Research Progress on Singlet Fission in Acenes and Their Derivatives
Abstract
:1. Introduction
2. Principles and Research Methods of SF
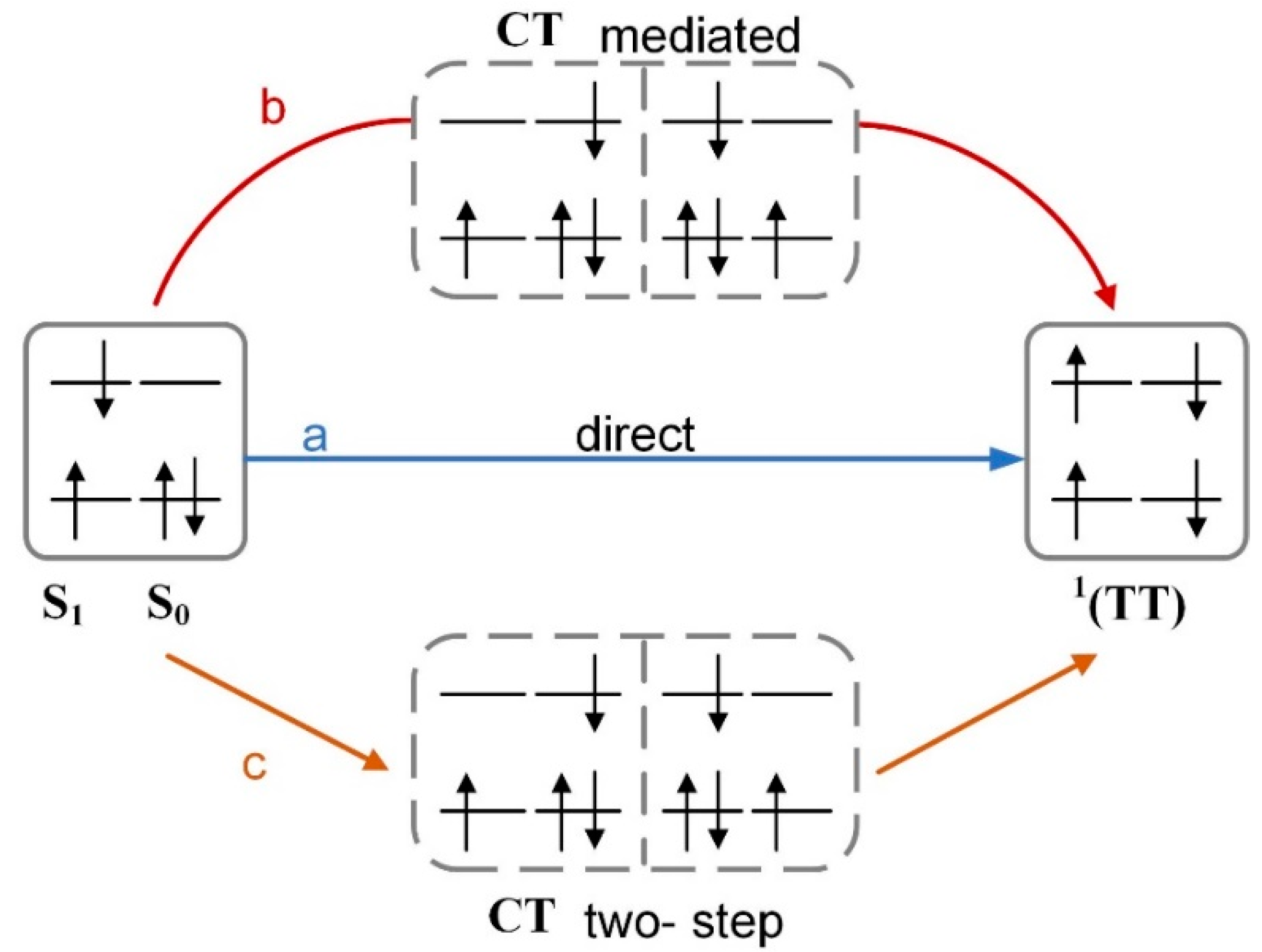
2.1. The SF Process
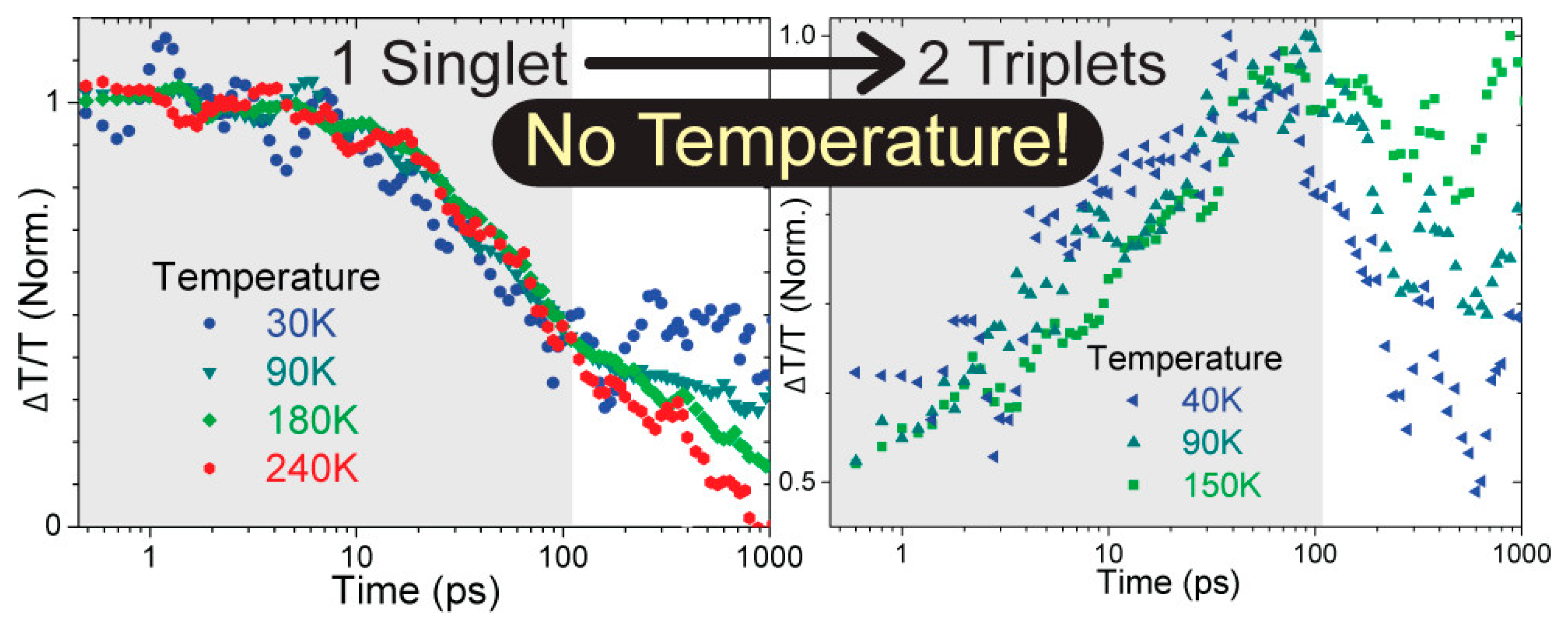
2.2. Condition for SF
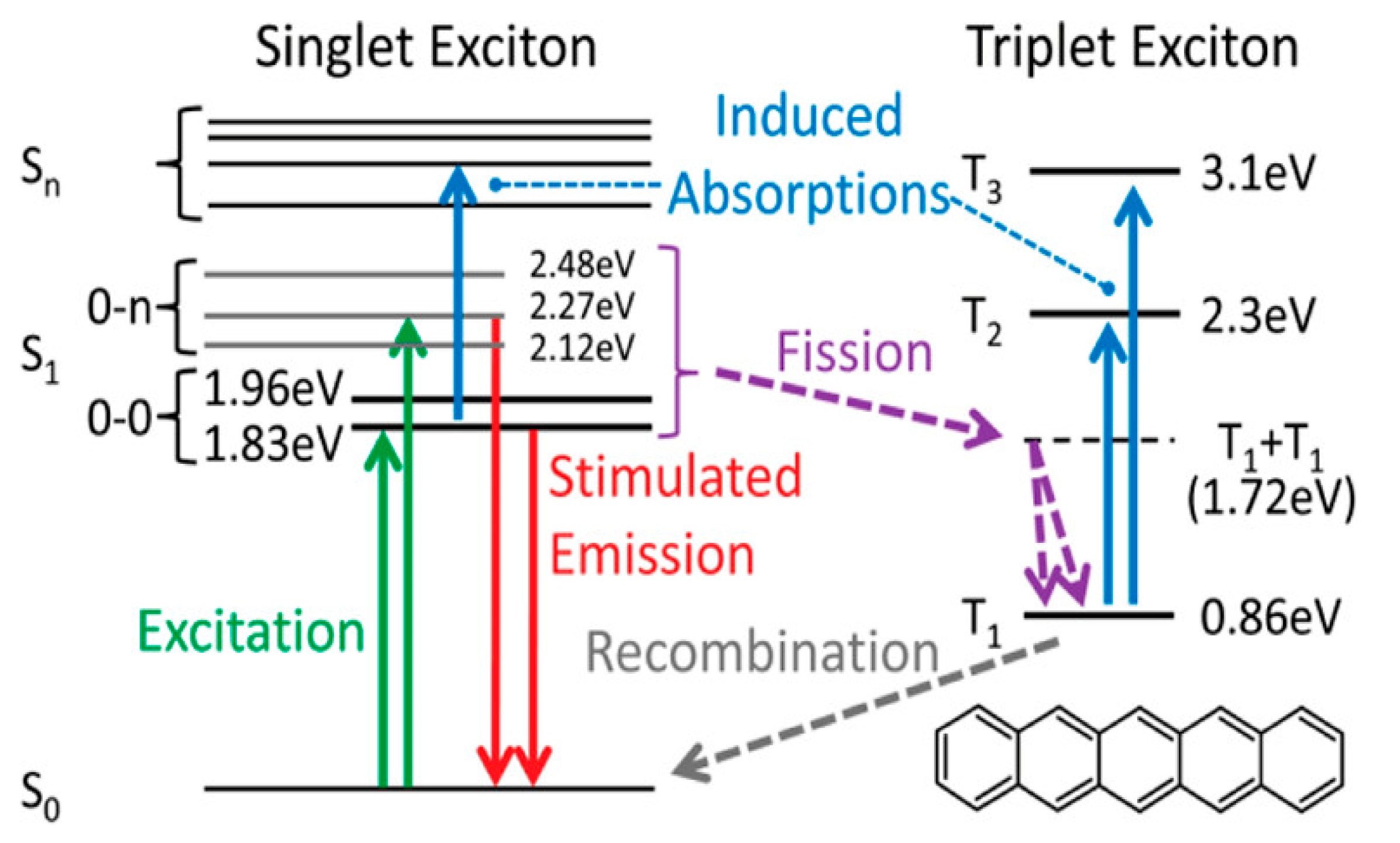
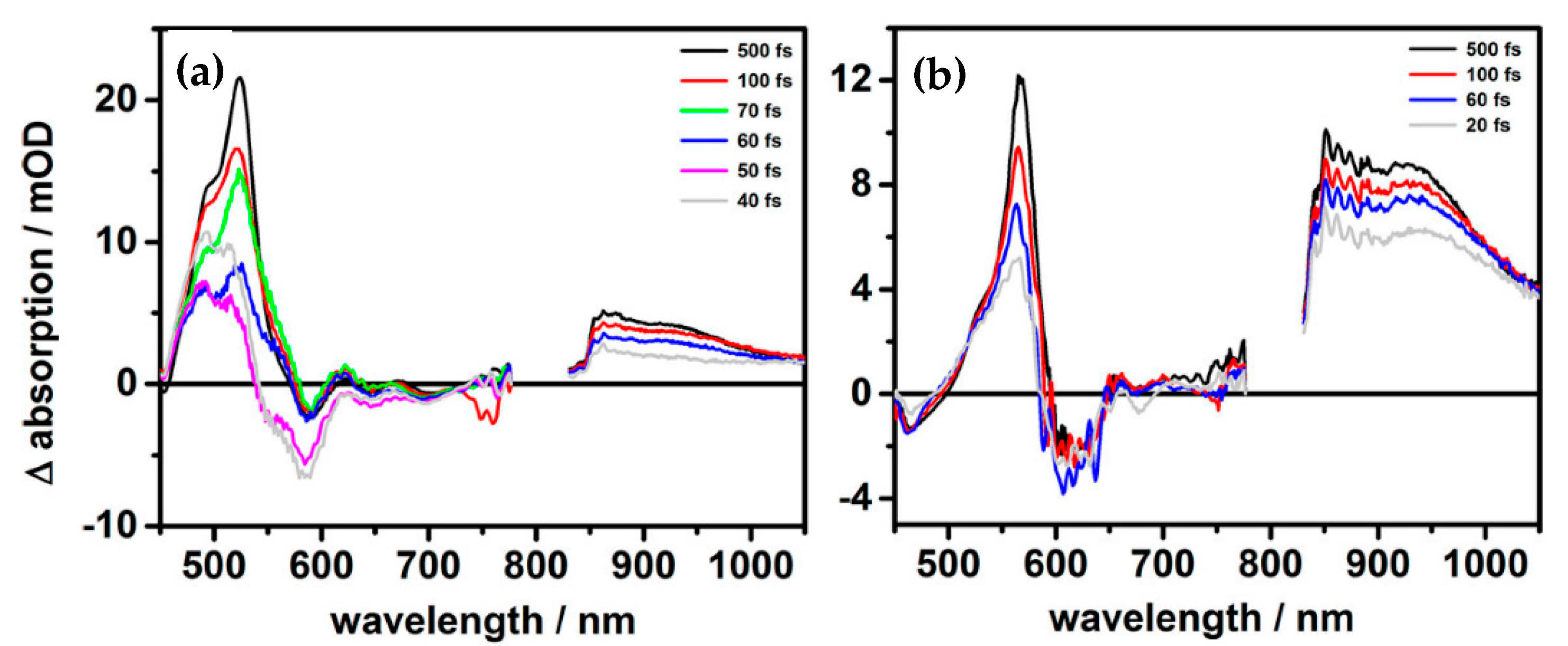
2.3. Experimental Method
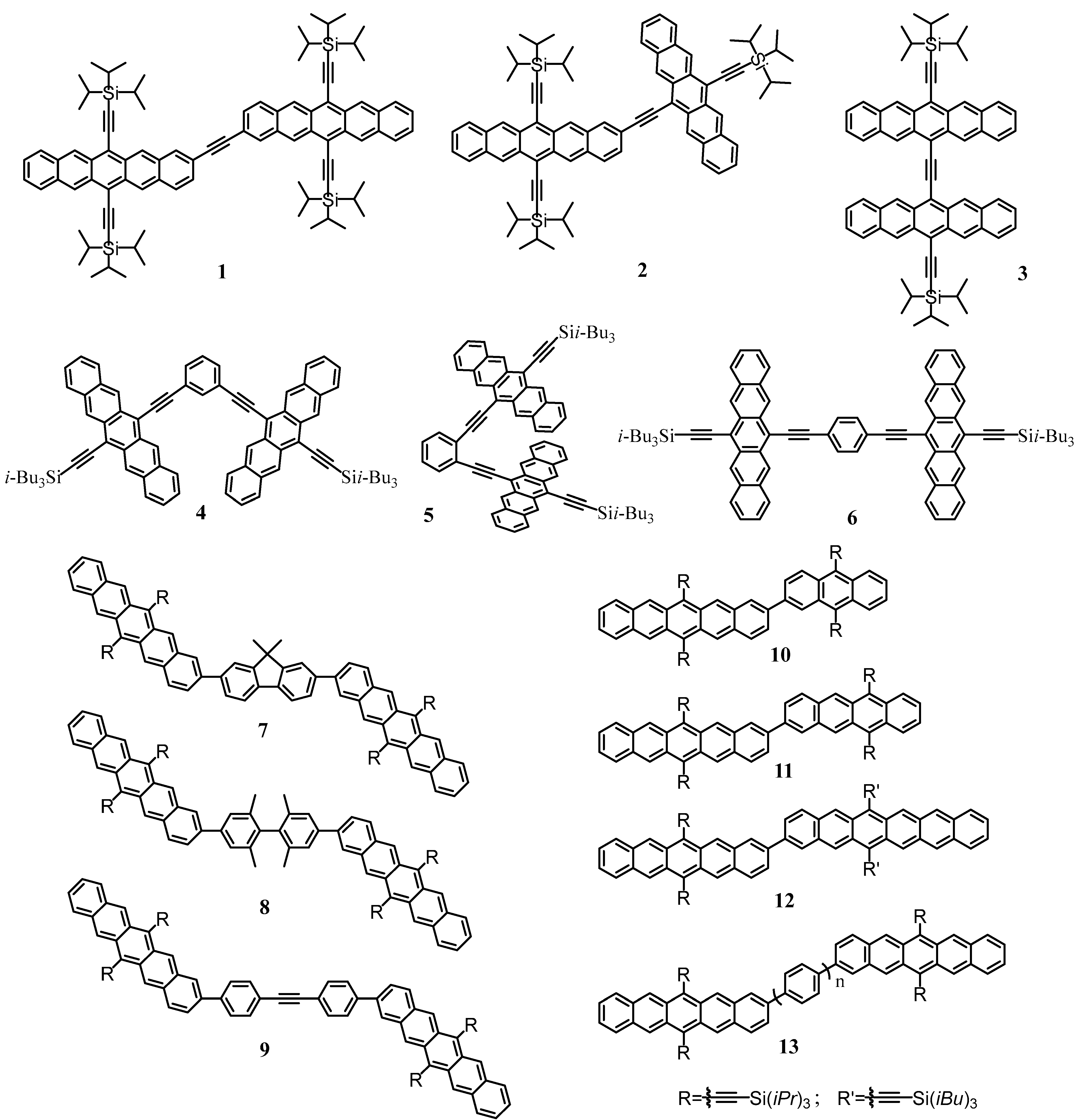
3. SF Materials
3.1. Monomer
| Monomer | Structure | Name | S1 Decay Time | Intermediate State Lifetime | TT Lifetime | T1 Lifetime | SF Yield | Reference |
|---|---|---|---|---|---|---|---|---|
| Tetracene and its derivatives | 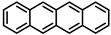 | Tetracene | — | ~200% | [56] | |||
 | PhTc | 1.9 ps | 49.5 ps (*S1) | 797.0 ps | 11.3 ns | 67 ± 15% | [72] | |
 | PhTc-PEG-1 (n = 1) | 1.94 ps | 409.14 ps (CT) | — | 14.62 ns | 60.6% | [71] | |
| PhTc-PEG-2 (n = 4) | 2.69 ps | 566.63 ps (CT) | — | 19.61 ns | 53.3% | |||
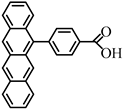 | PhTc-COOH | 17.2 ps | — | 229.9ps | 34.7 ns | 133 ± 10% | [72] | |
| Pentacene and its derivatives |  | Pentacene | ~80 fs | — | 160–180% | [44] | ||
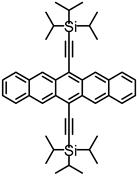 | TIPS– pentacene | 200 fs | 1.2 ps (S1/ME) | — | >1 ns | 144% | [77] | |
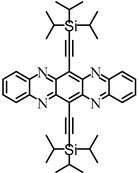 | Diaza–TIPS– pentacene | 110 fs | 1.0 ps (S1/ME) | — | >1 ns | — | [77] | |
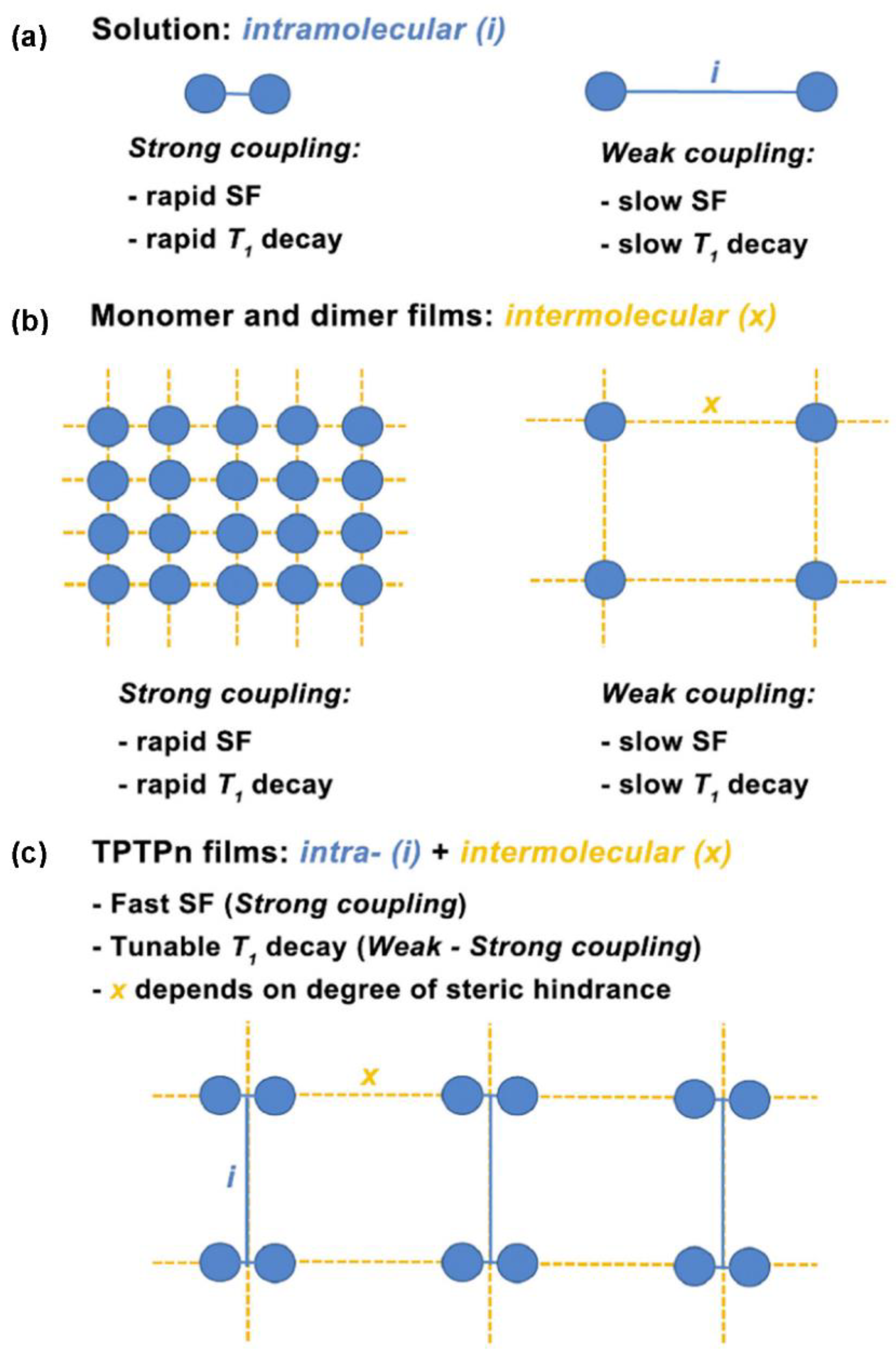
3.2. Dimers
| Dimers | Name | Solvent | SF Time Scale | TT Lifetime | Triplet Yield | References |
|---|---|---|---|---|---|---|
| Tetracene dimers | BET-B | THF | 2 ± 0.5 ps | — | 154 ± 10% (film) | [64] |
| BET-X | toluene | <180 fs | — | — | ||
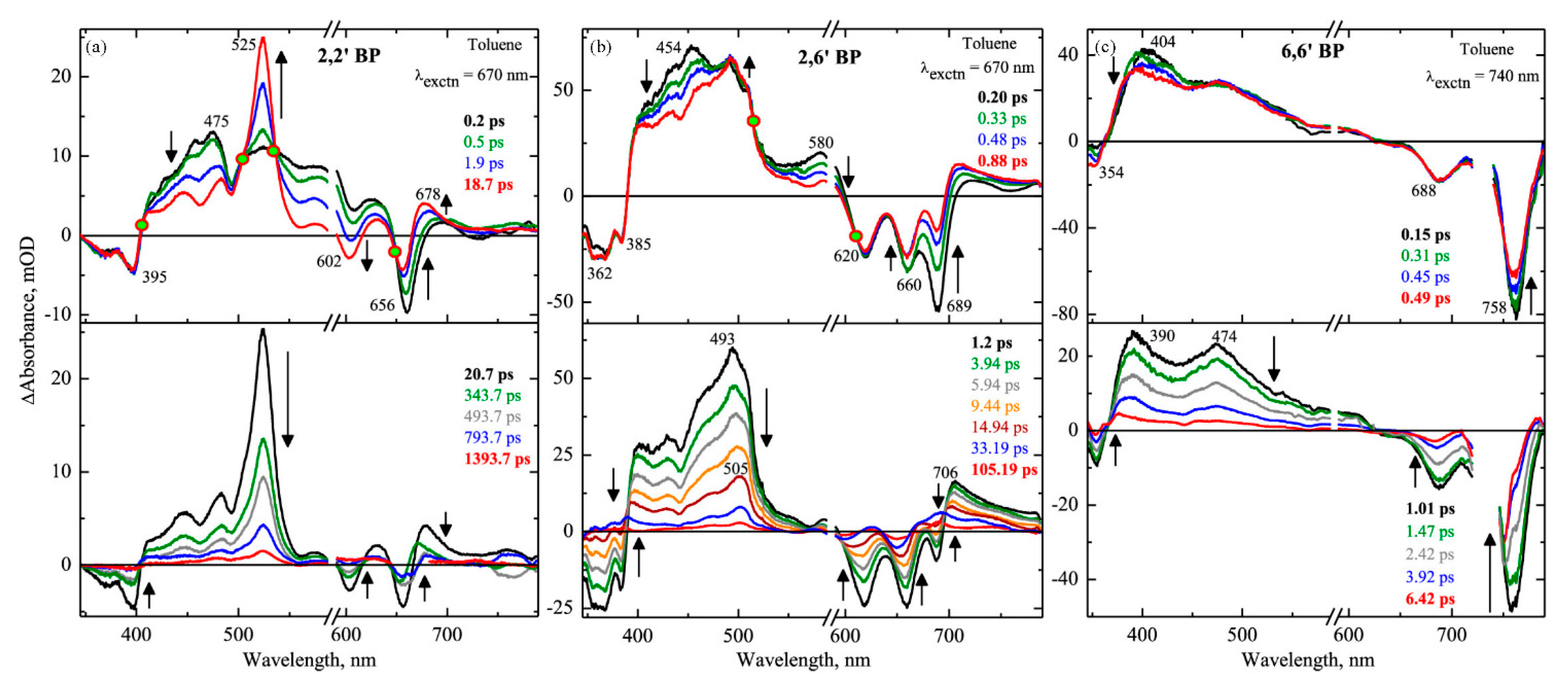
| Pentacene dimers | 2,2′ BP | toluene | 13.45 ± 0.5 ps | 495.41 ± 15 ps | 182% | [79] |
| 2,6′ BP | toluene | 5.85 ± 0.25 ps | 17.29 ± 0.8 ps | 97% | ||
| 6,6′ BP | toluene | 2.26 ± 0.25 ps | — | — | ||
| m-2 | benzonitrile | 63.0 ± 6.3 ps | 2.2 ± 0.1 ns | 156 ± 5% | [82] | |
| THF | 70.3 ± 7.0 ps | 2.5 ± 1.0 ns | 132 ± 2% | |||
| toluene | 90.2 ± 9.0 ps | 2.6 ± 0.1 ns | 125 ± 5% | |||
| o-2 | benzonitrile | 0.5 ± 0.2 ps | 12.0 ± 0.3 ps | — | ||
| toluene | — | — | — | |||
| p-2 | benzonitrile | 2.7 ± 1.0 ps | 17.3 ± 1.3 ps | 130 ± 10% | ||
| toluene | — | — | — | |||
| BP0 | chloroform | 760 fs | 0.45 ns | ~200% | [78] | |
| BP1 | chloroform | 20 ps | 16.5 ns | ~200% | ||
| BP2 | chloroform | 220 ps | 270 ns | ~200% | ||
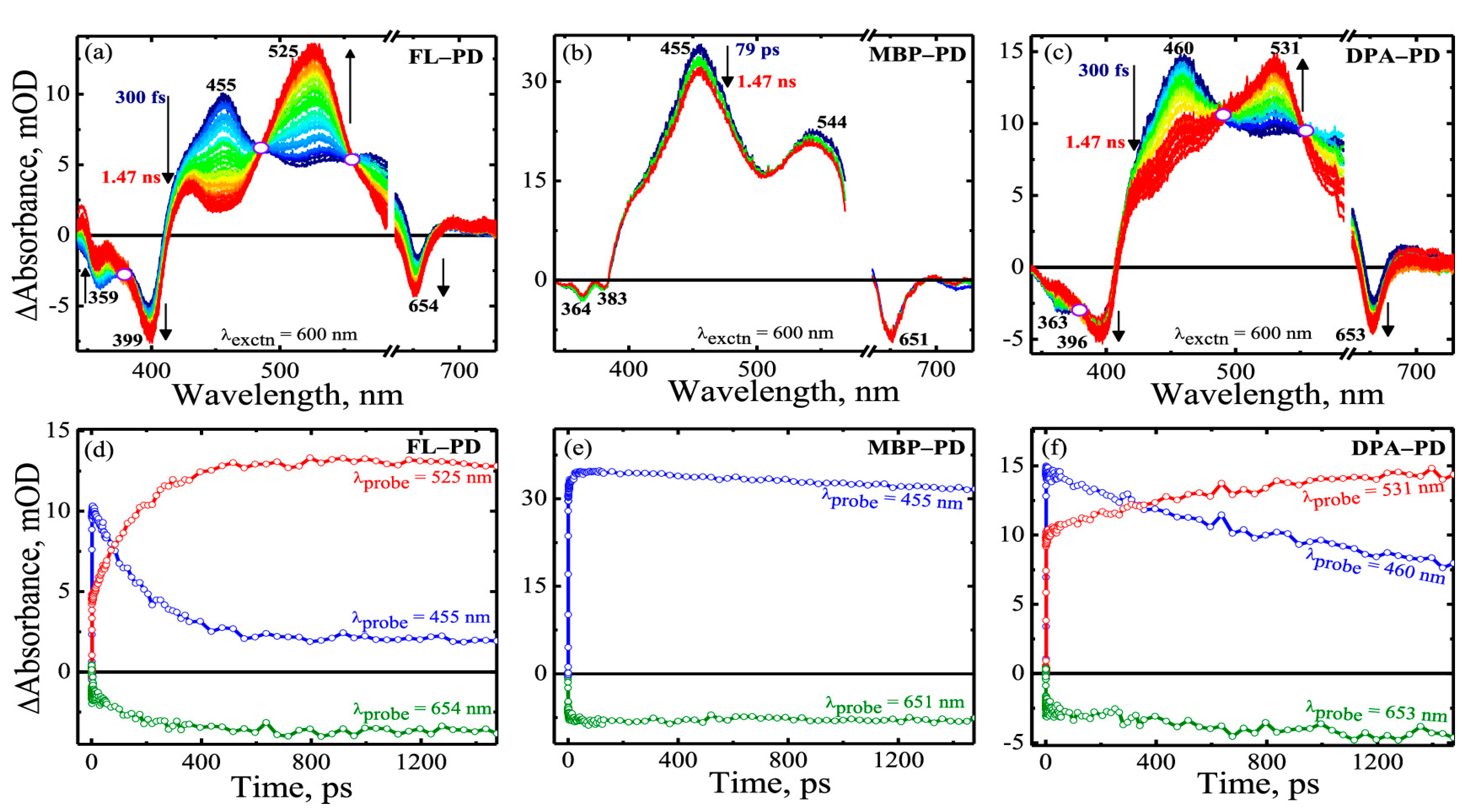
| FL-PD | toluene | 7.14 ± 0.3 ps | 186.67 ± 4.5 ps | 197.8 ± 5% | [83] | |
| MBP-PD | toluene | — | ~147.05 ns | 16 ± 2% | ||
| DPA-PD | toluene | 10.71 ± 0.5 ps | 1.09 ± 0.05 ns | 185.12 ± 6% | ||
| Heterodimers | PA | chloroform | (S1 lifetime = 11.5 ns) | — | — | [84] |
| PT | chloroform | 0.83 ps | 2.4 ns | — | ||
| PH | chloroform | 1.2 ps | 0.21 ns | — |
3.3. Trimers and Tetramers
4. Device Applications
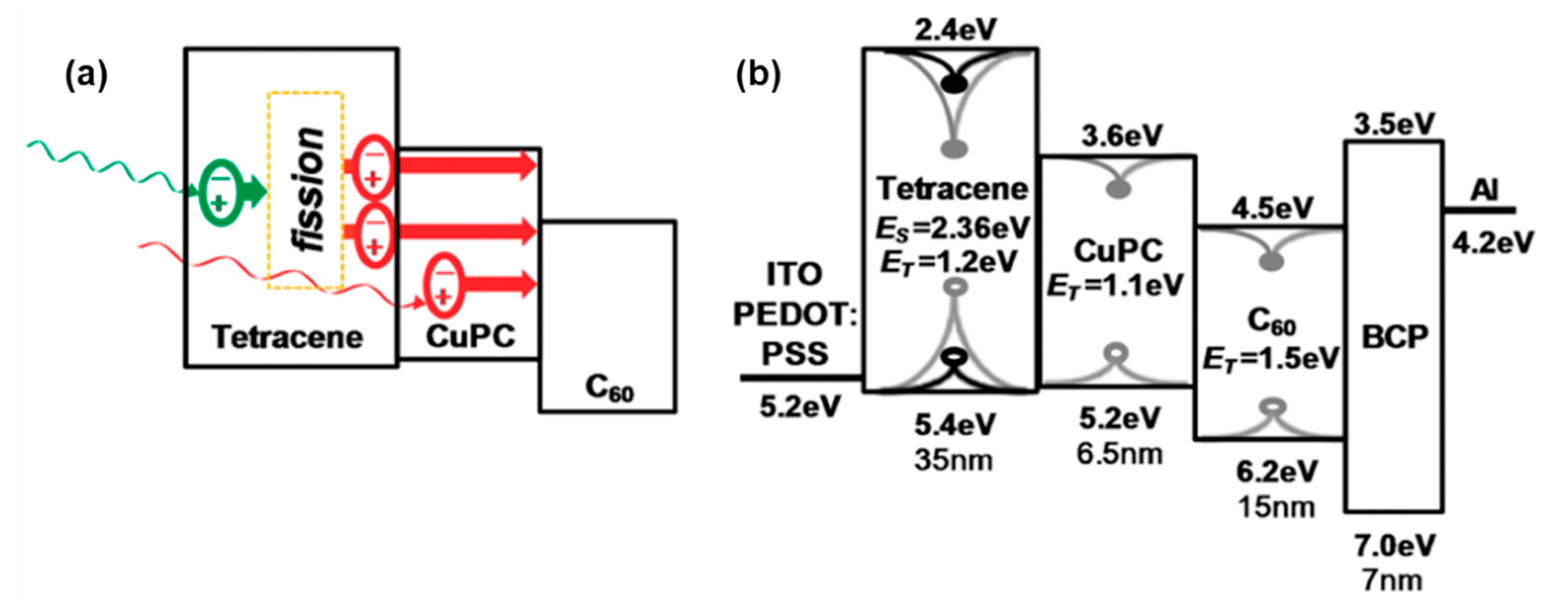
4.1. Organic Photovoltaic Devices
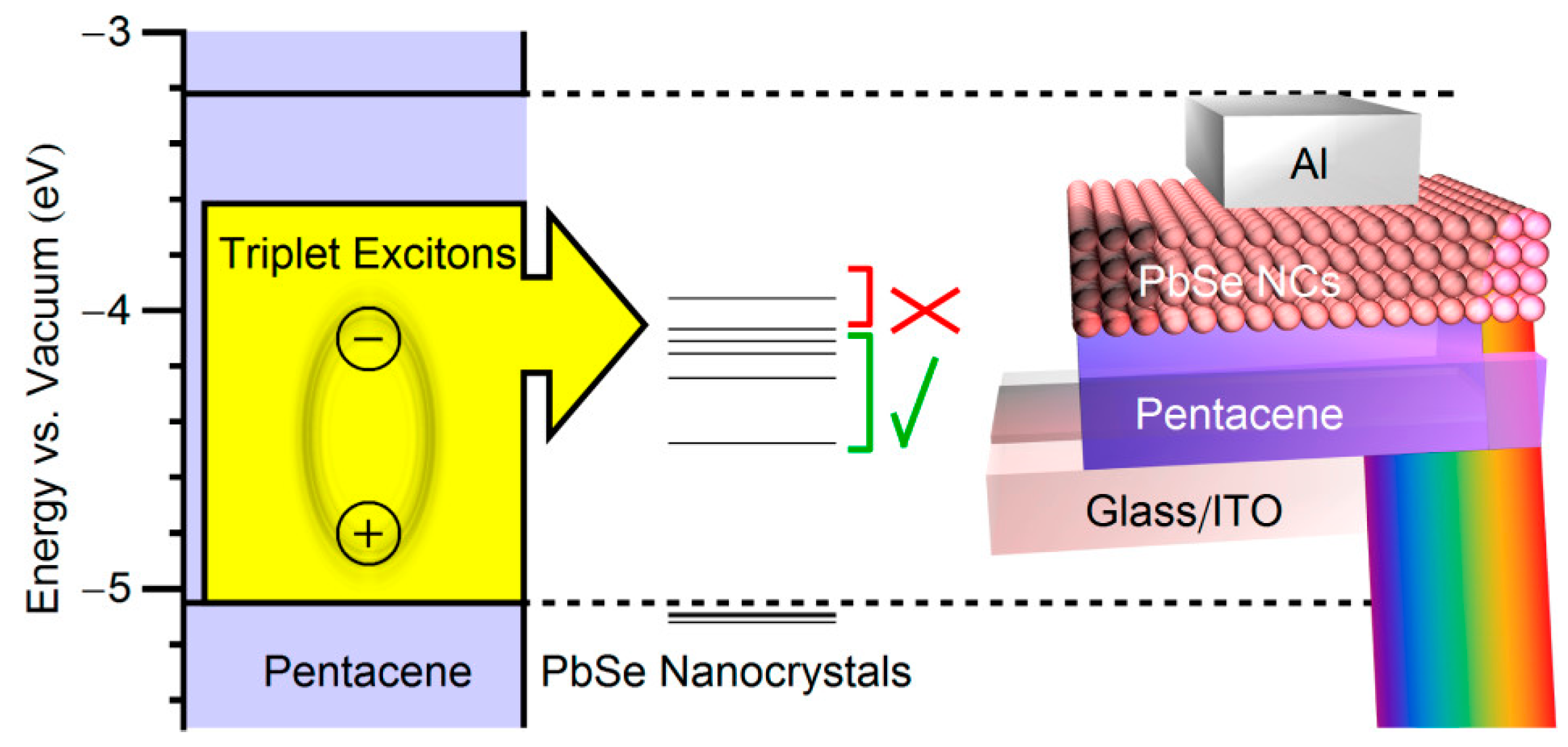
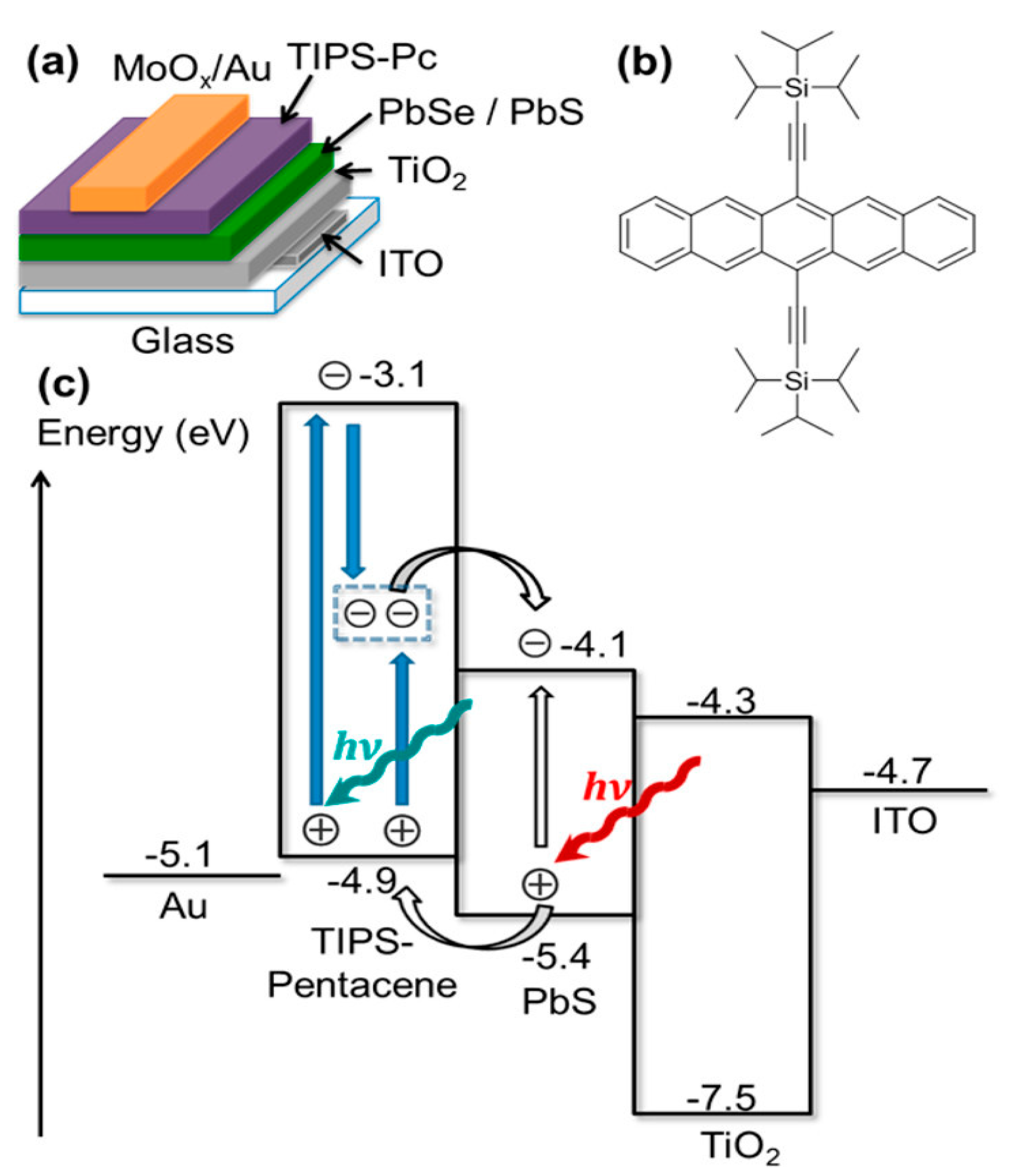
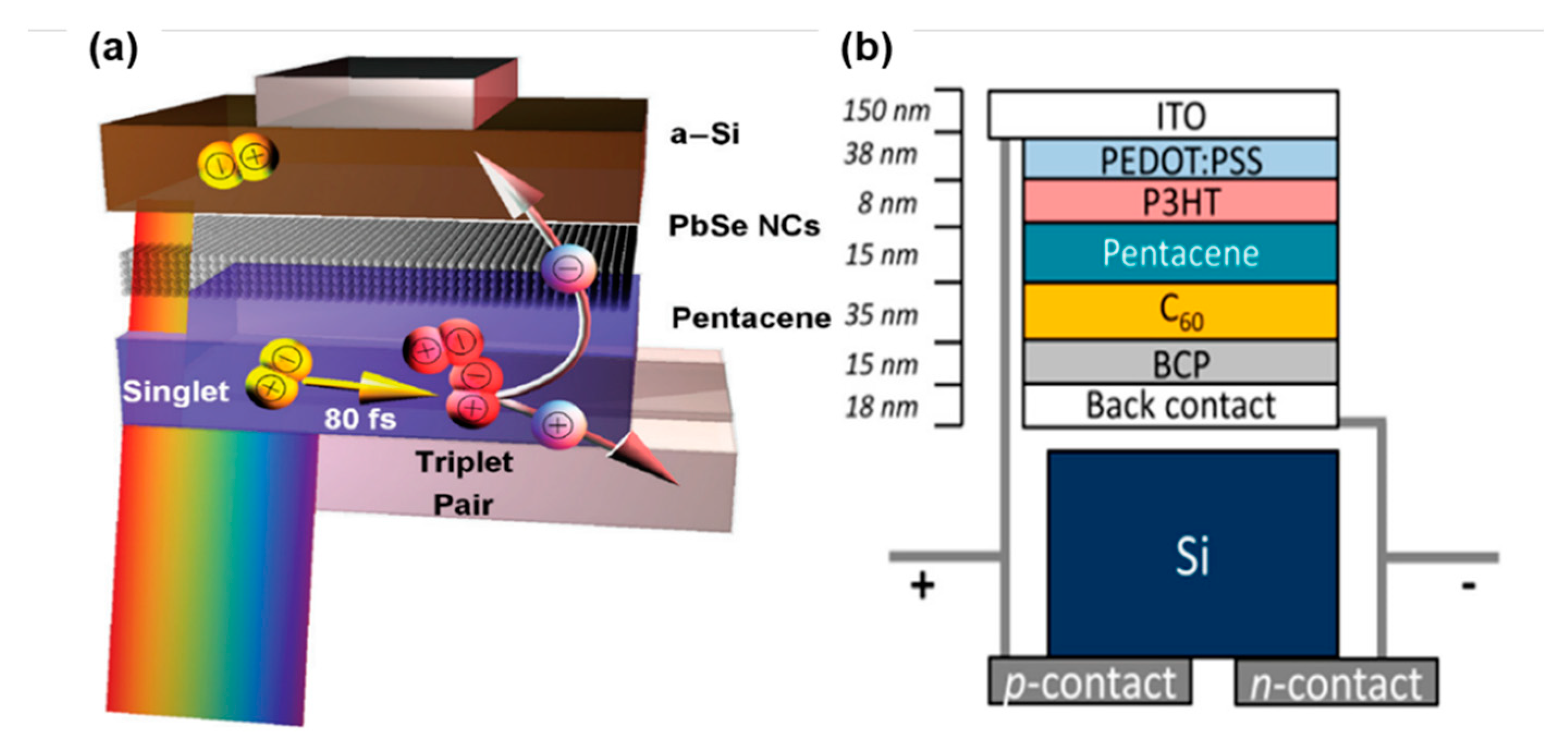
4.2. Inorganic Solar Cells
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wageh, S.; Raïssi, M.; Berthelot, T.; Laurent, M.; Rousseau, D.; Abusorrah, A.M.; Al-Hartomy, O.A.; Al-Ghamdi, A.A. Digital printing of a novel electrode for stable flexible organic solar cells with a power conversion efficiency of 8.5%. Sci. Rep. 2021, 11, 14212. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.D.; Suthar, R.; Pestrikova, A.A.; Nikolaev, A.Y.; Chen, F.C.; Keshtov, M.L. Efficient Ternary Polymer solar cells based ternary active layer consisting of conjugated polymers and non-fullerene acceptors with power conversion efficiency approaching near to 15.5%. Sol. Energy 2021, 216, 217–224. [Google Scholar] [CrossRef]
