Nano-Bismuth-Sulfide for Advanced Optoelectronics
Abstract
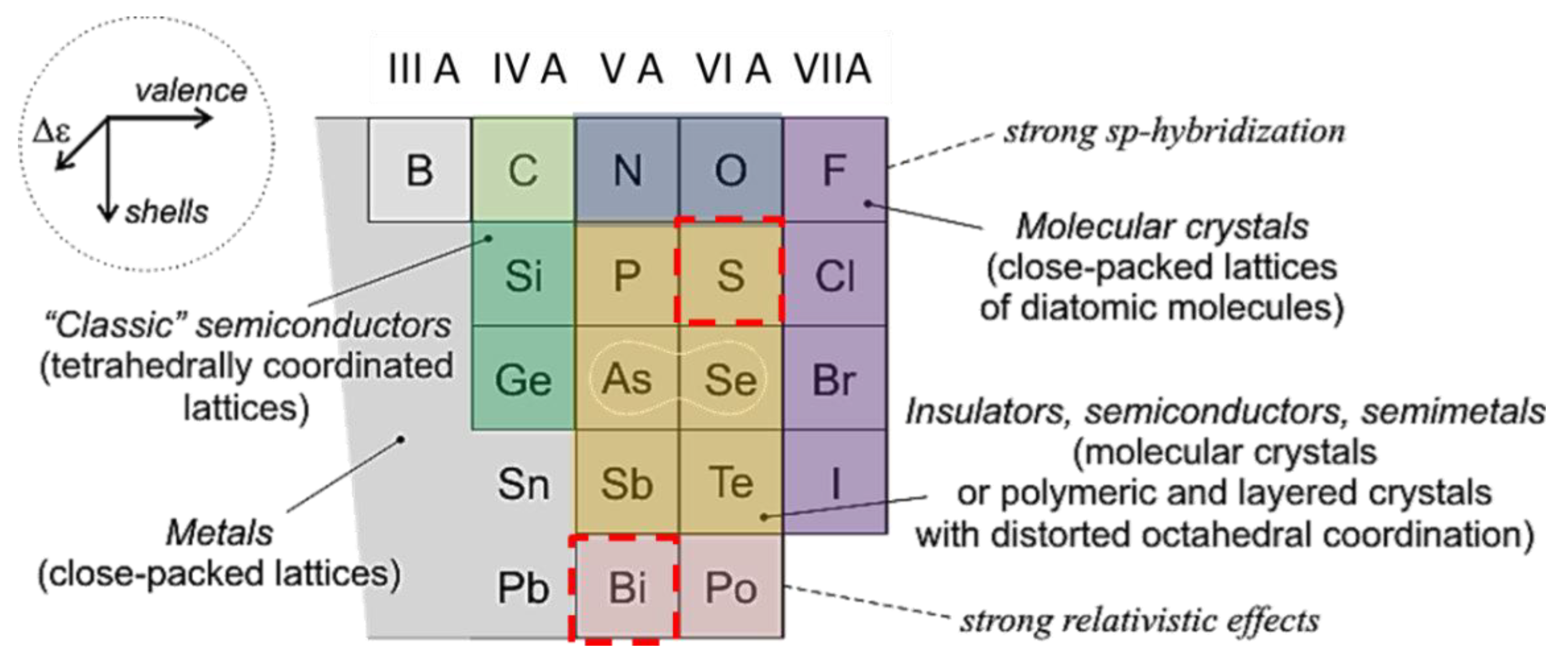
1. Introduction
2. Fabrications
| Method | Growth Condition | Starting Materials | Product | Size |
|---|---|---|---|---|
| “Top-down” fabrications | ||||
| Vapor phase deposition | ||||
| Thermal evaporation [26] | RT | Bi2S3 power | Amorphous film | 50 nm thickness |
| LP-MOCVD [56] | 450 °C | [Bi(S2CNMen-Hex)3] [Cd(S2CNMen-Hex)2] | Fiber-like particles | Length (L): 1 μm diameter (D): 50 nm |
| Pulsed laser deposition [14] | RT | Bi2S3 target | Quantum dots | D < 5 nm |
| Liquid phase deposition | ||||
| Cathodic electrodeposition [41] | RT | Na2S2O3, Na3C6H5O7Bi(NO3)3, | Thin film | / |
| Electrodeposition [62] | RT | Na2S2O3, Bi(NO3)3, EDTA | Thin film | / |
| Chemical bath deposition(CBD) [33] | RT | Bi(NO3)3, thioacetamide (TA), ammonium citrate (AC) | Nanowall Bi2S3 films | / |
| CBD [26] | RT | Bi(NO3)3,triethanolamie (TEA), TA, | Thin film | Thickness (T): 50–140 nm |
| Non-aqueous CBD [63] | RT | Bi(NO3)3,acetic acid, TA | Thin films | T: 241 nm |
| Non-aqueous CBD [42] | RT | Bi(NO3)3, Na2S2O3, formaldehyde, | Thin film | T: 50–100 nm |
| Surface Sulfurization | ||||
| High-temperature reaction of sulfur source with bismuth-based metal–organic framework [64] | 300~600 °C | Bi(BTC)(DMF)·DMF·(CH3OH)2 Trimesic acid (H3BTC) | Nanorod (NR) | D: 60 nm |
| Surface sulfurization [29] | 450 °C | Bi2O3 Nanosheets | 2D nanosheets | T: 2.5 nm |
| “Bottom-up” fabrications | ||||
| Solvothermal synthesis | ||||
| Hydrothermal synthesis [30] | 160 °C | Bi(NO3)3, NH2CSNH2, thiourea | NR | D: 50~100 nm L: 1~2 μm |
| Hydrothermal process [11] | 180 °C | BiCl3, HCl, TAA | Bi2S3 nanomeshes | L: 200 nm D: 20~40 nm |
| Hydrothermal process [65] | 180 °C | Bi(NO3)3, Thiourea, Urea, Methyl orange | Microsphere | D: 3 μm |
| Hydrothermal methods [59] | 180 °C | Bi(NO3)3, thiourea (TU), potassium thiocyanate (KSCN), TAA, sodium thiosulfate (Na2S2O3·5H2O) | Nanowires (NW), wire bundles, urchin-like nano-/microspheres microspheres with cavities | NW D:15~40 nm L: Tens μm bundles D: 2~3 μm L: 13~20 μm sphere D: ~1 μm/ |
| Solvothermal synthesis [66] | 160 °C | Bi(NO3)3, ethylene glycol (EG), TAA, TU, L-cysteine | Nanoparticles, urchin-like spheres | / |
| solvothermal method [67] | 80 °C | Bi(NO3)3, EG, TU, poly(vinylpyrrolidone) (K-30) | Chrysanthemum- like nano-Bi2S3 | D: ~500 nm. |
| Solvothermal method [58] | 150 °C 5 min | Oleyl amine, sulfur powder, BiCl3, oleic acid, hexane, 1-octadecene | Nanoribbons | D:10~80 nm L:100~500 nm |
| Hydrothermal route [68] | 160 °C | Bi(NO3)3, TAA, DA, ascorbic acid (AA), uric acid (UA), paracetamol | NR | L:100 nm |
