An Optical POCT Device for Colorimetric Detection of Urine Test Strips Based on Raspberry Pi Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of the Solutions
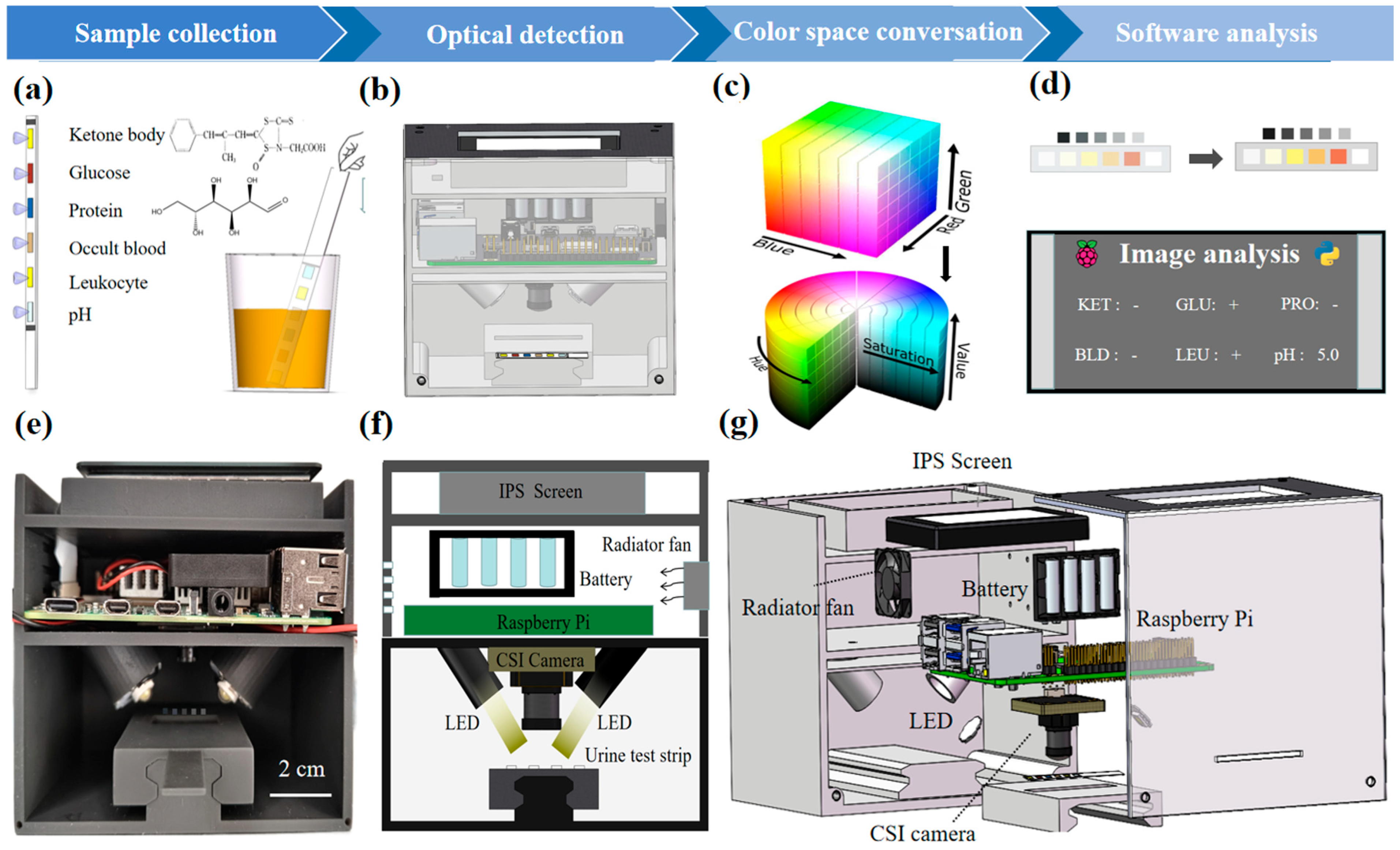
2.3. Design of the Optical POCT Device
2.4. Color Space Transformation
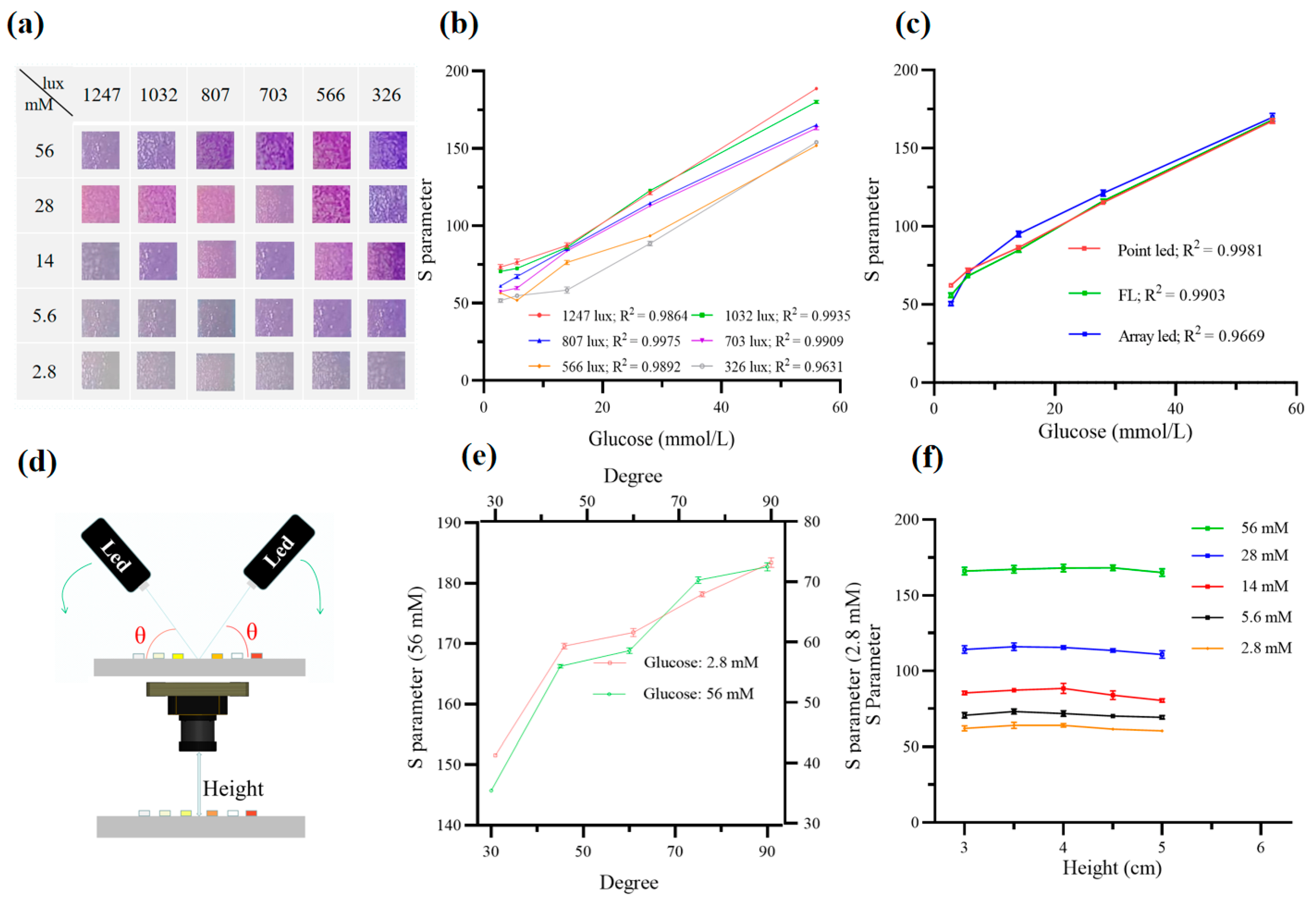
2.5. Optimization of Sensing Conditions for Best Illumination Uniformity
2.6. Automatic Recognition of the Paper-Based Sensors and the Image Processing Algorithm
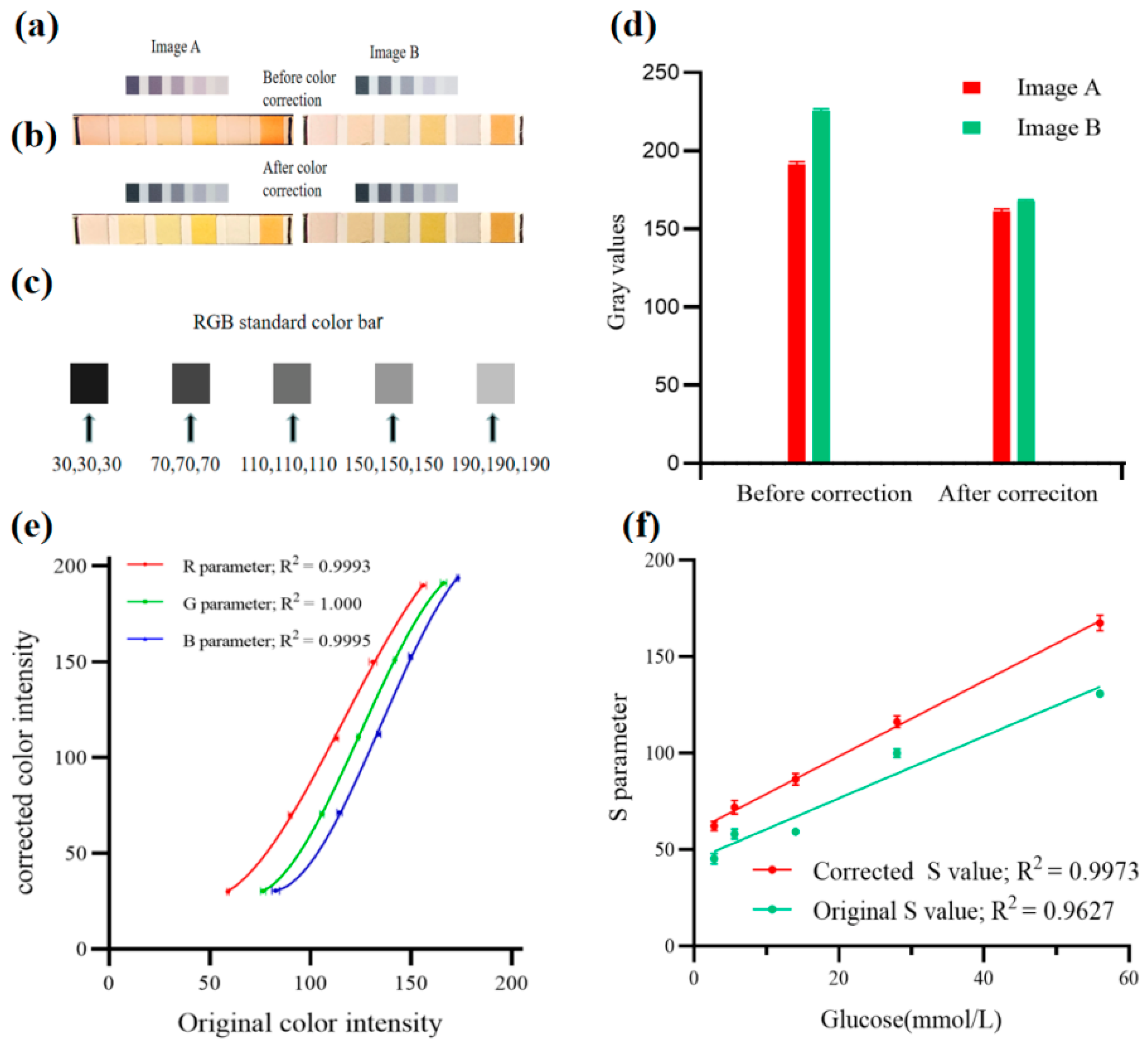
2.7. Color Correction
2.8. Detection of Clinical Samples and Statistical Analysis
3. Results and Discussions
3.1. Color Correction Using a Standard Color Bar
3.2. Optimization of the Sensing Conditions
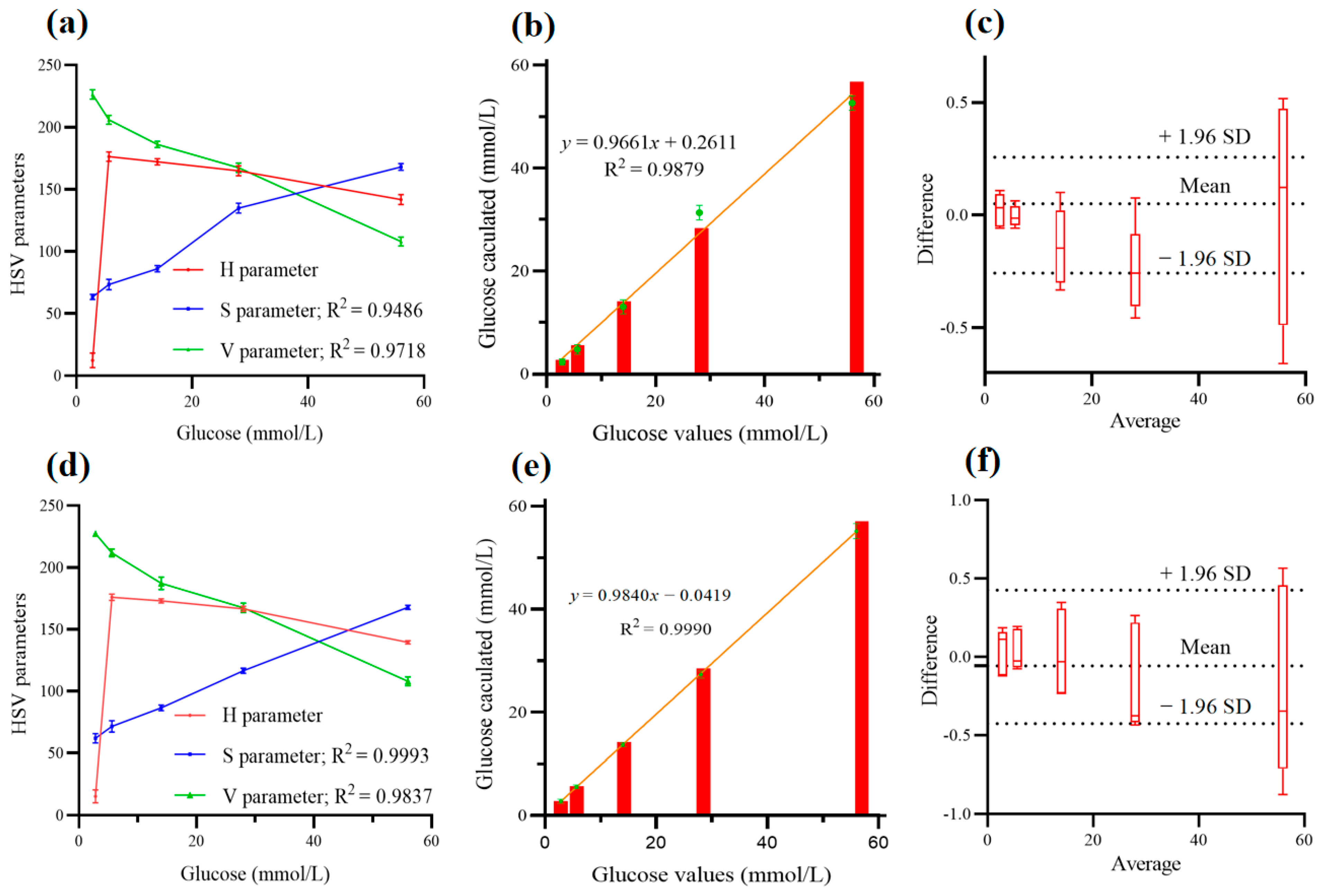
3.3. Optimization of the Algorithm
3.4. Conversion of the Correct RGB Color to Analytical Values
3.5. Determination of the Clinical Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aitekenov, S.; Gaipov, A.; Bukasov, R. Review: Detection and quantification of proteins in human urine. Talanta 2021, 223, 121718. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, Z.; Li, X. Non-invasive disease diagnosis using surface-enhanced Raman spectroscopy of urine and saliva. Appl. Spectrosc. Rev. 2020, 55, 197–219. [Google Scholar] [CrossRef]
- Tai, W.-C.; Chang, Y.-C.; Chou, D.; Fu, L.-M. Lab-on-Paper Devices for Diagnosis of Human Diseases Using Urine Samples—A Review. Biosensors 2021, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Mapp, I.D.; Davis, L.L.; Krowchuk, H. Prevention of unplanned intensive care unit admissions and hospital mortality by early warning systems. Dimens. Crit. Care Nurs. 2013, 32, 300–309. [Google Scholar] [CrossRef]