- An, Q.; Wang, J.; Gao, W.; Ma, X.; Hu, Z.; Gao, J.; Xu, C.; Hao, M.; Zhang, X.; Yang, C.; et al. Alloy-like ternary polymer solar cells with over 17.2% efficiency. Sci. Bull. 2020, 65, 538–545. [Google Scholar] [CrossRef]
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J.A.; Vilches, A.B.M.; Kasparavicius, E.; Smith, J.A.; et al. Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Pang, S.; Li, N.; Brabec, C.J.; Duan, C.; Huang, F.; Cao, Y. Ternary All-Polymer Solar Cells With 8.5% Power Conversion Efficiency and Excellent Thermal Stability. Front. Chem. 2020, 8, 302. [Google Scholar] [CrossRef]
- Li, M.; Gao, K.; Wan, X.; Zhang, Q.; Kan, B.; Xia, R.; Liu, F.; Yang, X.; Feng, H.; Ni, W.; et al. Solution-processed organic tandem solar cells with power conversion efficiencies >12%. Nat. Photonics 2016, 11, 85–90. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Hu, B.; Zhang, G.; Arunagiri, L.; Ma, L.K.; Huang, J.; Zhang, J.; Zhu, Z.; Bai, F.; et al. A Nonfullerene Semitransparent Tandem Organic Solar Cell with 10.5% Power Conversion Efficiency. Adv. Energy Mater. 2018, 8, 1800529. [Google Scholar] [CrossRef]
- You, J.; Dou, L.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.-C.; Gao, J.; Li, G.; et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Davis, N.J.L.K.; Böhm, M.L.; Tabachnyk, M.; Wisnivesky-Rocca-Rivarola, F.; Jellicoe, T.C.; Ducati, C.; Ehrler, B.; Greenham, N.C. Multiple-exciton generation in lead selenide nanorod solar cells with external quantum efficiencies exceeding 120%. Nat. Commun. 2015, 6, 8259. [Google Scholar] [CrossRef] [Green Version]
- Hanna, M.C.; Nozik, A.J. Solar conversion efficiency of photovoltaic and photoelectrolysis cells with carrier multiplication absorbers. J. Appl. Phys. 2006, 100, 074510. [Google Scholar] [CrossRef]
- Singh, S.; Jones, W.J.; Siebrand, W.; Stoicheff, B.P.; Schneider, W.G. Laser Generation of Excitons and Fluorescence in Anthracene Crystals. J. Chem. Phys. 1965, 42, 330–342. [Google Scholar] [CrossRef]
- Merrifield, R.E.; Avakian, P.; Groff, R.P. Fission of singlet excitons into pairs of triplet excitons in tetracene crystals. Chem. Phys. Lett. 1969, 3, 386–388. [Google Scholar] [CrossRef]
- Rademaker, H.; Hoff, A.J.; Grondelle, R.V.; Duysens, L.N. Carotenoid triplet yields in normal and deuterated Rhodospirillum rubrum. Biochim. Biophys. Acta 1980, 592, 240–257. [Google Scholar] [CrossRef]
- Austin, R.H.; Baker, G.L.; Etemad, S.; Thompson, R. Magnetic field effects on triplet exciton fission and fusion in a polydiacetylene. J. Chem. Phys. 1989, 90, 6642–6646. [Google Scholar] [CrossRef]
- Yarmus, L.; Rosenthal, J.; Chopp, M. EPR of triplet excitions in tetracene crystals: Spin polarization and the role of singlet exciton fission. Chem. Phys. Lett. 1972, 16, 477–481. [Google Scholar] [CrossRef]
- Jundt, C.; Klein, G.; Sipp, B.; Moigne, J.L.; Joucla, M.; Villaeys, A.A. Exciton dynamics in pentacene thin films studied by pump-probe spectroscopy. Chem. Phys. Lett. 1995, 24, 84–88. [Google Scholar] [CrossRef]
- Smith, M.B.; Michl, J. Singlet fission. Chem. Rev. 2010, 110, 6891–6936. [Google Scholar] [CrossRef]
- Ito, S.; Nagami, T.; Nakano, M. Molecular design for efficient singlet fission. J. Photochem. Photobiol. C 2018, 34, 85–120. [Google Scholar] [CrossRef]
