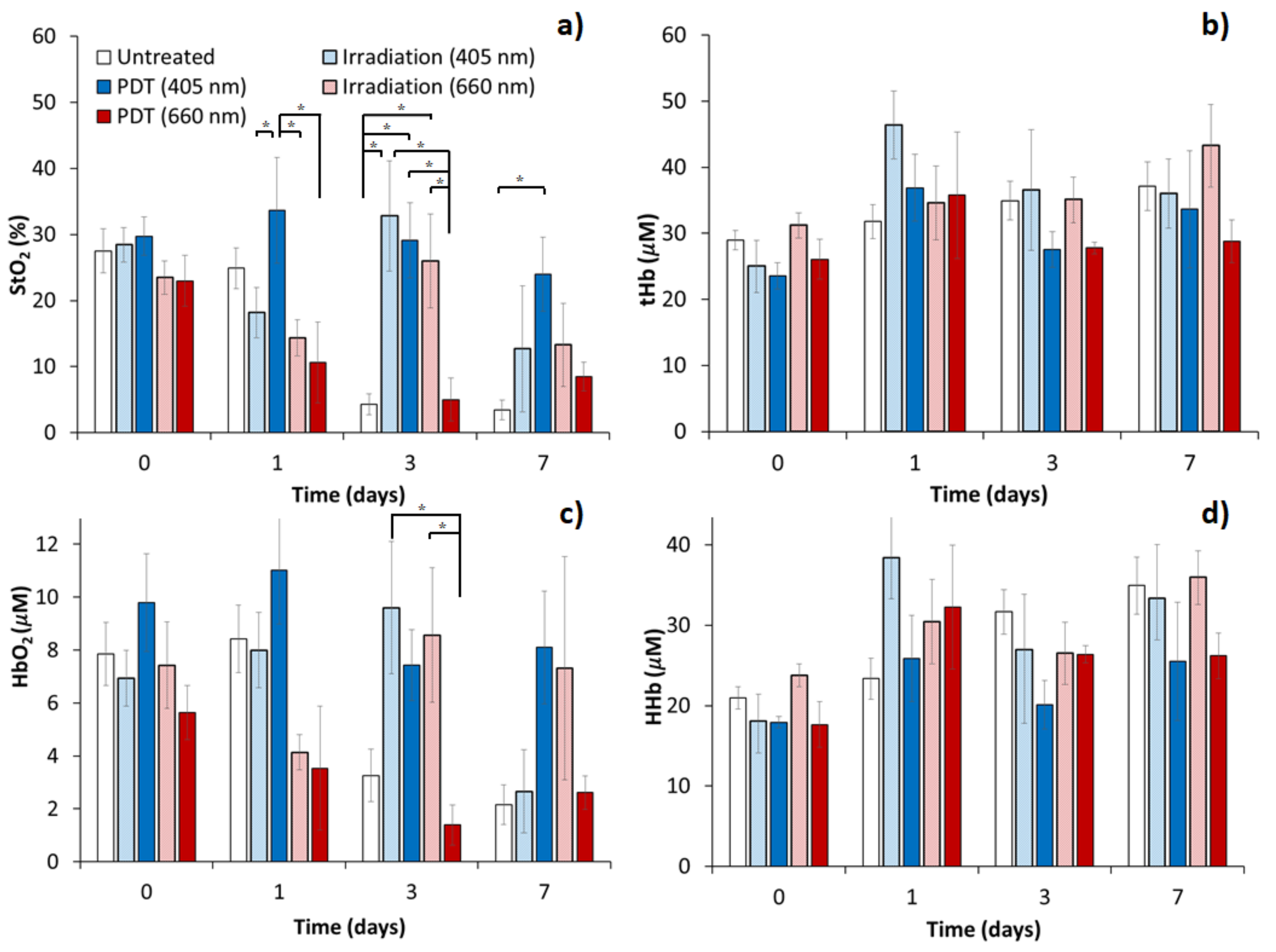
3.2. Tumor Oxygenation Dynamics Evaluated from DOS Measurements
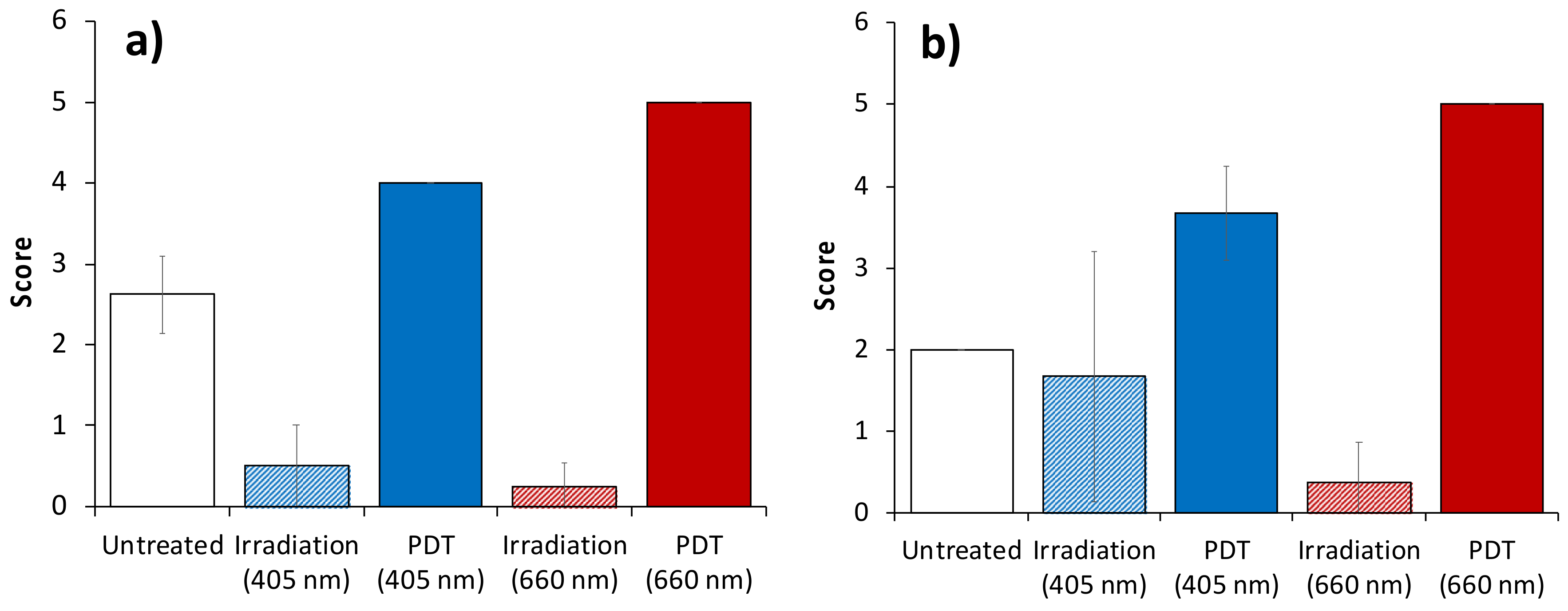
3.3. Histological Analysis of Hypoxia Associated Alterations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, J.H.; MacRobert, A.J.; Bown, S.G. The role of oxygen monitoring during photodynamic therapy and its potential for treatment dosimetry. Photochem. Photobiol. Sci. 2007, 6, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Ahn, P.H.; Finlay, J.C.; Gallagher-Colombo, S.M.; Quon, H.; O’Malley, B.W., Jr.; Weinstein, G.S.; Chalian, A.; Malloy, K.; Sollecito, T.; Greenberg, M. Lesion oxygenation associates with clinical outcomes in premalignant and early stage head and neck tumors treated on a phase 1 trial of photodynamic therapy. Photodiagnosis Photodyn. Ther. 2018, 21, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Krammer, B. Vascular effects of photodynamic therapy. Anticancer Res. 2001, 21, 4271–4277. [Google Scholar]
- Krzykawska-Serda, M.; Dąbrowski, J.M.; Arnaut, L.G.; Szczygieł, M.; Urbańska, K.; Stochel, G.; Elas, M. The role of strong hypoxia in tumors after treatment in the outcome of bacteriochlorin–based photodynamic therapy. Free Radic. Biol. Med. 2014, 73, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Sirotkina, M.; Matveev, L.; Shirmanova, M.; Zaitsev, V.; Buyanova, N.; Elagin, V.; Gelikonov, G.; Kuznetsov, S.; Kiseleva, E.; Moiseev, A. Photodynamic therapy monitoring with optical coherence angiography. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Kirillin, M.; Kurakina, D.; Khilov, A.; Orlova, A.; Shakhova, M.; Orlinskaya, N.; Sergeeva, E. Red and blue light in antitumor photodynamic therapy with chlorin–based photosensitizers: A comparative animal study assisted by optical imaging modalities. Biomed. Opt. Express 2021, 12, 872–892. [Google Scholar] [CrossRef]
- Thong, P.; Lee, K.; Toh, H.-J.; Dong, J.; Tee, C.-S.; Low, K.-P.; Chang, P.-H.; Bhuvaneswari, R.; Tan, N.-C.; Soo, K.-C. Early assessment of tumor response to photodynamic therapy using combined diffuse optical and diffuse correlation spectroscopy to predict treatment outcome. Oncotarget 2017, 8, 19902. [Google Scholar] [CrossRef]
- Redmond, R.W.; Gamlin, J.N. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem. Photobiol. 1999, 70, 391–475. [Google Scholar] [CrossRef]