- Luppa, P.B.; Mueller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. TrAC-Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Zhang, R.-Q.; Liu, S.-L.; Zhao, W.; Zhang, W.-P.; Yu, X.; Li, Y.; Li, A.-J.; Pang, D.-W.; Zhang, Z.-L. A Simple Point-of-Care Microfluidic Immunomagnetic Fluorescence Assay for Pathogens. Anal. Chem. 2013, 85, 2645–2651. [Google Scholar] [CrossRef]
- Fu, H.; Yang, J.; Guo, L.; Nie, J.; Yin, Q.; Zhang, L.; Zhang, Y. Using the Rubik’s Cube to directly produce paper analytical devices for quantitative point-of-care aptamer-based assays. Biosens. Bioelectron. 2017, 96, 194–200. [Google Scholar] [CrossRef]
- Kang, M.; Kim, E.; Winkler, T.E.; Banis, G.; Liu, Y.; Kitchen, C.A.; Kelly, D.L.; Ghodssi, R.; Payne, G.F. Reliable clinical serum analysis with reusable electrochemical sensor: Toward point-of-care measurement of the antipsychotic medication clozapine. Biosens. Bioelectron. 2017, 95, 55–59. [Google Scholar] [CrossRef]
- Hur, J.; Park, H.G.; Kim, M.I. Reagentless colorimetric biosensing platform based on nanoceria within an agarose gel matrix. Biosens. Bioelectron. 2017, 93, 226–233. [Google Scholar]
- Choodum, A.; Keson, J.; Kanatharana, P.; Limsakul, W.; Wongniramaikul, W. Selective pre and post blast trinitrotoluene detection with a novel ethylenediamine entrapped thin polymer film and digital image colorimetry. Sens. Actuators B Chem. 2017, 252, 463–469. [Google Scholar] [CrossRef]