- Korovina, N.V.; Pompetti, N.F.; Johnson, J.C. Lessons from intramolecular singlet fission with covalently bound chromophores. J. Chem. Phys. 2020, 152, 040904. [Google Scholar] [CrossRef] [Green Version]
- Miyata, K.; Conrad-Burton, F.S.; Geyer, F.L.; Zhu, X.Y. Triplet Pair States in Singlet Fission. Chem. Rev. 2019, 119, 4261–4292. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-L.; Berkelbach, T.C.; Provorse, M.R.; Monahan, N.R.; Tritsch, J.R.; Hybertsen, M.S.; Reichman, D.R.; Gao, J.; Zhu, X.-Y. The Quantum Coherent Mechanism for Singlet Fission: Experiment and Theory. Acc. Chem. Res. 2013, 46, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Felter, K.M.; Grozema, F.C. Singlet Fission in Crystalline Organic Materials: Recent Insights and Future Directions. J. Phys. Chem. Lett. 2019, 10, 7208–7214. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sanders, S.N.; Cheng, W.; Low, J.Z.; Liu, J.; Campos, L.M.; Sun, T. Singlet Fission: Progress and Prospects in Solar Cells. Adv. Mater. 2017, 29, 1601652. [Google Scholar] [CrossRef]
- Smith, M.B.; Michl, J. Recent Advances in Singlet Fission. Annu. Rev. Phys. Chem. 2013, 64, 361–386. [Google Scholar] [CrossRef]
- Burdett, J.J.; Bardeen, C.J. The Dynamics of Singlet Fission in Crystalline Tetracene and Covalent Analogs. Acc. Chem. Res. 2013, 46, 1312–1320. [Google Scholar] [CrossRef]
- Monahan, N.; Zhu, X.Y. Charge transfer-mediated singlet fission. Annu. Rev. Phys. Chem. 2015, 66, 601–618. [Google Scholar] [CrossRef]
- Parker, S.M.; Seideman, T.; Ratner, M.A.; Shiozaki, T. Model Hamiltonian Analysis of Singlet Fission from First Principles. J. Phys. Chem. C 2014, 118, 12700–12705. [Google Scholar] [CrossRef]
- Japahuge, A.; Zeng, T. Theoretical Studies of Singlet Fission: Searching for Materials and Exploring Mechanisms. Chempluschem 2018, 83, 146–182. [Google Scholar] [CrossRef]
- Rao, A.; Friend, R.H. Harnessing singlet exciton fission to break the Shockley–Queisser limit. Nat. Rev. Mater. 2017, 2, 17063. [Google Scholar] [CrossRef]
- Ullrich, T.; Munz, D.; Guldi, D.M. Unconventional singlet fission materials. Chem. Soc. Rev. 2021, 50, 3485–3518. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.W.; Rao, A.; Johnson, K.; Gelinas, S.; di Pietro, R.; Clark, J.; Friend, R.H. Temperature-Independent Singlet Exciton Fission in Tetracene. J. Am. Chem. Soc. 2013, 135, 16680–16688. [Google Scholar] [CrossRef] [PubMed]
- Tayebjee, M.J.Y.; Gray-Weale, A.A.; Schmidt, T.W. Thermodynamic Limit of Exciton Fission Solar Cell Efficiency. J. Phys. Chem. Lett. 2012, 3, 2749–2754. [Google Scholar] [CrossRef]
- Stern, H.L.; Cheminal, A.; Yost, S.R.; Broch, K.; Bayliss, S.L.; Chen, K.; Tabachnyk, M.; Thorley, K.; Greenham, N.; Hodgkiss, J.M.; et al. Vibronically coherent ultrafast triplet-pair formation and subsequent thermally activated dissociation control efficient endothermic singlet fission. Nat. Chem. 2017, 9, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Le, A.K.; Bender, J.A.; Arias, D.H.; Cotton, D.E.; Johnson, J.C.; Roberts, S.T. Singlet Fission Involves an Interplay between Energetic Driving Force and Electronic Coupling in Perylenediimide Films. J. Am. Chem. Soc. 2018, 140, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.K.; Musser, A.J.; Bayliss, S.L.; Lukman, S.; Tamura, H.; Bubnova, O.; Hallani, R.K.; Meneau, A.; Resel, R.; Maruyama, M.; et al. The Entangled Triplet Pair State in Acene and Heteroacene Materials. Nat. Commun. 2017, 8, 15953. [Google Scholar] [CrossRef] [PubMed]
- Pensack, R.D.; Tilley, A.J.; Grieco, C.; Purdum, G.E.; Ostroumov, E.E.; Granger, D.B.; Oblinsky, D.G.; Dean, J.C.; Doucette, G.S.; Asbury, J.B.; et al. Striking the right balance of intermolecular coupling for high-efficiency singlet fission. Chem. Sci. 2018, 9, 6240–6259. [Google Scholar] [CrossRef]