| Hydrothermal method [60] | 180 °C | Bi(NO3)3, Na2S2O3, glucose | Hollow nanotubes | L: dozens μm D: few μm |
| Hydrothermal method [43] | 180 °C 2 days | Bi(NO3)3, Na2S2O3, | NW | D: 20–60 nm |
| Hydrothermal method [39] | 180 °C 3 day | Tetramethylammonium Bi(NO3)3, hydroxide, Na2S | NW | D: 60 nm |
| Wet chemical synthesis [69] | 150 °C/1 h and then 240 °C/2 h | Bi(NO3)3,methanol, hydrochloric acid, thiourea | NR | D: 20–40 nm L: 200–600 nm |
| Other methods | ||||
| Chemical precipitation [32] | 70 °C | Bi(NO3)3,Thioacetamide (C2H5NS), HCl | Nanoparticle | D: 10~50 nm |
| Reflux [16] | 140 °C | Bi(NO3)3, citric acid, TU, CTAB DMF, EG, PEG | NR, nanoparticle | D < 40 nm |
| Sol-gel method [51,52] | 180 °C | Bi(NO3)3, TU, polyvinyl pyrrolidone, lithium hydroxide, EG | NR | D: 200 nm |
| Hot-injection [4] | 180 °C | Bismuth chloride thioacetamide | NR | D: 7~20 nm, L: 30~70 nm |
| High power sonication process [24] | RT | Bi2S3 powder | Nanoribbons | L: ~10 μm Width (W): ~40 nm |

3. Optoelectronic Properties of Nano-Bismuth-Sulfide
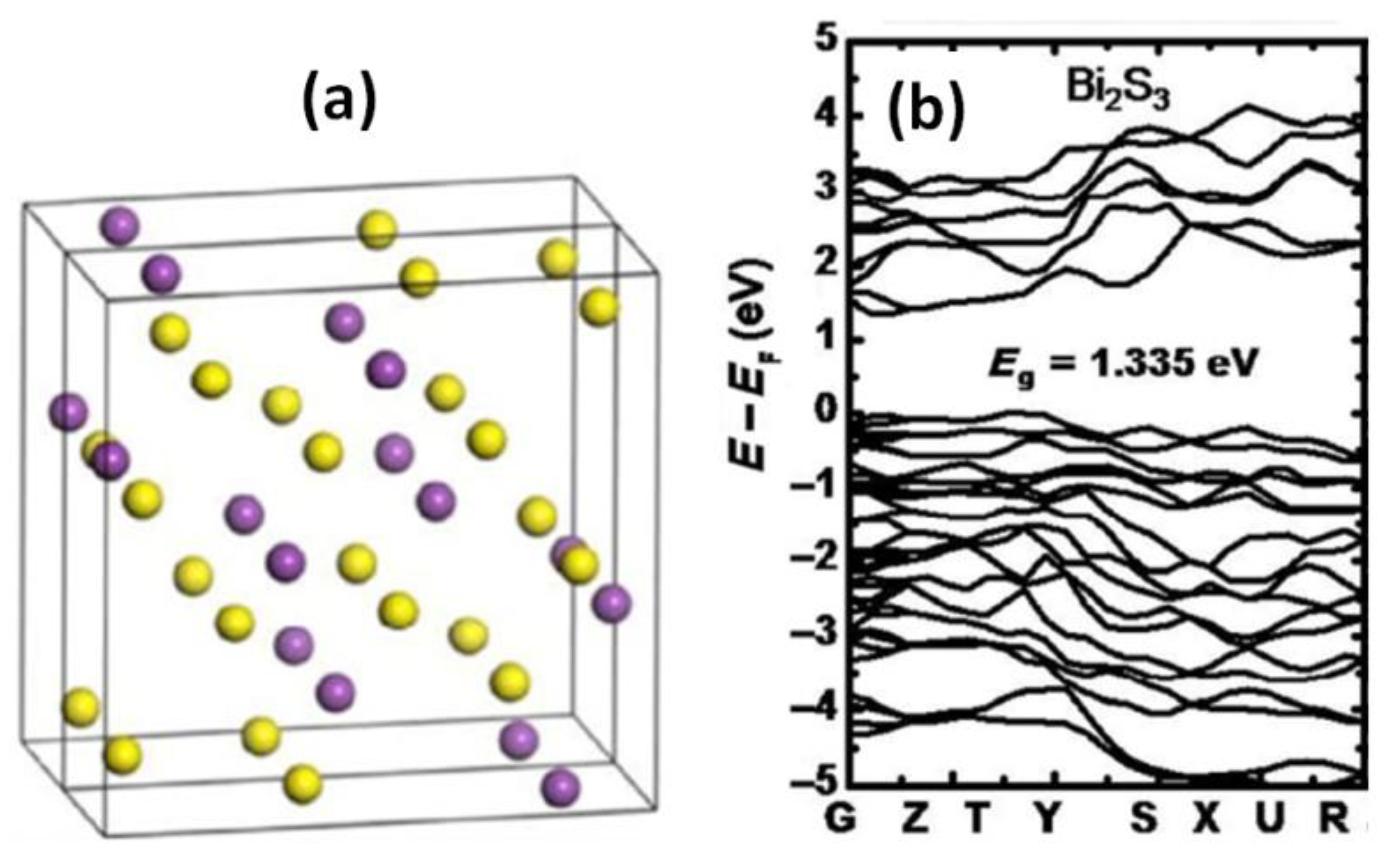
3.1. Electronic Band Structure and Conduction Properties
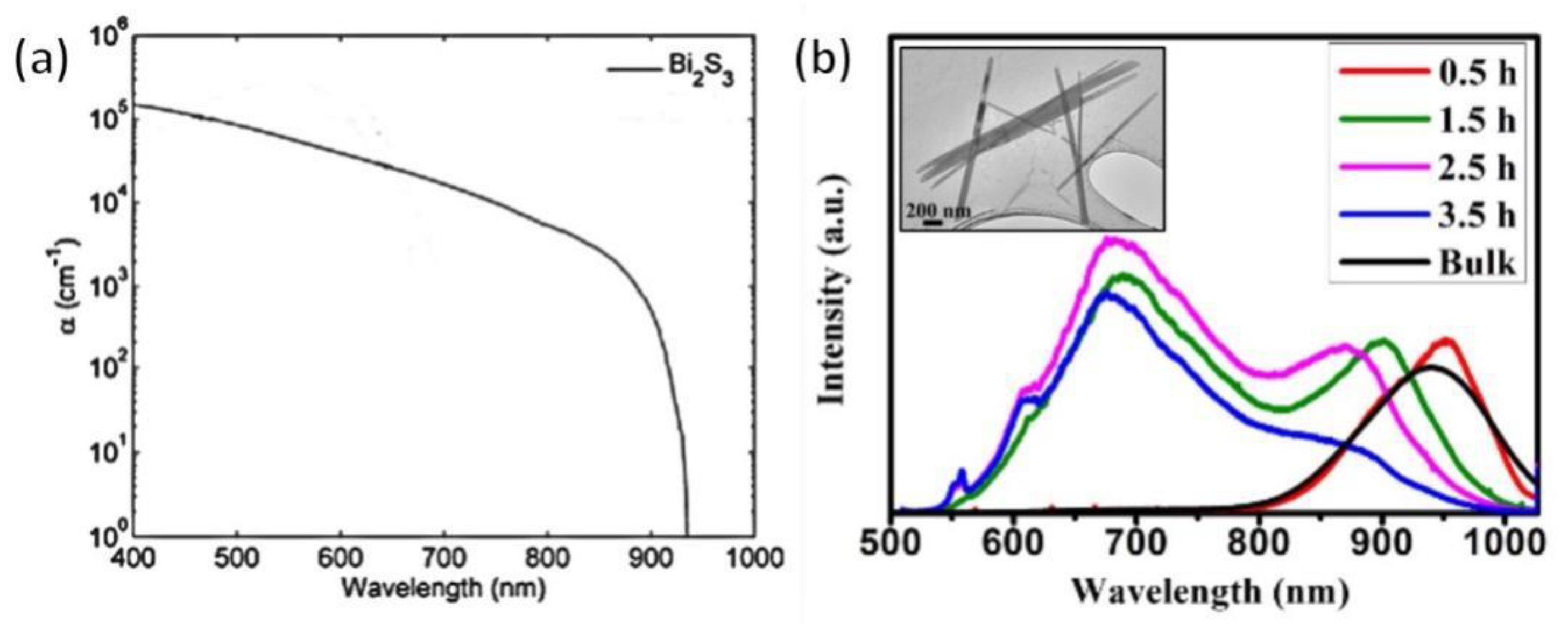
3.2. Optical Properties
4. Applications
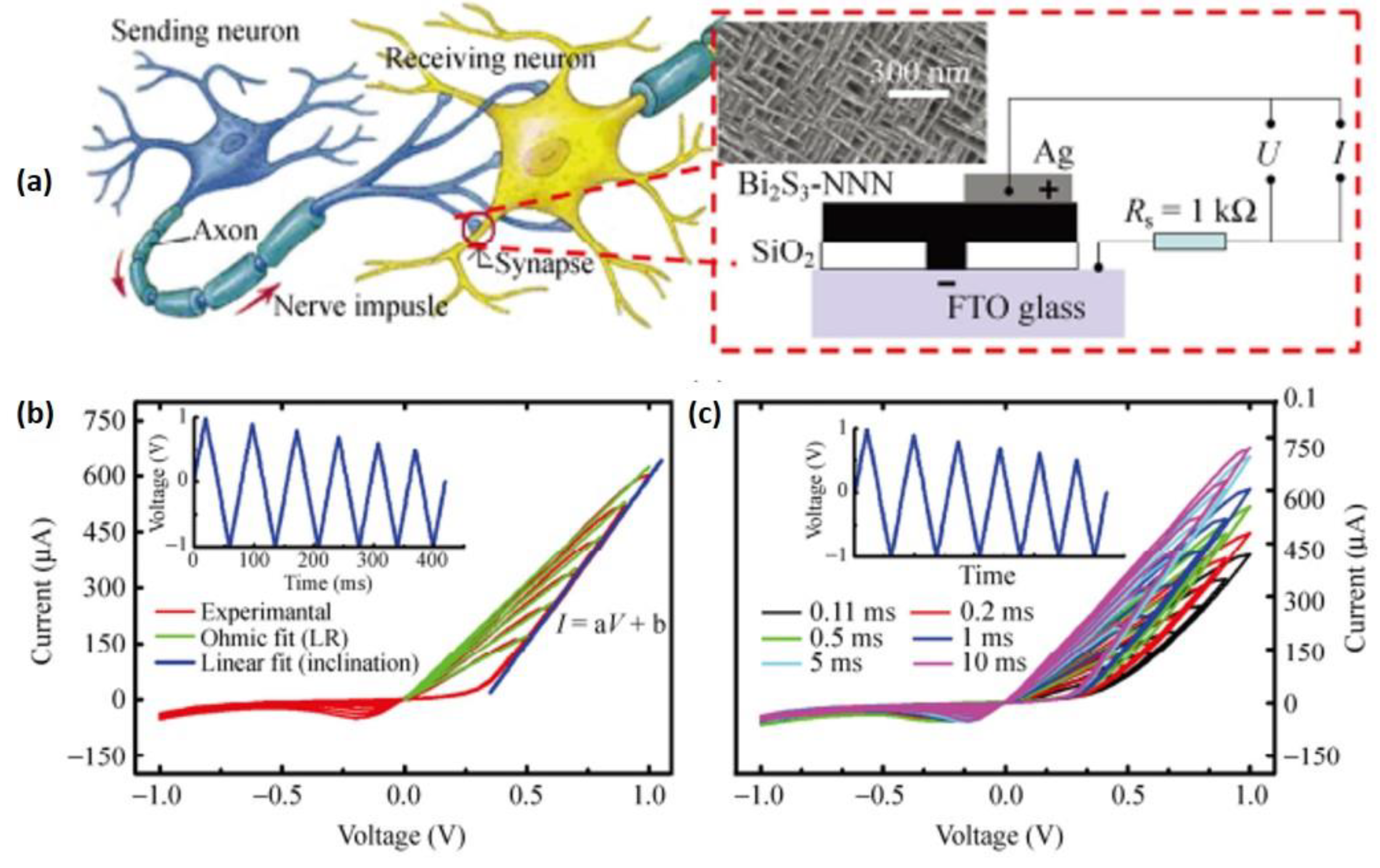
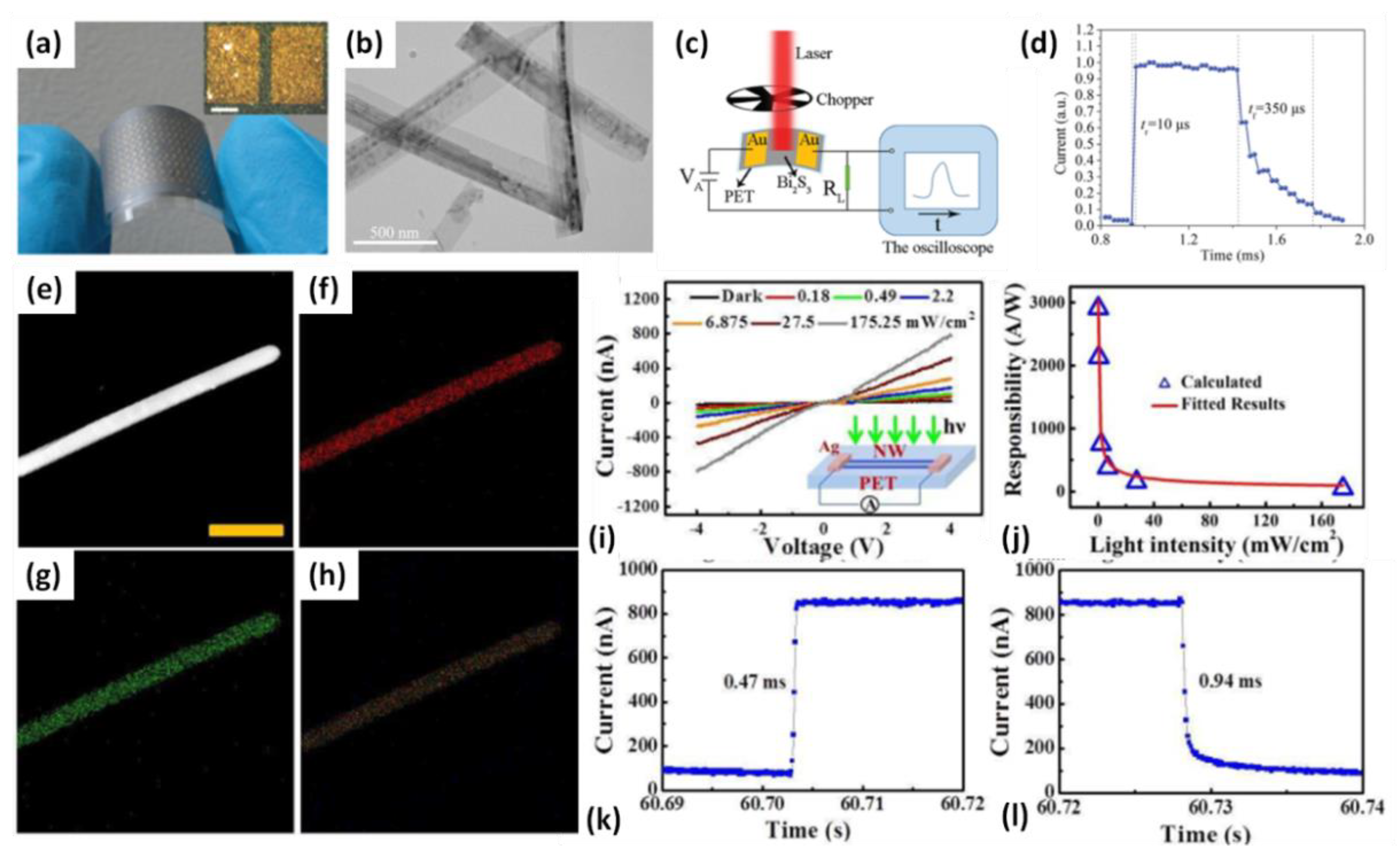
4.1. Photodetection
4.2. Photovoltaic Cell
| Samples | Wavelength | Ion/Ioff | Temporal Response (Rise/Decay) | Responsivity |
|---|---|---|---|---|
| Bi2S3 nano-networks [18] | 671 nm | / | ~3 s | / |
| Hierarchical Bi2S3 nanostructures [91] | / | / | 50/240 ms | / |
| Bi2S3/Bi2S3-xOx nanowire [50] | 475–650 nm | 44.6 | 0.47/0.93 ms | 2908.9 A/W |
| Bi2S3 nanocrystalline [47] | / | / | 23 ms | / |
| Bi2S3/BiOCl composites [99] | / | 330 | 70 ms | / |
| Bi2S3 nanorod [23,95] | 405 to 780 nm | / | 10/350 μs | 4.4 A/W |
| Bi2S3/SnS heterojunction thin film [93] | 400 to 800 nm | / | ~50 s | / |
| Bi2S3 nanorods and nanoflowers [44] | Laser@809 nm and 980 nm | ~100 | 2/3 s | / |
| Bi2S3 thin film [70] | 650 nm | / | 67.8 ms | / |
| Dandelion-shaped hierarchical Bi2S3 microsphere [92] | 650 nm | 567 | ~10 s | / |
| Bi2S3/BiOI p-n heterojunction [71] | visible | / | ~5 s | / |
| Bi2S3 Nanorods [94] | 475 nm/550 nm/650 nm | / | ~5 s | / |
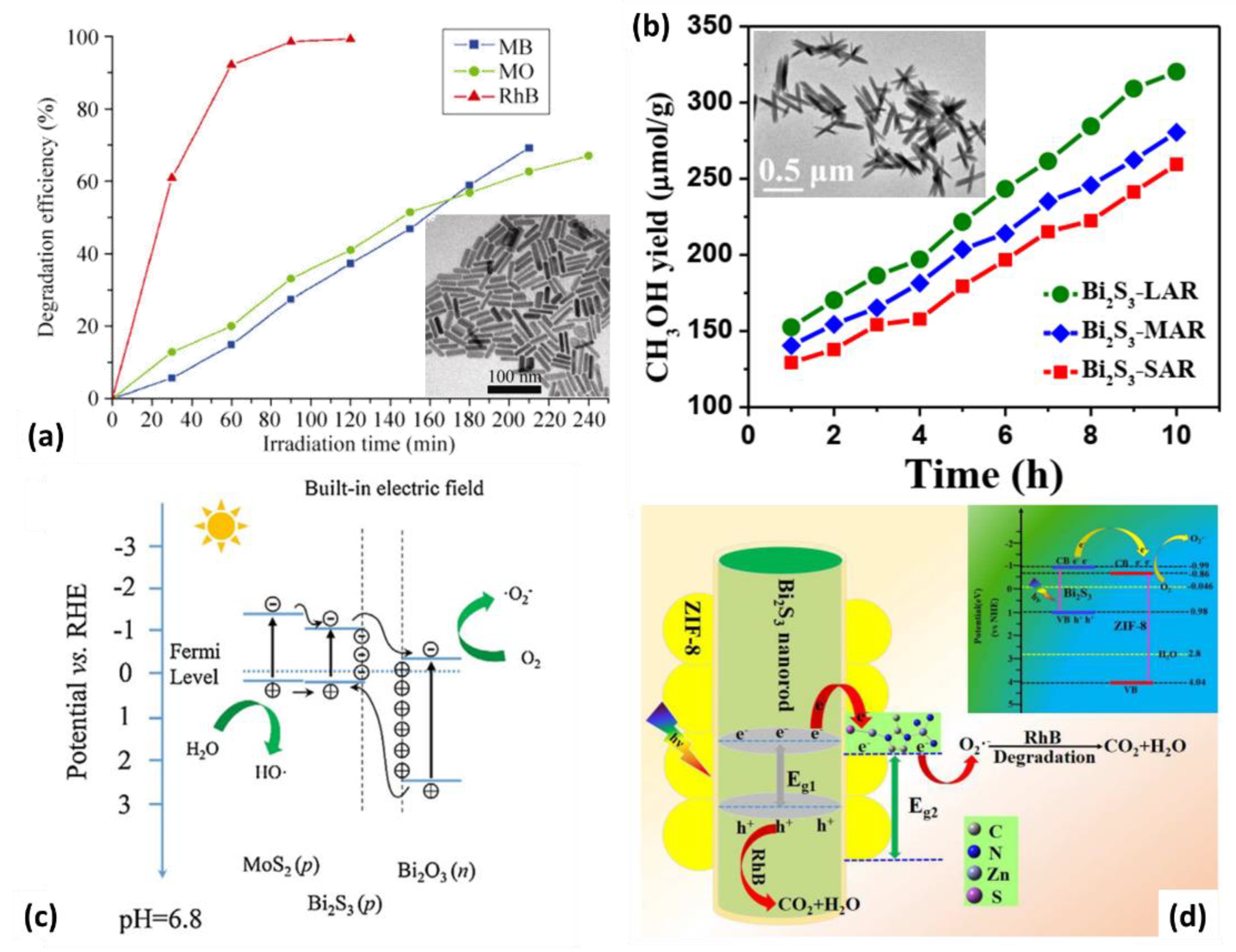
4.3. Photocatalysis
5. Conclusions and Perspectives
- (1)
- Vapor phase deposition, involving thermal evaporation, LP-MOCVD, and PLD; mainly used to prepare thin film.
- (2)
- Liquid phase deposition, involving chemical bath or electrochemical deposition, is also used to fabricate thin film.