- Li, F.; He, T.; Wu, S.; Peng, Z.; Qiu, P.; Tang, X. Visual and colorimetric detection of uric acid in human serum and urine using chitosan stabilized gold nanoparticles. Microchem. J. 2021, 164, 105987. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, K.; Sun, J.; Wu, J.; Ju, H. A pH-responsive colorimetric strategy for DNA detection by acetylcholinesterase catalyzed hydrolysis and cascade amplification. Biosens. Bioelectron. 2017, 94, 651–656. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, X.; Liu, X.; Li, H.; Nie, Z.; Qing, M.; Huang, Y.; Yao, S. A gold nanoparticles calorimetric assay for label-free detection of protein kinase activity based on phosphorylation protection against exopeptidase cleavage. Biosens. Bioelectron. 2014, 53, 295–300. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Hu, T.; Su, X. A novel fluorimetric sensing platform for highly sensitive detection of organophosphorus pesticides by using egg white-encapsulated gold nanoclusters. Biosens. Bioelectron. 2017, 91, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Chen, G.; Li, K.; Zhou, M.; Zhang, J.; Zhao, S. Dispersive liquid-liquid microextraction coupled with digital image colorimetric analysis for detection of total iron in water and food samples. Food Chem. 2017, 230, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Vilaivan, T.; Chailapakul, O.; Henry, C.S. Multiplex Paper-Based Colorimetric DNA Sensor Using Pyrrolidinyl Peptide Nucleic Acid-Induced AgNPs Aggregation for Detecting MERS-CoV, MTB, and HPV Oligonucleotides. Anal. Chem. 2017, 89, 5428–5435. [Google Scholar] [CrossRef]