- Margulies, E.A.; Miller, C.E.; Wu, Y.; Ma, L.; Schatz, G.C.; Young, R.M.; Wasielewski, M.R. Enabling singlet fission by controlling intramolecular charge transfer in pi-stacked covalent terrylenediimide dimers. Nat. Chem. 2016, 8, 1120–1125. [Google Scholar] [CrossRef]
- Monahan, N.R.; Sun, D.; Tamura, H.; Williams, K.W.; Xu, B.; Zhong, Y.; Kumar, B.; Nuckolls, C.; Harutyunyan, A.R.; Chen, G.; et al. Dynamics of the triplet-pair state reveals the likely coexistence of coherent and incoherent singlet fission in crystalline hexacene. Nat. Chem. 2017, 9, 341–346. [Google Scholar] [CrossRef]
- Yago, T.; Ishikawa, K.; Katoh, R.; Wakasa, M. Magnetic Field Effects on Triplet Pair Generated by Singlet Fission in an Organic Crystal: Application of Radical Pair Model to Triplet Pair. J. Phys. Chem. C 2016, 120, 27858–27870. [Google Scholar] [CrossRef]
- Piland, G.B.; Bardeen, C.J. How Morphology Affects Singlet Fission in Crystalline Tetracene. J. Phys. Chem. Lett. 2015, 6, 1841–1846. [Google Scholar] [CrossRef]
- Ryerson, J.L.; Schrauben, J.N.; Ferguson, A.J.; Sahoo, S.C.; Naumov, P.; Havlas, Z.; Michl, J.; Nozik, A.J.; Johnson, J.C. Two Thin Film Polymorphs of the Singlet Fission Compound 1,3-Diphenylisobenzofuran. J. Phys. Chem. C 2014, 118, 12121–12132. [Google Scholar] [CrossRef]
- Roberts, S.T.; McAnally, R.E.; Mastron, J.N.; Webber, D.H.; Whited, M.T.; Brutchey, R.L.; Thompson, M.E.; Bradforth, S.E. Efficient Singlet Fission Discovered in a Disordered Acene Film. J. Am. Chem. Soc. 2012, 134, 6388–6400. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.W.B.; Rao, A.; Clark, J.; Kumar, R.S.S.; Brida, D.; Cerullo, G.; Friend, R.H. Ultrafast Dynamics of Exciton Fission in Polycrystalline Pentacene. J. Am. Chem. Soc. 2011, 133, 11830–11833. [Google Scholar] [CrossRef] [PubMed]
- Busby, E.; Xia, J.; Low, J.Z.; Wu, Q.; Hoy, J.; Campos, L.M.; Sfeir, M.Y. Fast Singlet Exciton Decay in Push-Pull Molecules Containing Oxidized Thiophenes. J. Phys. Chem. B 2015, 119, 7644–7650. [Google Scholar] [CrossRef]
- Young, R.M.; Wasielewski, M.R. Mixed Electronic States in Molecular Dimers: Connecting Singlet Fission, Excimer Formation, and Symmetry-Breaking Charge Transfer. Acc. Chem. Res. 2020, 53, 1957–1968. [Google Scholar] [CrossRef]
- Busby, E.; Berkelbach, T.C.; Kumar, B.; Chernikov, A.; Zhong, Y.; Hlaing, H.; Zhu, X.-Y.; Heinz, T.F.; Hybertsen, M.S.; Sfeir, M.Y.; et al. Multiphonon Relaxation Slows Singlet Fission in Crystalline Hexacene. J. Am. Chem. Soc. 2014, 136, 10654–10660. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, L.; Guo, D.; Zhao, X.; Wang, C.; Lin, D.; Zhang, F.; Zhang, J.; Nie, Z. Ultrafast Dynamics of Long-Lived Bound Triplet Pair Generated by Singlet Fission in 6,13-Bis(triisopropylsilylethynyl) Pentacene. J. Phys. Chem. C 2020, 124, 14503–14509. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, W.; Ma, L.; Sun, L.; Gurzadyan, G.G. Enhancement of Singlet Fission Yield by Hindering Excimer Formation in Perylene Film. J. Phys. Chem. C 2021, 126, 396–403. [Google Scholar] [CrossRef]