- (3)
- Surface sulfurization can produce nano-Bi2S3 with better crystalline quality, but requires high processing temperature.
- (4)
- Chemical synthesis Bi2S3 nanostructures with a broad variety of shapes from 0D to 3D, as well as the hierarchical and heterogeneous structures of Bi2S3.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Popescu, M. Chalcogenides—Past, present, future. J. Non-Cryst. Solids 2006, 352, 887–891. [Google Scholar] [CrossRef]
- Sousa, V. Chalcogenide materials and their application to Non-Volatile Memories. Microelectron. Eng. 2011, 88, 807–813. [Google Scholar] [CrossRef]
- Moore, J.E. The birth of topological insulators. Nature 2010, 464, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhou, X.; Zhang, H.; Zhong, X. Bi2S3 nanostructures: A new photocatalyst. Nano Res. 2010, 3, 379–386. [Google Scholar] [CrossRef]
- Sanghera, J.S.; Shaw, L.B.; Aggarwal, I.D. Chalcogenide Glass-Fiber-Based Mid-IR Sources and Applications. IEEE J. Sel. Top. Quantum Electron. 2009, 15, 114–119. [Google Scholar] [CrossRef]
- Toudert, J.; Serna, R. Interband transitions in semi-metals, semiconductors, and topological insulators: A new driving force for plasmonics and nanophotonics [Invited]. Opt. Mater. Express 2017, 7, 2299–2325. [Google Scholar] [CrossRef]
- Tian, Y.; Toudert, J. Nanobismuth: Fabrication, Optical, and Plasmonic Properties—Emerging Applications. J. Nanotechnol. 2018, 2018, 3250932. [Google Scholar] [CrossRef]
- Panmand, R.P.; Kulkarni, M.V.; Valant, M.; Gosavi, S.W.; Kale, B.B. Quantum confinement of Bi2S3 in glass with magnetic behavior. AIP Adv. 2013, 3, 022123. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Z.; Sheng, L.; Shen, R.; Xing, D.Y. Magnetoresistance in an ultrathin Bi2Se3 film between two ferromagnetic insulators. Appl. Phys. Lett. 2011, 99, 182101. [Google Scholar] [CrossRef]
- Lu, C.; Li, Z.; Yu, L.; Zhang, L.; Xia, Z.; Jiang, T.; Yin, W.; Dou, S.; Liu, Z.; Sun, J. Nanostructured Bi2S3 encapsulated within three-dimensional N-doped graphene as active and flexible anodes for sodium-ion batteries. Nano Res. 2018, 11, 4614–4626. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, D.; Ni, J.; Gao, L.; Yang, J.; Dongliang, Y. One-pot facile fabrication of carbon-coated Bi2S3 nanomeshes with efficient Li-storage capability. Nano Res. 2014, 7, 765–773. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, Q.L.; Guo, J.; Feng, J.; Ge, Z.H. Enhanced thermoelectric properties of Bi2S3 polycrystals through an electroless nickel plating process. RSC Adv. 2019, 9, 23029–23035. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, H.; Ouerghui, W. Spin–orbit coupling effect on electronic, linear and nonlinear optical properties of Bi2S3 and the ternary bismuth sulfide Bi2S2.75Se0.25: Ab-initio calculations. Opt. Quantum Electron. 2021, 54, 20. [Google Scholar] [CrossRef]