- Losev, A.P.; Nichiporovich, I.N.; Zhuravkin, I.N.; Zhavrid, E.I. Energetics of chlorins as potent photosensitizers of PDT. In Proceedings of the SPIE European Conference on Biomedical Optics, Photochemotherapy: Photodynamic Therapy and Other Modalities II, Vienna, Austria, 4 December 1996; Volume 2924, pp. 40–48. [Google Scholar] [CrossRef]
- Kirillin, M.; Khilov, A.; Kurakina, D.; Orlova, A.; Perekatova, V.; Shishkova, V.; Malygina, A.; Mironycheva, A.; Shlivko, I.; Gamayunov, S.; et al. Dual–wavelength fluorescence monitoring of photodynamic therapy: From analytical models to clinical studies. Cancers 2021, 13, 5807. [Google Scholar] [CrossRef] [PubMed]
- Schouwink, H.; Oppelaar, H.; Ruevekamp, M.; van der Valk, M.; Hart, G.; Rijken, P.; Baas, P.; Stewart, F.A. Oxygen depletion during and after mthpc–mediated photodynamic therapy in rif1 and h–meso1 tumors. Radiat. Res. 2003, 159, 190–198. [Google Scholar] [CrossRef]
- Mallidi, S.; Watanabe, K.; Timerman, D.; Schoenfeld, D.; Hasan, T. Prediction of tumor recurrence and therapy monitoring using ultrasound–guided photoacoustic imaging. Theranostics 2015, 5, 289. [Google Scholar] [CrossRef]
- Karwicka, M.; Pucelik, B.; Gonet, M.; Elas, M.; Dąbrowski, J.M. Effects of photodynamic therapy with redaporfin on tumor oxygenation and blood flow in a lung cancer mouse model. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.; Spinelli, L.; Pifferi, A.; Taroni, P.; Cubeddu, R.; Danesini, G.M. Use of a nonlinear perturbation approach for in vivo breast lesion characterization by multiwavelength time–resolved optical mammography. Opt. Express 2003, 11, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Chance, B. Breast imaging technology: Probing physiology and molecular function using optical imaging–applications to breast cancer. Breast Cancer Res. 2000, 3, 1–6. [Google Scholar] [CrossRef]
- Tromberg, B.J.; Cerussi, A.; Shah, N.; Compton, M.; Durkin, A.; Hsiang, D.; Butler, J.; Mehta, R. Imaging in breast cancer: Diffuse optics in breast cancer: Detecting tumors in pre–menopausal women and monitoring neoadjuvant chemotherapy. Breast Cancer Res. 2005, 7, 1–7. [Google Scholar] [CrossRef]
- Durduran, T.; Choe, R.; Baker, W.B.; Yodh, A.G. Diffuse optics for tissue monitoring and tomography. Rep. Prog. Phys. 2010, 73, 076701. [Google Scholar] [CrossRef] [PubMed]