- Berera, R.; van Grondelle, R.; Kennis, J.T. Ultrafast transient absorption spectroscopy: Principles and application to photosynthetic systems. Photosynth. Res. 2009, 101, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Eaton, S.W.; Shoer, L.E.; Karlen, S.D.; Dyar, S.M.; Margulies, E.A.; Veldkamp, B.S.; Ramanan, C.; Hartzler, D.A.; Savikhin, S.; Marks, T.J.; et al. Singlet Exciton Fission in Polycrystalline Thin Films of a Slip-stacked Perylenediimide. J. Am. Chem. Soc. 2013, 135, 14701–14712. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, M.; Prasad, S.K.K.; Dover, C.B.; Forest, C.R.; Kaleem, A.; MacQueen, R.W.; Petty, A.J.; Forecast, R.; Beves, J.E.; Anthony, J.E.; et al. Singlet Fission in Concentrated TIPS-Pentacene Solutions: The Role of Excimers and Aggregates. J. Am. Chem. Soc. 2021, 143, 13749–13758. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, R.; Shen, L.; Xu, Y.; Xiao, M.; Zhang, C.; Li, X. A Covalently Linked Tetracene Trimer: Synthesis and Singlet Exciton Fission Property. Org. Lett. 2017, 19, 580–583. [Google Scholar] [CrossRef]
- Lukman, S.; Richter, J.M.; Yang, L.; Hu, P.; Wu, J.; Greenham, N.C.; Musser, A.J. Efficient Singlet Fission and Triplet-Pair Emission in a Family of Zethrene Diradicaloids. J. Am. Chem. Soc. 2017, 139, 18376–18385. [Google Scholar] [CrossRef]
- Nandi, A.; Manna, B.; Ghosh, R. Singlet Fission Dynamics in the 5,12-Bis(phenylethynyl)tetracene Thin Film. J. Phys. Chem. C 2021, 125, 2583–2591. [Google Scholar] [CrossRef]
- Chan, W.L.; Ligges, M.; Zhu, X.Y. The Energy Barrier in Singlet Fission can be Overcome through Coherent Coupling and Entropic Gain. Nat. Chem. 2012, 4, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Musser, A.J.; Beljonne, D.; Friend, R.H. Singlet exciton fission in solution. Nat. Chem. 2013, 5, 1019–1024. [Google Scholar] [CrossRef]
- Lee, J.; Bruzek, M.J.; Thompson, N.J.; Sfeir, M.Y.; Anthony, J.E.; Baldo, M.A. Singlet Exciton Fission in a Hexacene Derivative. Adv. Mater. 2013, 25, 1445–1448. [Google Scholar] [CrossRef]
- Watanabe, M.; Chang, Y.J.; Liu, S.W.; Chao, T.H.; Goto, K.; Islam, M.M.; Yuan, C.H.; Tao, Y.T.; Shinmyozu, T.; Chow, T.J. The Synthesis, Crystal Structure and Charge-Transport Properties of Hexacene. Nat. Chem. 2012, 4, 574–578. [Google Scholar] [CrossRef]
- Johnson, J.C.; Nozik, A.J.; Michl, J. High Triplet Yield from Singlet Fission in a Thin Film of 1,3-Diphenylisobenzofuran. J. Am. Chem. Soc. 2010, 132, 16302–16303. [Google Scholar] [CrossRef]
- Marciniak, H.; Pugliesi, I.; Nickel, B.; Lochbrunner, S. Ultrafast singlet and triplet dynamics in microcrystalline pentacene films. Phys. Rev. B 2009, 79, 235318. [Google Scholar] [CrossRef]
- Marciniak, H.; Fiebig, M.; Huth, M.; Schiefer, S.; Nickel, B.; Selmaier, F.; Lochbrunner, S. Ultrafast exciton relaxation in microcrystalline pentacene films. Phys. Rev. Lett. 2007, 99, 176402. [Google Scholar] [CrossRef] [PubMed]
- Thorsmolle, V.K.; Averitt, R.D.; Demsar, J.; Smith, D.L.; Tretiak, S.; Martin, R.L.; Chi, X.; Crone, B.K.; Ramirez, A.P.; Taylor, A.J. Morphology effectively controls singlet-triplet exciton relaxation and charge transport in organic semiconductors. Phys. Rev. Lett. 2009, 102, 017401. [Google Scholar] [CrossRef] [PubMed]
- Korovina, N.V.; Das, S.; Nett, Z.; Feng, X.; Joy, J.; Haiges, R.; Krylov, A.I.; Bradforth, S.E.; Thompson, M.E. Singlet Fission in a Covalently Linked Cofacial Alkynyltetracene Dimer. J. Am. Chem. Soc. 2016, 138, 617–627. [Google Scholar] [CrossRef]
- Feng, X.; Krylov, A.I. On couplings and excimers: Lessons from studies of singlet fission in covalently linked tetracene dimers. Phys. Chem. Chem. Phys. 2016, 18, 7751–7761. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Casanova, D.; Krylov, A.I. Intra- and Intermolecular Singlet Fission in Covalently Linked Dimers. J. Phys. Chem. C 2016, 120, 19070–19077. [Google Scholar] [CrossRef]