- Han, M.; Guo, H.; Li, B.; Jia, J.; Wang, W. Controllable coverage of Bi2S3 quantum dots on one-dimensional TiO2 nanorod arrays by pulsed laser deposition technique for high photoelectrochemical properties. New J. Chem. 2017, 41, 4820–4827. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Salimi, A. Facile Synthesis of Ultra-wide Two Dimensional Bi2S3 Nanosheets: Characterizations, Properties and Applications in Hydrogen Peroxide Sensing and Hydrogen Storage. Electroanalysis 2017, 29, 2027–2035. [Google Scholar] [CrossRef]
- Arumugam, J.; Raj, A.D.; Irudayaraj, A.A. Solvent effects on the properties of Bi2S3 nanoparticles: Photocatalytic application. J. Mater. Sci. Mater. Electron. 2017, 28, 3487–3494. [Google Scholar] [CrossRef]
- Chitara, B.; Kolli, B.S.; Yan, F. Near-Infrared Photodetectors Based on 2D Bi2S3. Chem. Phys. Lett. 2022, 804, 139876. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, S.; Tan, W. Improved optical and electrical switching in Bi2S3 nested nano-networks with broad trap distribution. Appl. Nanosci. 2022, 12, 2023–2030. [Google Scholar] [CrossRef]
- Tian, Y.; Pan, L.; Guo, C.F.; Liu, Q. Atomic origin of the traps in memristive interface. Nano Res. 2017, 10, 1924–1931. [Google Scholar] [CrossRef]
- Schricker, A.D.; Sigman, M.B., Jr.; Korgel, B.A. Electrical transport, Meyer–Neldel rule and oxygen sensitivity of Bi2S3 nanowires. Nanotechnology 2005, 16, S508. [Google Scholar] [CrossRef]
- Stallinga, P.; Gomes, H. Trap states as an explanation for the Meyer–Neldel rule in semiconductors. Org. Electron. 2005, 6, 137–141. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, C.; Guo, S.; Yu, T.; Liu, Q. Bivariate-continuous-tunable interface memristor based on Bi2S3 nested nano-networks. Nano Res. 2014, 7, 953–962. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, C.F.; Zhang, J.; Liu, Q. Operable persistent photoconductivity of Bi2S3 nested nano-networks. Phys. Chem. Chem. Phys. 2015, 17, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Schricker, A.D. Electrical Properties of Single GaAs, Bi2S3 and Ge Nanowires; University of Texas Libraries: San Antonio, TX, USA, 2005. [Google Scholar]
- Yu, H.; Wang, J. Bismuth Sulfide (Bi2S3) Nanorods as Efficient Photodetection Materials. In Proceedings of the 2016 5th International Conference on Advanced Materials and Computer Science, Qingdao, China, 26–27 March; pp. 935–938.