- Hong, J.I.; Chang, B.-Y. Development of the smartphone-based colorimetry for multi-analyte sensing arrays. Lab A Chip 2014, 14, 1725–1732. [Google Scholar] [CrossRef]
- Mei, Q.; Jing, H.; Li, Y.; Yisibashaer, W.; Chen, J.; Li, B.N.; Zhang, Y. Smartphone based visual and quantitative assays on upconversional paper sensor. Biosens. Bioelectron. 2016, 75, 427–432. [Google Scholar] [CrossRef]
- Ye, J.; Li, N.; Lu, Y.; Cheng, J.; Xu, Y. A portable urine analyzer based on colorimetric detection. Anal. Methods 2017, 9, 2464–2471. [Google Scholar] [CrossRef]
- Jalal, U.M.; Jin, G.J.; Shim, J.S. Paper-Plastic Hybrid Microfluidic Device for Smartphone-Based Colorimetric Analysis of Urine. Anal. Chem. 2017, 89, 13160–13166. [Google Scholar] [CrossRef]
- Yang, R.; Cheng, W.; Chen, X.; Qian, Q.; Zhang, Q.; Pan, Y.; Duan, P.; Miao, P. Color Space Transformation-Based Smartphone Algorithm for Colorimetric Urinalysis. Acs Omega 2018, 3, 12141–12146. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D. Buffers for pH and Metal Ion Control; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Lin, B.; Yu, Y.; Cao, Y.; Guo, M.; Zhu, D.; Dai, J.; Zheng, M. Point-of-care testing for streptomycin based on aptamer recognizing and digital image colorimetry by smartphone. Biosens. Bioelectron. 2018, 100, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Chien-Hsuan, K.; Chan-Chiung, L.; Kuan-Hong, C.; Fuu, S.; Lung-Ming, F.; Szu-Jui, C. Microfluidic colorimetric analysis system for sodium benzoate detection in foods. Food Chem. 2021, 345, 128773. [Google Scholar] [CrossRef]