- Jones, A.C.; Kearns, N.M.; Ho, J.J.; Flach, J.T.; Zanni, M.T. Impact of non-equilibrium molecular packings on singlet fission in microcrystals observed using 2D white-light microscopy. Nat. Chem. 2020, 12, 40–47. [Google Scholar] [CrossRef]
- Ribson, R.D.; Choi, G.; Hadt, R.G.; Agapie, T. Controlling Singlet Fission with Coordination Chemistry-Induced Assembly of Dipyridyl Pyrrole Bipentacenes. ACS Cent. Sci. 2020, 6, 2088–2096. [Google Scholar] [CrossRef]
- Munson, K.T.; Gan, J.; Grieco, C.; Doucette, G.S.; Anthony, J.E.; Asbury, J.B. Ultrafast Triplet Pair Separation and Triplet Trapping following Singlet Fission in Amorphous Pentacene Films. J. Phys. Chem. C 2020, 124, 23567–23578. [Google Scholar] [CrossRef]
- Luo, X.; Han, Y.; Chen, Z.; Li, Y.; Liang, G.; Liu, X.; Ding, T.; Nie, C.; Wang, M.; Castellano, F.N.; et al. Mechanisms of triplet energy transfer across the inorganic nanocrystal/organic molecule interface. Nat. Commun. 2020, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Liu, H.; Wang, X.; Liu, S.; Zhao, D.; Tang, Z.; Zhou, J.; Li, X. Singlet Fission in Self-assembled Amphipathic Tetracene Nanoparticles: Probing the Role of Charge-transfer State. J. Photochem. Photobiol. A 2020, 397, 112597. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, S.; Wang, X.; Liu, H.; Yan, X.; Liu, S.; Lu, X.; Li, X. Enhancing the intermolecular singlet fission efficiency by controlling the self-assembly of amphipathic tetracene derivatives in aqueous solution. J. Mater. Chem. C 2019, 7, 11090–11098. [Google Scholar] [CrossRef]
- Wilson, M.W.B.; Rao, A.; Ehrler, B.; Friend, R.H. Singlet Exciton Fission in Polycrystalline Pentacene: From Photophysics toward Devices. Acc. Chem. Res. 2013, 46, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Sheraw, C.D.; Jackson, T.N.; Eaton, D.L.; Anthony, J.E. Functionalized Pentacene Active Layer Organic Thin-Film Transistors. Adv. Mater. 2003, 15, 2009–2011. [Google Scholar] [CrossRef]
- Grieco, C.; Doucette, G.S.; Munson, K.T.; Swartzfager, J.R.; Munro, J.M.; Anthony, J.E.; Dabo, I.; Asbury, J.B. Vibrational probe of the origin of singlet exciton fission in TIPS-pentacene solutions. J. Chem. Phys. 2019, 151, 154701. [Google Scholar] [CrossRef]
- Cui, M.; Jiang, Y.; Zhang, B.; Song, D.; Yuan, M.; Qin, C.; Liu, Y. Multiexciton state of singlet fission in triisopropylsilylethynyl-pentacene. Microw. Opt. Technol. Lett. 2021, 63, 1399–1405. [Google Scholar] [CrossRef]
- Herz, J.; Buckup, T.; Paulus, F.; Engelhart, J.; Bunz, U.H.; Motzkus, M. Acceleration of Singlet Fission in an Aza-Derivative of TIPS-Pentacene. J. Phys. Chem. Lett. 2014, 5, 2425–2430. [Google Scholar] [CrossRef]
- Sanders, S.N.; Kumarasamy, E.; Pun, A.B.; Trinh, M.T.; Choi, B.; Xia, J.; Taffet, E.J.; Low, J.Z.; Miller, J.R.; Roy, X.; et al. Quantitative Intramolecular Singlet Fission in Bipentacenes. J. Am. Chem. Soc. 2015, 137, 8965–8972. [Google Scholar] [CrossRef]
- Paul, S.; Karunakaran, V. Excimer Formation Inhibits the Intramolecular Singlet Fission Dynamics: Systematic Tilting of Pentacene Dimers by Linking Positions. J. Phys. Chem. B 2022, 126, 1054–1062. [Google Scholar] [CrossRef]
- Alagna, N.; Perez Lustres, J.L.; Wollscheid, N.; Luo, Q.; Han, J.; Dreuw, A.; Geyer, F.L.; Brosius, V.; Bunz, U.H.F.; Buckup, T.; et al. Singlet Fission in Tetraaza-TIPS-Pentacene Oligomers: From fs Excitation to mus Triplet Decay via the Biexcitonic State. J. Phys. Chem. B 2019, 123, 10780–10793. [Google Scholar] [CrossRef]
- Vallett, P.J.; Snyder, J.L.; Damrauer, N.H. Tunable electronic coupling and driving force in structurally well-defined tetracene dimers for molecular singlet fission: A computational exploration using density functional theory. J. Phys. Chem. A 2013, 117, 10824–10838. [Google Scholar] [CrossRef]