- Dhar, N.; Syed, N.; Mohiuddin, M.; Jannat, A.; Zavabeti, A.; Zhang, B.Y.; Datta, R.S.; Atkin, P.; Mahmood, N.; Esrafilzadeh, D.; et al. Exfoliation Behavior of van der Waals Strings: Case Study of Bi2S3. ACS Appl. Mater. Interfaces 2018, 10, 42603–42611. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, J.; Tian, Y.; Liu, Q. A General Strategy to Superstructured Networks and Nested Self-Similar Networks of Bismuth Compounds. ACS Nano 2012, 6, 8746–8752. [Google Scholar] [CrossRef]
- Li, D.-B.; Hu, L.; Xie, Y.; Niu, G.; Liu, T.; Zhou, Y.; Gao, L.; Yang, B.; Tang, J. Low-Temperature-Processed Amorphous Bi2S3 Film as an Inorganic Electron Transport Layer for Perovskite Solar Cells. ACS Photonics 2016, 3, 2122–2128. [Google Scholar] [CrossRef]
- Martinez, L.; Bernechea, M.; de Arquer, F.P.G.; Konstantatos, G. Near IR-Sensitive, Non-toxic, Polymer/Nanocrystal Solar Cells Employing Bi2S3 as the Electron Acceptor. Adv. Energy Mater. 2011, 1, 1029–1035. [Google Scholar] [CrossRef]
- Li, L.; Sun, N.; Huang, Y.; Qin, Y.; Zhao, N.; Gao, J.; Li, M.; Zhou, H.; Qi, L. Topotactic Transformation of Single-Crystalline Precursor Discs into Disc-Like Bi2S3 Nanorod Networks. Adv. Funct. Mater. 2008, 18, 1194–1201. [Google Scholar] [CrossRef]
- Messalea, K.A.; Zavabeti, A.; Mohiuddin, M.; Syed, N.; Jannat, A.; Atkin, P.; Ahmed, T.; Walia, S.; McConville, C.F.; Kalantar-Zadeh, K.; et al. Two-Step Synthesis of Large-Area 2D Bi2S3 Nanosheets Featuring High In-Plane Anisotropy. Adv. Mater. Interfaces 2020, 7, 2001131. [Google Scholar] [CrossRef]
- Chen, X.L.; Yan, K.; Chu, H.Y. Preheating Effects on Bi2S3 Morphology Synthesized via Hydrothermal Method. Adv. Mater. Res. 2013, 631–632, 298–302. [Google Scholar] [CrossRef]
- Wang, Z.J.; Qu, S.C.; Xu, Y.; Chen, Y.H.; Zeng, X.B.; Liu, J.P.; Wu, J.; Wang, Z.G. Solventless Synthesis of Bi2S3 Nanowires and their Application to Solar Cells. Adv. Mater. Res. 2007, 26–28, 601–607. [Google Scholar] [CrossRef]
- Reynoso, Y.R.; Martinez-Ayala, A.; Pal, M.; Paraguay-Delgado, F.; Mathews, N. Bi2S3 nanoparticles by facile chemical synthesis: Role of pH on growth and physical properties. Adv. Powder Technol. 2018, 29, 3561–3568. [Google Scholar] [CrossRef]
- Gao, C.; Shen, H.; Sun, L. Preparation of nanowall Bi2S3 films by chemical bath deposition. Appl. Surf. Sci. 2011, 258, 89–92. [Google Scholar] [CrossRef]
- Maria, C.C.S.; Patil, R.A.; Hasibuan, D.P.; Saragih, C.S.; Lai, C.-C.; Liou, Y.; Ma, Y.-R. White-light photodetection enhancement and thin film impediment in Bi2S3 nanorods/thin-films homojunction photodetectors. Appl. Surf. Sci. 2022, 584, 152608. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Zhou, Y.; Pang, Q.; Shao, J.; Wang, G.; Wang, J.; Zhao, L.-D. Enhancing the thermoelectric performance of Bi2S3: A promising earth-abundant thermoelectric material. Front. Phys. 2018, 14, 13601. [Google Scholar] [CrossRef]
- Bernechea, M.; Cao, Y.; Konstantatos, G. Size and bandgap tunability in Bi2S3 colloidal nanocrystals and its effect in solution processed solar cells. J. Mater. Chem. A 2015, 3, 20642–20648. [Google Scholar] [CrossRef]
- Whittaker-Brooks, L.; Gao, J.; Hailey, A.K.; Thomas, C.R.; Yao, N.; Loo, Y.L. Bi2S3 nanowire networks as electron acceptor layers in solution-processed hybrid solar cells. J. Mater. Chem. C 2015, 3, 2686–2692. [Google Scholar] [CrossRef]
- Peter, L.M.; Wijayantha, K.U.; Riley, D.J.; Waggett, J.P. Band-edge tuning in self-assembled layers of Bi2S3 nanoparticles used to photosensitize nanocrystalline TiO2. J. Phys. Chem. B 2003, 107, 8378–8381. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, C.H.; Wang, R.H.; Chen, Q.; Peng, L.M. High-Quality Ultralong Bi2S3 Nanowires: Structure, Growth, and Properties. J. Phys. Chem. B 2005, 109, 18772–18776. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Pramanik, P. Semiconductor Liquid Junction Solar Cell Based on Chemically Deposited Bi2S3 Thin Film and Some Semiconducting Properties of Bismuth Chalcogenides. J. Electrochem. Soc. 1982, 129, 332–335. [Google Scholar] [CrossRef]
- Huang, J.F.; Wang, Y.; Cao, L.Y.; Zhu, H.; Zeng, X.R. Effects of pH Value on the Structures and Optical Properties of Bi2S3 Thin Films. Key Eng. Mater. 2010, 434–435, 397–399. [Google Scholar] [CrossRef]
- Desai, J.D.; Lokhande, C.D. Nonaqueous chemical bath deposition of Bi2S3 thin films. Mater. Chem. Phys. 1993, 34, 313–316. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, W.-T. Uniform Bi2S3 nanowires: Structure, growth, and field-effect transistors. Mater. Lett. 2009, 63, 1917–1920. [Google Scholar] [CrossRef]
- Chao, J.; Xing, S.; Liu, Z.; Zhang, X.; Zhao, Y.; Zhao, L.; Fan, Q. Large-scale synthesis of Bi2S3 nanorods and nanoflowers for flexible near-infrared laser detectors and visible light photodetectors. Mater. Res. Bull. 2018, 98, 194–199. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Lei, Y.; Li, P.; Jia, H.; Hou, H.; Zheng, Z. In situ fabrication of Bi2S3 nanocrystal film for photovoltaic devices. Mater. Sci. Eng. B 2012, 177, 1764–1768. [Google Scholar] [CrossRef]
- Liu, W.; Guo, C.F.; Yao, M.; Lan, Y.; Zhang, H.; Zhang, Q.; Chen, S.; Opeil, C.P.; Ren, Z. Bi2S3 nanonetwork as precursor for improved thermoelectric performance. Nano Energy 2014, 4, 113–122. [Google Scholar] [CrossRef]
- Konstantatos, G.; Levina, L.; Tang, J.; Sargent, E.H. Sensitive solution-processed Bi2S3 nanocrystalline photodetectors. Nano Lett. 2008, 8, 4002–4006. [Google Scholar] [CrossRef]
- He, D.; Wang, Y.; Song, S.; Liu, S.; Luo, Y.; Deng, Y. Polymer-based nanocomposites employing Bi2S3@SiO2 nanorods for high dielectric performance: Understanding the role of interfacial polarization in semiconductor-insulator core-shell nanostructure. Compos. Sci. Technol. 2017, 151, 25–33. [Google Scholar] [CrossRef]