- Stubbings, T.C.; Hutter, H. Combining multispectral image information using color. Anal. Chem. 2000, 72, 282A–288A. [Google Scholar] [CrossRef][Green Version]
- Park, H.; Koh, Y.G.; Lee, W. Smartphone-based colorimetric analysis of structural colors from pH-responsive photonic gel. Sens. Actuators B-Chem. 2021, 345, 130359. [Google Scholar] [CrossRef]
- Luka, G.S.; Nowak, E.; Kawchuk, J.; Hoorfar, M.; Najjaran, H. Portable device for the detection of colorimetric assays. R. Soc. Open Sci. 2017, 4, 171025. [Google Scholar] [CrossRef]
- Zhu, W.; Gong, C.; Kulkarni, N.; Nguyen, C.D.; Kang, D. Smartphone-based microscopes. In Smartphone Based Medical Diagnostics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 159–175. [Google Scholar] [CrossRef]
- Hattori, M.; Shirane, S.; Matsuda, T.; Nagayama, K.; Nagai, T. Smartphone-Based Portable Bioluminescence Imaging System Enabling Observation at Various Scales from Whole Mouse Body to Organelle. Sensors 2020, 20, 7166. [Google Scholar] [CrossRef]
- Di, W.; Da-Wen, S. Colour measurements by computer vision for food quality control—A review. Trends Food Sci. Technol. 2013, 29, 5–20. [Google Scholar] [CrossRef]
- Lott, J.A.; Turner, K. Evaluation of Trinder’s glucose oxidase method for measuring glucose in serum and urine. Clin. Chem. 1975, 21, 1754–1760. [Google Scholar] [CrossRef]