- Zirzlmeier, J.; Lehnherr, D.; Coto, P.B.; Chernick, E.T.; Casillas, R.; Basel, B.S.; Thoss, M.; Tykwinski, R.R.; Guldi, D.M. Singlet fission in pentacene dimers. Proc. Natl. Acad. Sci. USA 2015, 112, 5325–5330. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Govind, C.; Karunakaran, V. Planarity and Length of the Bridge Control Rate and Efficiency of Intramolecular Singlet Fission in Pentacene Dimers. J. Phys. Chem. B 2021, 125, 231–239. [Google Scholar] [CrossRef]
- Sanders, S.N.; Kumarasamy, E.; Pun, A.B.; Steigerwald, M.L.; Sfeir, M.Y.; Campos, L.M. Intramolecular Singlet Fission in Oligoacene Heterodimers. Angew. Chem., Int. Ed. 2016, 55, 3373–3377. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Wang, X.; Shen, L.; Zhang, C.; Xiao, M.; Li, X. Singlet exciton fission in a linear tetracene tetramer. J. Mater. Chem. C 2018, 6, 3245–3253. [Google Scholar] [CrossRef]
- Kuroda, K.; Yazaki, K.; Tanaka, Y.; Akita, M.; Sakai, H.; Hasobe, T.; Tkachenko, N.V.; Yoshizawa, M. A Pentacene-based Nanotube Displaying Enriched Electrochemical and Photochemical Activities. Angew. Chem. Int. Ed. 2019, 58, 1115–1119. [Google Scholar] [CrossRef]
- Huang, H.; He, G.; Xu, K.; Wu, Q.; Wu, D.; Sfeir, M.Y.; Xia, J. Achieving Long-Lived Triplet States in Intramolecular SF Films through Molecular Engineering. Chem 2019, 5, 2405–2417. [Google Scholar] [CrossRef]
- Lee, J.; Jadhav, P.; Baldo, M.A. High efficiency organic multilayer photodetectors based on singlet exciton fission. Appl. Phys. Lett. 2009, 95, 033301. [Google Scholar] [CrossRef]
- Congreve, D.N.; Lee, J.; Thompson, N.J.; Hontz, E.; Yost, S.R.; Reusswig, P.D.; Bahlke, M.E.; Reineke, S.; Van Voorhis, T.; Baldo, M.A. External Quantum Efficiency Above 100% in a Singlet-Exciton-Fission–Based Organic Photovoltaic Cell. Science 2013, 340, 334–337. [Google Scholar] [CrossRef]
- Thompson, N.J.; Congreve, D.N.; Goldberg, D.; Menon, V.M.; Baldo, M.A. Slow light enhanced singlet exciton fission solar cells with a 126% yield of electrons per photon. Appl. Phys. Lett. 2013, 103, 263302. [Google Scholar] [CrossRef]
- Rao, A.; Wilson, M.W.B.; Hodgkiss, J.M.; Albert-Seifried, S.; Bässler, H.; Friend, R.H. Exciton Fission and Charge Generation via Triplet Excitons in Pentacene/C60Bilayers. J. Am. Chem. Soc. 2010, 132, 12698–12703. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-L.; Ligges, M.; Jailaubekov, A.; Kaake, L.; Miaja-Avila, L.; Zhu, X.-Y. Observing the Multiexciton State in Singlet Fission and Ensuing Ultrafast Multielectron Transfer. Science 2011, 334, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.J.; Brown, P.R.; Thompson, N.; Wunsch, B.; Mohanty, A.; Yost, S.R.; Hontz, E.; Van Voorhis, T.; Bawendi, M.G.; Bulović, V.; et al. Triplet Exciton Dissociation in Singlet Exciton Fission Photovoltaics. Adv. Mater. 2012, 24, 6169–6174. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tabachnyk, M.; Bayliss, S.L.; Böhm, M.L.; Broch, K.; Greenham, N.C.; Friend, R.H.; Ehrler, B. Solution-Processable Singlet Fission Photovoltaic Devices. Nano Lett. 2014, 15, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.J.; Mohanty, A.; Sussman, J.; Lee, J.; Baldo, M.A. Singlet Exciton Fission in Nanostructured Organic Solar Cells. Nano Lett. 2011, 11, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Editorial A decade of perovskite photovoltaics. Nat. Energy 2019, 4, 1. [CrossRef]
- Ehrler, B.; Musselman, K.P.; Böhm, M.L.; Friend, R.H.; Greenham, N.C. Hybrid pentacene/a-silicon solar cells utilizing multiple carrier generation via singlet exciton fission. Appl. Phys. Lett. 2012, 101, 153507. [Google Scholar] [CrossRef]