- Ding, Y.H.; Zhang, X.L.; Zhang, N.; Zhang, J.Y.; Zhang, R.; Liu, Y.F.; Fang, Y.Z. A visible-light driven Bi2S3@ZIF-8 core–shell heterostructure and synergistic photocatalysis mechanism. Dalton Trans. 2018, 47, 684–692. [Google Scholar] [CrossRef]
- Errando-Herranz, C.; Takabayashi, A.Y.; Edinger, P.; Sattari, H.; Gylfason, K.B.; Quack, N. MEMS for Photonic Integrated Circuits. IEEE J. Sel. Top. Quantum Electron. 2020, 26, 1–16. [Google Scholar] [CrossRef]
- Xie, Y.-Y.; Ni, P.-N.; Wang, Q.-H.; Kan, Q.; Briere, G.; Chen, P.-P.; Zhao, Z.-Z.; Delga, A.; Ren, H.-R.; Chen, H.-D.; et al. Metasurface-integrated vertical cavity surface-emitting lasers for programmable directional lasing emissions. Nat. Nanotechnol. 2020, 15, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Rife, J.C.; Miller, M.M.; Sheehan, P.E.; Tamanaha, C.R.; Tondra, M.; Whitman, L.J. Design and performance of GMR sensors for the detection of magnetic microbeads in biosensors. Sens. Actuators A Phys. 2003, 107, 209–218. [Google Scholar] [CrossRef]
- Monteiro, O.C.; Trindade, T.; Park, J.-H.; O’Brien, P. The LP-MOCVD of CdS/Bi2S3 bilayers using single-molecule precursors. Mater. Lett. 2004, 58, 119–122. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, S.; Tan, W. Oxygen-doping to Bi2S3 thin film and its substrate-dependent resistive switching. Mater. Res. Express 2019, 6, 116324. [Google Scholar] [CrossRef]
- Jin, J.; He, T. Facile synthesis of Bi2S3 nanoribbons for photocatalytic reduction of CO2 into CH3OH. Appl. Surf. Sci. 2017, 394, 364–370. [Google Scholar] [CrossRef]
- Ma, J.; Yang, J.; Jiao, L.; Wang, T.; Lian, J.; Duan, X.; Zheng, W. Bi2S3 nanomaterials: Morphology manipulation and related properties. Dalton Trans. 2011, 40, 10100–10108. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, M.; Zhang, Z.; Zapien, J.A.; Wang, X.; Lee, J.-M. Hierarchical self-assembled Bi2S3 hollow nanotubes coated with sulfur-doped amorphous carbon as advanced anode materials for lithium ion batteries. Nanoscale 2018, 10, 13343–13350. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, C.F.; Guo, Y.; Wang, Q.; Liu, Q. BiOCl nanowire with hierarchical structure and its Raman features. Appl. Surf. Sci. 2012, 258, 1949–1954. [Google Scholar] [CrossRef]
- Saitou, M.; Yamaguchi, R.; Oshikawa, W. Novel process for electrodeposition of Bi2S3 thin films. Mater. Chem. Phys. 2002, 73, 306–309. [Google Scholar] [CrossRef][Green Version]
- Mane, R.; Sankapal, B.; Lokhande, C. A chemical method for the deposition of Bi2S3 thin films from a non-aqueous bath. Thin Solid Film. 2000, 359, 136–140. [Google Scholar] [CrossRef]
- Chai, W.; Yang, F.; Yin, W.; You, S.; Wang, K.; Ye, W.; Rui, Y.; Tang, B. Bi2S3/C nanorods as efficient anode materials for lithium-ion batteries. Dalton Trans. 2019, 48, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-Q.; Zheng, Y.-J.; He, Z.-G.; Lu, Z.-X.; Wang, L.; Li, C.-F.; Jiao, F.-P.; Deng, C.-Y. Synthesis of Bi2S3 microsphere and its efficient photocatalytic activity under visible-light irradiation. Trans. Nonferrous Met. Soc. China 2018, 28, 2002–2010. [Google Scholar] [CrossRef]
- Chen, J.; Qin, S.; Song, G.; Xiang, T.; Xin, F.; Yin, X. Shape-controlled solvothermal synthesis of Bi2S3 for photocatalytic reduction of CO2 to methyl formate in methanol. Dalton Trans. 2013, 42, 15133–15138. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, Z.; Zhou, J.; Pan, A.; Liang, S. Chrysanthemum-like Bi2S3 nanostructures: A promising anode material for lithium-ion batteries and sodium-ion batteries. J. Alloys Compd. 2017, 715, 432–437. [Google Scholar] [CrossRef]
- Yan, X.; Gu, Y.; Li, C.; Zheng, B.; Li, Y.; Zhang, T.; Zhang, Z.; Yang, M. Morphology-controlled synthesis of Bi2S3 nanorods-reduced graphene oxide composites with high-performance for electrochemical detection of dopamine. Sens. Actuators B Chem. 2018, 257, 936–943. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, H.; Li, X.; Gong, Z.; Wang, X.; Peng, X.; He, M.; Sheng, Z. Wet chemical synthesis of Bi2S3 nanorods for efficient photocatalysis. Mater. Lett. 2013, 105, 12–15. [Google Scholar] [CrossRef]
- Song, H.; Zhan, X.; Li, D.; Zhou, Y.; Yang, B.; Zeng, K.; Zhong, J.; Miao, X.; Tang, J. Rapid thermal evaporation of Bi2S3 layer for thin film photovoltaics. Sol. Energy Mater. Sol. Cells 2016, 146, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Dai, J.Y.; Zhu, H.X.; Hong, A.J.; Zhou, X.H.; Ren, Z.F.; Liu, J.M. Thermoelectric property studies on CuxBi2SeS2 with nano-scale precipitates Bi2S3. Nano Energy 2015, 12, 447–456. [Google Scholar] [CrossRef]
- Ke, J.; Liu, J.; Sun, H.; Zhang, H.; Duan, X.; Liang, P.; Li, X.; Tade, M.O.; Liu, S.; Wang, S. Facile assembly of Bi2O3/Bi2S3/MoS2 n-p heterojunction with layered n-Bi2O3 and p-MoS2 for enhanced photocatalytic water oxidation and pollutant degradation. Appl. Catal. B Environ. 2017, 200, 47–55. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.; Feng, H.; Jin, R.; Zhang, S.; Gao, S. Engineering Bi2S3/BiOI p-n heterojunction to sensitize TiO2 nanotube arrays photoelectrodes for highly efficient solar cells and photocatalysts. Ceram. Int. 2019, 45, 3995–4002. [Google Scholar] [CrossRef]
- Adhikari, S.; Kim, D.-H. Synthesis of Bi2S3/Bi2WO6 hierarchical microstructures for enhanced visible light driven photocatalytic degradation and photoelectrochemical sensing of ofloxacin. Chem. Eng. J. 2018, 354, 692–705. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Xing, C.; Jiang, D.; Xie, J.; Chen, M. Controlled synthesis of Bi2S3/ZnS microspheres by an in situ ion-exchange process with enhanced visible light photocatalytic activity. Dalton Trans. 2013, 42, 12980–12988. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Zai, J.; Xu, M.; Zhu, Q.; Wei, X.; Qian, X. Novel Bi2S3/Bi2O2CO3 heterojunction photocatalysts with enhanced visible light responsive activity and wastewater treatment. J. Mater. Chem. A 2014, 2, 4208–4216. [Google Scholar] [CrossRef]