- Olofsen, E.; Dahan, A.; Borsboom, G.; Drummond, G. Improvements in the application and reporting of advanced Bland-Altman methods of comparison. J. Clin. Monit. Comput. 2015, 29, 127–139. [Google Scholar] [CrossRef]
- Gong, X.; Feng, S.; Qiao, Z.; Chen, Y.-C. Imaging-Based Optofluidic Biolaser Array Encapsulated with Dynamic Living Organisms. Anal. Chem. 2021, 93, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.; Erenas, M.M.; de Orbe-Paya, I.; Capitan-Vallvey, L.F. Use of the Hue Parameter of the Hue, Saturation, Value Color Space As a Quantitative Analytical Parameter for Bitonal Optical Sensors. Anal. Chem. 2010, 82, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhang, Y.; Cai, Z.; Wang, W.; Wang, C. A 3D printed centrifugal microfluidic platform for automated colorimetric urinalysis. Microsyst. Technol.-Micro-Nanosyst.-Inf. Storage Process. Syst. 2020, 26, 291–299. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, L.; Yu, H.; Xin, Y.; Cong, Z. Wavelet denoising method for laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2013, 28, 1884–1893. [Google Scholar] [CrossRef]
- Hore, A.; Ziou, D. Image Quality Metrics: PSNR vs. SSIM. In Proceedings of the 2010 20th International Conference on Pattern Recognition, Istanbul, Turkey, 23–26 August 2010; pp. 2366–2369. [Google Scholar] [CrossRef]
- Deville, W.L.J.M.; Yzermans, J.C.; van Duijn, N.P.; Bezemer, P.D.; van der Windt, D.A.W.M.; Bouter, L.M. The urine dipstick test useful to rule out infections. A meta-analysis of the accuracy. BMC Urol. 2004, 4, 4. [Google Scholar] [CrossRef]