- De Blasi, R.A.; Cope, M.; Elwell, C.; Safoue, F.; Ferrari, M. Noninvasive measurement of human forearm oxygen consumption by near infrared spectroscopy. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 67, 20–25. [Google Scholar] [CrossRef]
- Lu, H.; Golay, X.; Pekar, J.J.; Van Zijl, P.C. Sustained poststimulus elevation in cerebral oxygen utilization after vascular recovery. J. Cereb. Blood Flow Metab. 2004, 24, 764–770. [Google Scholar] [CrossRef]
- Gibson, A.P.; Hebden, J.C.; Arridge, S.R. Recent advances in diffuse optical imaging. Phys. Med. Biol. 2005, 50, R1. [Google Scholar] [CrossRef]
- Zhou, C.; Choe, R.; Shah, N.S.; Durduran, T.; Yu, G.; Durkin, A.; Hsiang, D.; Mehta, R.; Butler, J.A.; Cerussi, A.E. Diffuse optical monitoring of blood flow and oxygenation in human breast cancer during early stages of neoadjuvant chemotherapy. J. Biomed. Opt. 2007, 12, 051903. [Google Scholar] [CrossRef] [PubMed]
- Cochran, J.M.; Busch, D.R.; Leproux, A.; Zhang, Z.; O’Sullivan, T.D.; Cerussi, A.E.; Carpenter, P.M.; Mehta, R.S.; Roblyer, D.; Yang, W. Tissue oxygen saturation predicts response to breast cancer neoadjuvant chemotherapy within 10 days of treatment. J. Biomed. Opt. 2018, 24, 021202. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, M.V.; Kalganova, T.I.; Lyubimtseva, Y.S.; Plekhanov, V.I.; Golubyatnikov, G.Y.; Ilyinskaya, O.Y.; Orlova, A.G.; Subochev, P.V.; Safonov, D.V.; Shakhova, N.M. Multimodal approach in assessment of the response of breast cancer to neoadjuvant chemotherapy. J. Biomed. Opt. 2018, 23, 091410. [Google Scholar] [CrossRef]
- Zhang, Y.; Moy, A.J.; Feng, X.; Nguyen, H.T.; Reichenberg, J.S.; Markey, M.K.; Tunnell, J.W. Physiological model using diffuse reflectance spectroscopy for nonmelanoma skin cancer diagnosis. J. Biophotonics 2019, 12, e201900154. [Google Scholar] [CrossRef]
- Blondel, W.; Delconte, A.; Khairallah, G.; Marchal, F.; Gavoille, A.; Amouroux, M. Spatially–Resolved Multiply–Excited Autofluorescence and Diffuse Reflectance Spectroscopy: SpectroLive Medical Device for Skin In Vivo Optical Biopsy. Electronics 2021, 10, 243. [Google Scholar] [CrossRef]
- Vishwanath, K.; Yuan, H.; Barry, W.T.; Dewhirst, M.W.; Ramanujam, N. Using optical spectroscopy to longitudinally monitor physiological changes within solid tumors. Neoplasia 2009, 11, 889–900. [Google Scholar] [CrossRef]