- Zeng, Q.; Bai, J.; Li, J.; Li, Y.; Li, X.; Zhou, B. Combined nanostructured Bi2S3/TNA photoanode and Pt/SiPVC photocathode for efficient self-biasing photoelectrochemical hydrogen and electricity generation. Nano Energy 2014, 9, 152–160. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, C.; Tian, Y.; Liu, Q. Superstructure transformations from hexagonal to tetragonal microplates and nested two-dimensional nanonetworks. Chin. Sci. Bull. 2014, 59, 1787–1793. [Google Scholar] [CrossRef]
- Chen, L.; He, J.; Yuan, Q.; Zhang, Y.W.; Wang, F.; Au, C.T.; Yin, S.F. CuS–Bi2S3 hierarchical architectures: Controlled synthesis and enhanced visible-light photocatalytic performance for dye degradation. RSC Adv. 2015, 5, 33747–33754. [Google Scholar] [CrossRef]
- Zhao, Y.; Chua, K.T.E.; Gan, C.K.; Zhang, J.; Peng, B.; Peng, Z.; Xiong, Q. Phonons in Bi2S3 nanostructures: Raman scattering and first-principles studies. Phys. Rev. B 2011, 84, 205330. [Google Scholar] [CrossRef]
- Shaban, H.; Nassary, M.; El-Sadek, M.S.A. Transport properties of Bi2S3 single crystals. Phys. B Condens. Matter 2008, 403, 1655–1659. [Google Scholar] [CrossRef]
- Yang, J.; Liu, G.; Yan, J.; Zhang, X.; Shi, Z.; Qiao, G. Enhanced the thermoelectric properties of n-type Bi2S3 polycrystalline by iodine doping. J. Alloy. Compd. 2017, 728, 351–356. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, L.; Zhang, X.; Zhang, G. A trap-limited-current-based model of Meyer–Neldel rule and its connection to persistent photocurrent. J. Phys. D Appl. Phys. 2016, 49, 405107. [Google Scholar] [CrossRef]
- Liu, Z.; Pei, Y.; Geng, H.; Zhou, J.; Meng, X.; Cai, W.; Liu, W.; Sui, J. Enhanced thermoelectric performance of Bi2S3 by synergistical action of bromine substitution and copper nanoparticles. Nano Energy 2015, 13, 554–562. [Google Scholar] [CrossRef]
- Kulbashinskii, V.; Kytin, V.; Maslov, N.; Singha, P.; Das, S.; Deb, A.; Banerjee, A. Thermoelectrical properties of Bi2Te3 nanocomposites. Mater. Today Proc. 2019, 8, 573–581. [Google Scholar] [CrossRef]
- Fang, J.; Chen, F.; Stokes, K.; He, J.; Tang, J.; O’Connor, C.J. Synthesis and Thermoelectric Properties of Bi2S3 Nanobeads. MRS Online Proc. Libr. 2011, 730, 55. [Google Scholar] [CrossRef]
- Xi, H.; Liu, Q.; Tian, Y.; Wang, Y.; Guo, S.; Chu, M. Ge2Sb1.5Bi0.5Te5 thin film as inorganic photoresist. Opt. Mater. Express 2012, 2, 461–468. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, C.; Wang, Y.; Miao, J.; Tian, Y.; Liu, Q. Micro-optical elements fabricated by metal-transparent-metallic-oxides grayscale photomasks. Appl. Opt. 2012, 51, 6606–6611. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, W.; Zhao, H.; Zhang, X.; Liang, X.; Dai, S.; Chen, F. Third-order nonlinear optical properties of Bi2S3 nanocrystals doped in sodium borosilicate glass studied with Z-scan technique. Mater. Res. Bull. 2011, 46, 355–360. [Google Scholar] [CrossRef]
- Yu, X.; Cao, C.; Zhu, H. Synthesis and photoluminescence properties of Bi2S3 nanowires via surfactant micelle-template inducing reaction. Solid State Commun. 2005, 134, 239–243. [Google Scholar] [CrossRef]
- Jariwala, S.; Sun, H.; Adhyaksa, G.W.; Lof, A.; Muscarella, L.; Ehrler, B.; Garnett, E.C.; Ginger, D.S. Local Crystal Misorientation Influences Non-radiative Recombination in Halide Perovskites. Joule 2019, 3, 3048–3060. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, J.; Guo, C.F.; Zhang, B.; Liu, Q. Photoconductive probing of the trap distribution in switchable interfaces. Nanoscale 2016, 8, 915–920. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Zhang, J.; Zhou, M. Facile synthesis of hierarchical Bi2S3 nanostructures for photodetector and gas sensor. RSC Adv. 2012, 2, 6258–6261. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Zhang, P.; Deng, Q.; Zhang, J.; Jiang, K.; Hu, Z.; Chu, J. Superior adsorption and photoinduced carries transfer behaviors of dandelion-shaped Bi2S3@MoS2: Experiments and theory. Sci. Rep. 2017, 7, 42484. [Google Scholar] [CrossRef] [PubMed]
- Jamali-Sheini, F.; Cheraghizade, M.; Heshmatynezhad, L. An efficient wide range photodetector fabricated using a bilayer Bi2S3/SnS heterojunction thin film. Semicond. Sci. Technol. 2019, 34, 045008. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Wang, T.; Yu, H.; Yang, J.; Liu, G.; Qiao, G.; Yang, Q.; Cheng, X. Scalable colloidal synthesis of uniform Bi2S3 nanorods as sensitive materials for visible-light photodetectors. CrystEngComm 2017, 19, 727–733. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.; Dai, G.; Su, W.; Sun, Y.; Hou, J.; Zhang, N.; Zhao, G.; Fang, Y.; Dai, N. Single Bi2S3/Bi2S3-xOx nanowire photodetector with broadband response from ultraviolet to near-infrared range. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 120, 114041. [Google Scholar] [CrossRef]
- Enke, L.; Bingsheng, Z.; Jinsheng, L. Semiconductor Physics; Publishing House of Electronics Industry: Beijing, China, 2003. [Google Scholar]
- Chao, J.; Li, H.; Xing, S. Selective synthesis of Bi2S3/BiOCl composites as electrode for high performance photodetector. Solid State Sci. 2019, 98, 106034. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Zheng, K.; Ding, T.; Wang, W.; Jiang, Y.; Yang, Q. Fabrication of Ultrathin Bi2S3 Nanosheets for High-Performance, Flexible, Visible–NIR Photodetectors. Small 2015, 11, 2848–2855. [Google Scholar] [CrossRef]
- Esparza, D.; Zarazúa, I.; López-Luke, T.; Carriles, R.; Castro, A.T.; De la Rosa, E. Photovoltaic Properties of Bi2S3 and CdS Quantum Dot Sensitized TiO2 Solar Cells. Electrochim. Acta 2015, 180, 486–492. [Google Scholar] [CrossRef]
- Moreno-García, H.; Nair, M.; Nair, P. All-chemically deposited Bi2S3/PbS solar cells. Thin Solid Films 2011, 519, 7364–7368. [Google Scholar] [CrossRef]