| Analyte | Sensitivity |
|---|---|
| KET | 0.33 mM |
| GLU | 1.16 mM |
| PRO | 0.10 g/L |
| BLD | 0.37 cells/μL |
| pH | 4.72 |
| LEU | 9.03 cells/μL |
| Analyte | Negative | Positive | |||
|---|---|---|---|---|---|
| Level1+ | Level2+ | Level3+ | Level4+ | ||
| KET | 86.7% | 80.0% | - | - | - |
| GLU | 90.1% | 85.7% | 87.5% | 83.3% | 83.3% |
| PRO | 84.2% | 80.1% | 83.3% | 80.0% | - |
| BLD | 91.5% | 84.0% | 84.2% | 88.9% | - |
| pH | 86.0% | - | - | - | - |
| LEU | 85.5% | 81.9% | 84.2% | 81.8% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Cai, G.; Zhao, J.; Feng, S. An Optical POCT Device for Colorimetric Detection of Urine Test Strips Based on Raspberry Pi Imaging. Photonics 2022, 9, 784. https://doi.org/10.3390/photonics9100784
Yang Z, Cai G, Zhao J, Feng S. An Optical POCT Device for Colorimetric Detection of Urine Test Strips Based on Raspberry Pi Imaging. Photonics. 2022; 9(10):784. https://doi.org/10.3390/photonics9100784
Chicago/Turabian StyleYang, Zixin, Gaozhe Cai, Jianlong Zhao, and Shilun Feng. 2022. "An Optical POCT Device for Colorimetric Detection of Urine Test Strips Based on Raspberry Pi Imaging" Photonics 9, no. 10: 784. https://doi.org/10.3390/photonics9100784
APA StyleYang, Z., Cai, G., Zhao, J., & Feng, S. (2022). An Optical POCT Device for Colorimetric Detection of Urine Test Strips Based on Raspberry Pi Imaging. Photonics, 9(10), 784. https://doi.org/10.3390/photonics9100784