- Spliethoff, J.W.; Evers, D.J.; Jaspers, J.E.; Hendriks, B.H.; Rottenberg, S.; Ruers, T.J. Monitoring of tumor response to cisplatin using optical spectroscopy. Transl. Oncol. 2014, 7, 230–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orlova, A.; Kirillin, M.Y.; Volovetsky, A.; Shilyagina, N.Y.; Sergeeva, E.; Golubiatnikov, G.Y.; Turchin, I. Diffuse optical spectroscopy monitoring of oxygen state and hemoglobin concentration during skbr–3 tumor model growth. Laser Phys. Lett. 2016, 14, 015601. [Google Scholar] [CrossRef]
- Orlova, A.; Maslennikova, A.; Golubiatnikov, G.Y.; Suryakova, A.; Kirillin, M.Y.; Kurakina, D.; Kalganova, T.; Volovetsky, A.; Turchin, I. Diffuse optical spectroscopy assessment of rodent tumor model oxygen state after single–dose irradiation. Biomed. Phys. Eng. Express 2019, 5, 035010. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Hornung, R.; Berns, M.W.; Tadir, Y.; Tromberg, B.J. Monitoring tumor response during photodynamic therapy using near-infrared photon-migration spectroscopy. Photochem. Photobiol. 2001, 73, 669–677. [Google Scholar] [CrossRef]
- Wang, H.W.; Putt, M.E.; Emanuele, M.J.; Shin, D.B.; Glatstein, E.; Yodh, A.G.; Busch, T.M. Treatment–induced changes in tumor oxygenation predict photodynamic therapy outcome. Cancer Res. 2004, 64, 7553–7561. [Google Scholar] [CrossRef]
- Dong, J.; Toh, H.J.; Thong, P.S.; Tee, C.S.; Bi, R.; Soo, K.C.; Lee, K. Hemodynamic monitoring of chlorin e6–mediated photodynamic therapy using diffuse optical measurements. J. Photochem. Photobiol. B Biol. 2014, 140, 163–172. [Google Scholar] [CrossRef]
- Kleshnin, M.S.; Turchin, I.V. Evaluation of oxygenation in the surface layers of biological tissues based on diffuse optical spectroscopy with automated calibration of measurements. Quantum Electron. 2019, 49, 628. [Google Scholar] [CrossRef]
- Farrell, T.J.; Patterson, M.S.; Wilson, B. A diffusion theory model of spatially resolved, steady–state diffuse reflectance for the noninvasive determination of tissue optical properties in vivo. Med. Phys. 1992, 19, 879–888. [Google Scholar] [CrossRef]
- Turchin, I.V. Methods of biomedical optical imaging: From subcellular structures to tissues and organs. Phys.–Uspekhi 2016, 59, 487. [Google Scholar] [CrossRef]
- Kleshnin, M.S. Deep learning neural network estimation of tissue oxygenation based on diffuse optical spectroscopy. Laser Phys. 2019, 29, 085603. [Google Scholar] [CrossRef]