- Moreno-García, H.; Nair, M.T.S.; Nair, P.K. Chemically deposited lead sulfide and bismuth sulfide thin films and Bi2S3/PbS solar cells. Thin Solid Films 2011, 519, 2287–2295. [Google Scholar] [CrossRef]
- Fang, M.; Jia, H.; He, W.; Lei, Y.; Zhang, L.; Zheng, Z. Construction of flexible photoelectrochemical solar cells based on ordered nanostructural BiOI/Bi2S3 heterojunction films. Phys. Chem. Chem. Phys. 2015, 17, 13531–13538. [Google Scholar] [CrossRef]
- Salunkhe, D.B.; Dubal, D.P.; Sali, J.V.; Sankapal, B.R. Linker free synthesis of TiO2/Bi2S3 heterostructure towards solar cell application: Facile chemical routes. Mater. Sci. Semicond. Process. 2015, 30, 335–342. [Google Scholar] [CrossRef]
- Han, M.; Jia, J. 3D Bi2S3/TiO2 cross-linked heterostructure: An efficient strategy to improve charge transport and separation for high photoelectrochemical performance. J. Power Sources 2016, 329, 23–30. [Google Scholar] [CrossRef]




| Method | Growth Condition | Start Materials | Product | Size |
|---|---|---|---|---|
| Topotactical transformation [72] | 180 °C | Bi(NO3)3, TU | Bi2S3/Bi2WO6 hierarchical microstructures | D: ~2 μm |
| Topotactical transformation [76] | 80 °C | BiOCl, TAA | Bi2S3 hierarchical microstructures | D: 30~200 nm |
| In situ ion-exchange process [73] | 120 °C | BiCl3, ethanol. | Bi2S3/ZnS microspheres | D: 200~500 nm |
| Solvothermal method [77] | 160 °C | Bi(NO3)3, glycol, L-lysine, CuCl2 | CuS–Bi2S3 microspheres and cockscomb-like structures | D: 500–5 μm |
| Hydrothermal route [10] | 180 °C | Thioacetamide, ethanol, glycerol, BiCl3 | Nanostructured Bi2S3 encapsulated within 3D N-doped graphene | 500–2000 nm |
| Sample | Electrical Conductivity (S/cm) | Thermal Conductivity (W·m−1·K−1) | Seebeck Coefficient (μV/K) | Power Factor (μW·cm−1·K−2) | ZT Value |
|---|---|---|---|---|---|
| Bi2S3 powder [12] | 7.153@628 K | 0.54~0.75 | 390~440 | ~1.15@628 K | ~0.11@628 K |
| Bi2S3@Ni powder [12] | 28.9~38.4 | 0.4~0.48 | 180~291 | 2.44@628 K | 0.38@628 K |
| Pristine Bi2S3 [80] | 2.6 | 0.45~0.85 | 455@673 K | ~1.6 | ~0.15@773 K |
| I-doping Bi2S3 [80] | ~30 | 0.42~0.82 | 375@773 K | 3.1 | 0.58@773 K |
| Bi2S3 nanobeads [84] | ~160@RT | / | ~65 | / | / |
| Bi2S3 nanoparticles [32] | / | / | 315~375 | / | / |
| CuBr2 doping Bi2S3 [82] | 2.2 | 1.3 | 418.5 | 1. | ~0.1@773 K |
| 187.6 | 1.0 | 155.9 | / | ~0.4@773 K | |
| 309.6 | 1.0 | 113.9 | / | 0.72@773 K | |
| 225.2 | 0.7 | 114.6 | / | ~0.5@773 K | |
| Se and Cl doping Bi2S3 [35] | / | / | / | 2.0 | ~0.6@723 K |
| Surface-treated Bi2S3 nanonetwork [76] | 333 | / | 56.8 | / | 0.5@723 K |
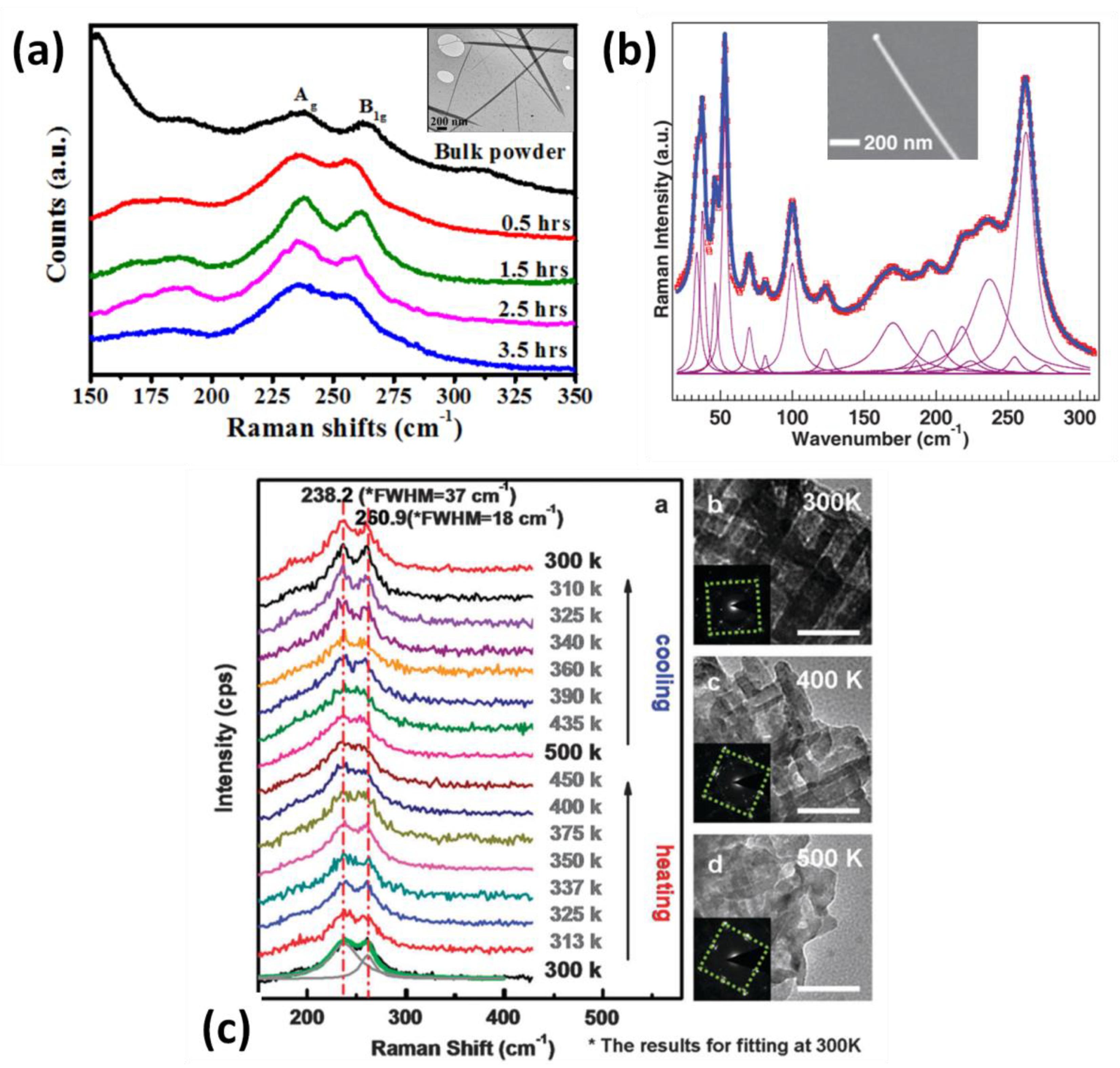
| Raman Modes | Theoretical Peak Site (cm−1) | Experimental Peak Site (cm−1) |
|---|---|---|
| B1g | 32.8 | 33.6 |
| B2g | 38.1 | 37.6 |
| Ag | 40.4 | 46.3 |
| Ag | 53.5 | 53.1 |
| Ag | 70.9 | 70.1 |
| B1g | 86.0 | 81.1 |
| Ag | 99.3 | 100.0, |
| B1g | 173.4 | 168.7 |
| Ag | 184.0 | 186.0, 187 a, 190.2 b |
| Ag | 195.5 | 196.0 |
| Ag | 211.1 | 218.7 |
| B3g | 228.2 | 224.1 |
| Ag | 237.2 | 237.1, 237 a, 235.4 b, 235 c, 238.2 d |
| Ag | 253.3 | 254.5 |
| B1g | 260.7 | 262.4, 264 a, 262.4 b, 263 c, 260.9 d |
| B1g | 277.3 | 276.3 |
| Sample | Voc(V) | Jsc(mA/cm2) | Filing Factor | Conversion Efficiency (%) |
| Bi2S3/PbS thin film [99] | 0.13–0.31 | 0.5–5 | 0.25–0.42 | 0.1–0.4 |
| Bi2S3/PbS thin film [100] | 0.28 | 2.1 | 0.34 | 0.19 |
| Bi2S3 thin film [70] | 0.23 | 10 | 0.33 | 0.75 |
| Bi2S3 quantum dot-sensitized TiO2 solar cells [98] | 0.502 | 7.9 | 0.537 | 2.52 |
| Bi2S3nanowire networks/ P3HT hybrid solar cells [37] | 0.7 | 10.7 | 0.45 | 3.3 |
| Bi2S3/P3OT solar cells [104] | 0.44 | 0.022 | / | / |
| BiOI/Bi2S3 heterojunction films [101] | 0.5 | 1.82 | 0.4 | 0.36 |
| TiO2/Bi2S3 heterostructure [102] | 0.33 | 0.57 | 0.39 | 0.148 |
| Bi2S3 nanocrystal film [45] | 0.058 | 0.33 | 0.283 | 0.0054 |
| Bi2S3 colloidal nanocrystals [36] | 0.36 | 3.21 | 0.52 | 0.60 |
| Polymer/Bi2S3 nanocrystal solar cells [27] | 0.32 | 3 | 0.49 | 0.46 |
| Bi2S3/TiO2 cross-linked heterostructure [103] | 0.48 | 14.48 | 0.47 | 3.29 |
| Bi2S3/TiO2 nanotube array cell [75] | 0.766 | 1.56 | 0.602 | 0.718 |
| NiO/CH3NH3PbI3/Bi2S3 solar cell [26] | 0.949 | 18.6 | 74.2 | 13 |
| Bi2S3 quantum dots/TiO2 nanorod QDSSC [14] | 0.46 | 14.51 | 0.46 | 3.06 |
| Sample | Photocatalytic Reaction | Spectral Region |
|---|---|---|
| TiO2 nanotubes/Bi2S3-BiOI [71] | RhB, methyl orange (MO), methylene blue (MB) and Cr (VI) | Visible (Xe lamp) |
| Bi2S3 nanoparticles [16] | MB | Visible |
| Bi2S3 microsphere [65] | MO | Visible |
| Bi2S3 nanorod [4] | MB, MO, RhB | UV |
| Bi2S3 nanoparticles [59] | CO2 | Visible (mercury lamp) |
| Bi2S3/Bi2WO6 hierarchical microstructures [72] | Ofloxacin | Visible |
| Bi2S3/ZnS microspheres [73] | RhB, oxytetracycline (OTC) | Visible |
| CuS–Bi2S3 hierarchical architectures [77] | Rh-B and crystal violet (CV) | Visible |
| Bi2S3@ZIF-8 core-shell heterostructure [52] | RhB | Visible |
| Bi2S3 nanoribbons [58] | CO2 | Visible |
| Bi2O3/Bi2S3/MoS2 n-p heterojunction [58] | Oxidizing water molecules, MB | Simulated solar light |
| Bi2S3 nanorods [69] | RhB | UV-vis |
| Bi2S3/Bi2O2CO3 heterojunction [74] | RhB | Visible (Xe lamp) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Tian, Y. Nano-Bismuth-Sulfide for Advanced Optoelectronics. Photonics 2022, 9, 790. https://doi.org/10.3390/photonics9110790
Li Z, Tian Y. Nano-Bismuth-Sulfide for Advanced Optoelectronics. Photonics. 2022; 9(11):790. https://doi.org/10.3390/photonics9110790
Chicago/Turabian StyleLi, Zimin, and Ye Tian. 2022. "Nano-Bismuth-Sulfide for Advanced Optoelectronics" Photonics 9, no. 11: 790. https://doi.org/10.3390/photonics9110790
APA StyleLi, Z., & Tian, Y. (2022). Nano-Bismuth-Sulfide for Advanced Optoelectronics. Photonics, 9(11), 790. https://doi.org/10.3390/photonics9110790