- Pazos-Outón, L.M.; Lee, J.M.; Futscher, M.H.; Kirch, A.; Tabachnyk, M.; Friend, R.H.; Ehrler, B. A Silicon–Singlet Fission Tandem Solar Cell Exceeding 100% External Quantum Efficiency with High Spectral Stability. ACS Energy Lett. 2017, 2, 476–480. [Google Scholar] [CrossRef]
- Sundin, E.; Ringström, R.; Johansson, F.; Küçüköz, B.; Ekebergh, A.; Gray, V.; Albinsson, B.; Mårtensson, J.; Abrahamsson, M. Singlet Fission and Electron Injection from the Triplet Excited State in Diphenylisobenzofuran–Semiconductor Assemblies: Effects of Solvent Polarity and Driving Force. J. Phys. Chem. C 2020, 124, 20794–20805. [Google Scholar] [CrossRef]
- Daiber, B.; van den Hoven, K.; Futscher, M.H.; Ehrler, B. Realistic Efficiency Limits for Singlet-Fission Silicon Solar Cells. ACS Energy Lett. 2021, 6, 2800–2808. [Google Scholar] [CrossRef]
- MacQueen, R.W.; Liebhaber, M.; Niederhausen, J.; Mews, M.; Gersmann, C.; Jäckle, S.; Jäger, K.; Tayebjee, M.J.Y.; Schmidt, T.W.; Rech, B.; et al. Crystalline silicon solar cells with tetracene interlayers: The path to silicon-singlet fission heterojunction devices. Mater. Horiz. 2018, 5, 1065–1075. [Google Scholar] [CrossRef]
- Cheung, J.C.F.; Kaake, L.G. Detailed balance analysis of advanced geometries for singlet fission solar cells. Appl. Phys. Lett. 2021, 119, 013301. [Google Scholar] [CrossRef]
- Einzinger, M.; Wu, T.; Kompalla, J.F.; Smith, H.L.; Perkinson, C.F.; Nienhaus, L.; Wieghold, S.; Congreve, D.N.; Kahn, A.; Bawendi, M.G.; et al. Sensitization of silicon by singlet exciton fission in tetracene. Nature 2019, 571, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, B.; Madu, I.K.; Kim, H.; Cai, Z.; Jiang, H.; Muthike, A.K.; Yu, L.; Zimmerman, P.M.; Goodson, T., 3rd. Activating intramolecular singlet exciton fission by altering pi-bridge flexibility in perylene diimide trimers for organic solar cells. Chem. Sci. 2020, 11, 8757–8770. [Google Scholar] [CrossRef] [PubMed]
- Korovina, N.V.; Chang, C.H.; Johnson, J.C. Spatial separation of triplet excitons drives endothermic singlet fission. Nat. Chem. 2020, 12, 391–398. [Google Scholar] [CrossRef]
- Hu, J.; Xu, K.; Shen, L.; Wu, Q.; He, G.; Wang, J.Y.; Pei, J.; Xia, J.; Sfeir, M.Y. New insights into the design of conjugated polymers for intramolecular singlet fission. Nat. Commun. 2018, 9, 2999. [Google Scholar] [CrossRef]
- Wang, L.; Cai, W.; Sun, J.; Wu, Y.; Zhang, B.; Tian, X.; Guo, S.; Liang, W.; Fu, H.; Yao, J. H-Type-like Aggregation-Accelerated Singlet Fission Process in Dipyrrolonaphthyridinedione Thin Film: The Role of Charge Transfer/Excimer Mixed Intermediate State. J. Phys. Chem. Lett. 2021, 12, 12276–12282. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Cao, H.; Zhang, Z.; Liu, S.; Xia, Y. Research Progress on Singlet Fission in Acenes and Their Derivatives. Photonics 2022, 9, 689. https://doi.org/10.3390/photonics9100689
Li J, Cao H, Zhang Z, Liu S, Xia Y. Research Progress on Singlet Fission in Acenes and Their Derivatives. Photonics. 2022; 9(10):689. https://doi.org/10.3390/photonics9100689
Chicago/Turabian StyleLi, Jingjing, He Cao, Zhibin Zhang, Shuo Liu, and Yuanqin Xia. 2022. "Research Progress on Singlet Fission in Acenes and Their Derivatives" Photonics 9, no. 10: 689. https://doi.org/10.3390/photonics9100689
APA StyleLi, J., Cao, H., Zhang, Z., Liu, S., & Xia, Y. (2022). Research Progress on Singlet Fission in Acenes and Their Derivatives. Photonics, 9(10), 689. https://doi.org/10.3390/photonics9100689