- Greening, G.; Mundo, A.; Rajaram, N.; Muldoon, T.J. Sampling depth of a diffuse reflectance spectroscopy probe for in–vivo physiological quantification of murine subcutaneous tumor allografts. J. Biomed. Opt. 2018, 23, 085006. [Google Scholar] [CrossRef] [PubMed]
- Kurakina, D.; Khilov, A.; Shakhova, M.; Orlinskaya, N.; Sergeeva, E.; Meller, A.; Turchin, I.; Kirillin, M. Comparative analysis of single–and dual–wavelength photodynamic therapy regimes with chlorin–based photosensitizers: Animal study. J. Biomed. Opt. 2019, 25, 063804. [Google Scholar] [CrossRef]
- Hino, H.; Murayama, Y.; Nakanishi, M.; Inoue, K.; Nakajima, M.; Otsuji, E. 5–aminolevulinic acid–mediated photodynamic therapy using light–emitting diodes of different wavelengths in a mouse model of peritoneally disseminated gastric cancer. J. Surg. Res. 2013, 185, 119–126. [Google Scholar] [CrossRef]
- Calderhead, R.G. The photobiological basics behind light-emitting diode (led) phototherapy. Laser Ther. 2007, 16, 97–108. [Google Scholar] [CrossRef][Green Version]
- Ottaviani, G.; Martinelli, V.; Rupel, K.; Caronni, N.; Naseem, A.; Zandonà, L.; Perinetti, G.; Gobbo, M.; Di Lenarda, R.; Bussani, R. Laser therapy inhibits tumor growth in mice by promoting immune surveillance and vessel normalization. EBioMedicine 2016, 11, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Park, H.; Griffin, R.J. Improvement of tumor oxygenation by mild hyperthermia. Radiat. Res. 2001, 155, 515–528. [Google Scholar] [CrossRef]
- Sun, X.; Xing, L.; Clifton Ling, C.; Li, G.C. The effect of mild temperature hyperthermia on tumour hypoxia and blood perfusion: Relevance for radiotherapy, vascular targeting and imaging. Int. J. Hyperth. 2010, 26, 224–231. [Google Scholar] [CrossRef]
- Griffin, R.J.; Dings, R.P.; Jamshidi–Parsian, A.; Song, C.W. Mild temperature hyperthermia and radiation therapy: Role of tumour vascular thermotolerance and relevant physiological factors. Int. J. Hyperth. 2010, 26, 256–263. [Google Scholar] [CrossRef] [PubMed]
- DaCosta, R.S.; Andersson, H.; Wilson, B.C. Molecular fluorescence excitation–emission matrices relevant to tissue spectroscopy. Photochem. Photobiol. 2003, 78, 384–392. [Google Scholar] [CrossRef]
- Maslennikova, A.V.; Orlova, A.G.; Golubiatnikov, G.Y.; Kamensky, V.A.; Shakhova, N.M.; Babaev, A.A.; Snopova, L.B.; Ivanova, I.P.; Plekhanov, V.I.; Prianikova, T.I.; et al. Comparative study of tumor hypoxia by diffuse optical spectroscopy and immunohistochemistry in two tumor models. J. Biophotonics 2010, 3, 743–751. [Google Scholar] [CrossRef]



| Grade | Myocytes | Microcirculatory System |
|---|---|---|
| 0 | No alterations | No alterations |
| 1 | Single fragmentation within several fields of view | Single occurrences of endothelium alterations, endothelium atrophy, presence of plethora |
| 2 | Single fragmentation within single field of view | Several occurrences of endothelium atrophy, endothelium destruction, plethora, stasis |
| 3 | Several fragmented myocytes within single field of view | Several occurrences of endothelium atrophy, endothelium destruction, presence of stasis, sludges |
| 4 | Prevalence of fragmented myocytes within single field of view | Multiple occurrences of endothelium destruction, presence of stasis, sludges, single occurrences of thrombosis |
| 5 | Prevalence of fragmented myocytes within several fields of view | Multiple occurrences of endothelium destruction, presence of stasis, sludges, multiple occurrences of thrombosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlova, A.; Perevalova, Y.; Pavlova, K.; Orlinskaya, N.; Khilov, A.; Kurakina, D.; Shakhova, M.; Kleshnin, M.; Sergeeva, E.; Turchin, I.; et al. Diffuse Optical Spectroscopy Monitoring of Experimental Tumor Oxygenation after Red and Blue Light Photodynamic Therapy. Photonics 2022, 9, 19. https://doi.org/10.3390/photonics9010019
Orlova A, Perevalova Y, Pavlova K, Orlinskaya N, Khilov A, Kurakina D, Shakhova M, Kleshnin M, Sergeeva E, Turchin I, et al. Diffuse Optical Spectroscopy Monitoring of Experimental Tumor Oxygenation after Red and Blue Light Photodynamic Therapy. Photonics. 2022; 9(1):19. https://doi.org/10.3390/photonics9010019
Chicago/Turabian StyleOrlova, Anna, Yulia Perevalova, Ksenia Pavlova, Natalia Orlinskaya, Aleksandr Khilov, Daria Kurakina, Maria Shakhova, Mikhail Kleshnin, Ekaterina Sergeeva, Ilya Turchin, and et al. 2022. "Diffuse Optical Spectroscopy Monitoring of Experimental Tumor Oxygenation after Red and Blue Light Photodynamic Therapy" Photonics 9, no. 1: 19. https://doi.org/10.3390/photonics9010019
APA StyleOrlova, A., Perevalova, Y., Pavlova, K., Orlinskaya, N., Khilov, A., Kurakina, D., Shakhova, M., Kleshnin, M., Sergeeva, E., Turchin, I., & Kirillin, M. (2022). Diffuse Optical Spectroscopy Monitoring of Experimental Tumor Oxygenation after Red and Blue Light Photodynamic Therapy. Photonics, 9(1), 19. https://doi.org/10.3390/photonics9010019






