Abstract
A simple co-precipitation method was used to create pure tungsten oxide (WO3) nanoparticles using phthalic acid, citric acid, and oxalic acid as chelating agents. The influences of chelating agents on the structural, morphological, and optical properties were investigated. X-ray diffraction (XRD) patterns of WO3 nanoparticles showed the existence of combined phase of anorthic-monoclinic for all the samples, and the crystalline size was found to be reduced while using oxalic acid. The vibrational band observed in the region around (500–800 cm−1) in the FTIR spectra indicates the formation of WO3 nanoparticles. SEM images revealed the formation of WO3 agglomerates. The energy-dispersive X-ray (EDX) spectra of the WO3 nanoparticles confirmed the purity of synthesized nanoparticles. The enhanced light-absorption ability of oxalic-acid-assisted WO3 nanoparticles are inferred from the decreased band gap energy in UV–vis absorption spectra. The PL spectra showed emission in both the UV and visible regions. The optimized reaction parameters for obtaining high catalytic efficiency are identified by varying the concentrations of oxidant, catalyst, and dye during the catalytic reaction. The synthesized WO3 nanoparticles exhibited better catalytic degradation of CV than MB and RB dyes even with the lesser quantity of catalyst material.
1. Introduction
Over the last decade, disposal of wastewater containing organic dye molecules released from various industries, especially from textiles, into natural water resources is the major cause of water contamination and disturbance of aquatic and human life. Some of the most commonly used industrial synthetic cationic dyes are crystal violet (CV), methylene blue (MB), and rhodamine B (RB). All these dyes have adverse effects on both aquatic and human health. Excessive inhalation of CV dye causes irritation of the respiratory tracts, vomiting, diarrhea, headaches, and dizziness, and its long-term exposure might damage the mucous membrane. MB dye causes anemia, dizziness, jaundice, tissue necrosis, gastrointestinal and eye problems in humans. Further, the RB has the potential to cause neurotoxicity, reproductive and developmental toxicity, and also act as a respiratory tract, skin, and eye irritant. Among the various methods adopted for the degradation of these toxic dye molecules, an advanced oxidation process based on metal oxide nanoparticles seems to be the most efficient method since it can exhibit complete degradation of a wide range of toxic contaminants into a harmless by-product via the production of hydroxyl radicals upon light illumination on the catalyst surface [1,2,3,4].
Tungsten oxide (WO3) has attracted significant attention due to its excellent physical and chemical properties in different phases with applications in photochromics [5], gas sensors [6], electrochromic windows [7], photocatalysis [8], lithium-ion batteries [9], and solar energy devices [10]. It is a well-known n-type semiconductor photocatalyst with indirect transition and has strong absorption in the far-ultraviolet and visible regions, with a relatively narrow band gap energy (2.6 to 3.25 eV), stability in acid, small size, high surface energy, and good thermal stability [11,12]. Various chemical methods have been employed for the preparation of WO3 nanoparticles with different morphologies, including the hydrothermal method [13], sol-gel [14], and chemical deposition method [15], etc. Among these preparation methods, the co-precipitation method stands out because of its homogeneity, low cost, high purity of product, high yield, low temperature, and easy manipulation [16]. WO3 nanoparticles can exhibit various polymorphs, such as monoclinic, triclinic, hexagonal, orthorhombic, tetragonal, and cubic. Researchers have also reported the formation of mixed phases such as monoclinic–hexagonal and monoclinic–triclinic with enhanced catalytic properties [17,18]. The crystalline phase and morphology of the WO3 nanoparticles can be controlled by using various capping agents such as sodium sulphate, potassium sulphate, and sodium nitrate and chelating agents such as citric acid, tartaric acid, phthalic acid, and oxalic acid [19]. The chelating agents are the structure-directing agents and are used to bind the metal ions in the reaction and are responsible for the formation of the crystalline phase. In general, the bond formed between carboxylic acid group and metal ion is responsible for the formation of nanoparticles with different morphologies. Ghasemi et al. reported the morphological evolution of WO3 nanoparticles from rod shaped to regular and spherical shapes by varying the amount of chelating agent and polyethylene glycol [20]. Aliasghari et al., obtained different morphologies of WO3 nanoparticles while using various organic fuels such as oxalic acid, glycine, and citric acid in the solution combustion method [21]. However, to our knowledge, no reports were found on focusing the effect of chelating agents on the formation of crystal phases and morphologies of WO3 nanostructures in the co-precipitation method. Hence, in the present work, an effort is made to investigate the effect of chelating agents such as phthalic acid, citric acid, and oxalic acid on the formation of crystalline phase and dye-degradation efficiency towards CV, MB, and RB dyes in the presence of H2O2. The effects of some operational parameters, such as concentration of H2O2, catalyst dose, and dye on catalytic degradation of CV, are studied for the prepared material.
2. Experimental Methods
2.1. Preparation of WO3 by Co-Precipitation Method
First, 0.1 M of sodium tungstate (Na2WO4.2H2O) was dissolved in 100 mL of distilled water and stirred for 30 min to obtain a transparent solution. Then the chelating agent, 0.01 M of citric acid (C6H8O7), was added and stirred vigorously until it dissolved completely. Then, by adding HNO3, the pH of the solution was adjusted to 1 and stirred for few hours. The obtained white solution was ultra-sonicated and dried at 100 °C for 24 h. To remove impurities, the yellow precipitate was filtered and washed three times with ethanol and distilled water. The resulting powder was calcined for 3 h at 500 °C. The same method was followed to synthesize WO3 nanoparticles, with oxalic acid (C2H2O4) and phthalic acid (C8H6O4) as chelating agents.
2.2. Characterization Techniques
The structural properties of the synthesized WO3 nanoparticles are determined by X-ray diffraction (XRD, XPERT-PRO; CuKα1 wavelength of 1.5406 Å). The functional groups were identified by using Fourier transform infra-red spectroscopy (FTIR, Shimadzu00719 (A221355)). A scanning electron microscope (SEM) VEGA3, TESCAN (Brno, Czech Republic) was used to identify the morphology and composition of synthesized nanoparticles. The ultraviolet visible (UV–vis) absorption spectroscopy (JASCO V-770, Tokyo, Japan) was used to investigate the optical absorption properties. The emission properties were studied by using photoluminescence (PL) spectroscopy (JASCO FP-8300). The photocatalytic activity of the synthesized nanoparticles was investigated by monitoring the absorbance spectra using UV–vis absorption spectrophotometer (Shimadzu UV-1800, Kyoto, Japan).
2.3. Dye Degradation
The catalytic activity of the samples was investigated by monitoring the decomposition of textile dyes (CV, MB, and RB) in the presence of hydrogen peroxide (H2O2) at room temperature. In a typical experiment, first, a dye solution is prepared for the required ppm (1 mg/100 mL), and then, the specific dose of WO3 nanoparticles is added to the dye solution and stirred at room temperature. To initiate the catalytic reaction, an appropriate amount of H2O2 is added to the prepared solution under continuous stirring. At regular time intervals, 3 mL of the solution is taken for concentration analysis using a UV–visible spectrophotometer. The complete experiment is performed under normal light conditions without using any concentrated light source, such as a UV lamp, to initiate the catalytic reaction. The only light source that could provide a small amount of light to the reaction is a fluorescent lamp located on the ceiling to brighten the laboratory. The dye-degradation efficiency is calculated by using
where A0 and A are the absorbance of the dye at time t = 0 min and after time t [22].
3. Results and Discussions
3.1. Catalyst Characterization
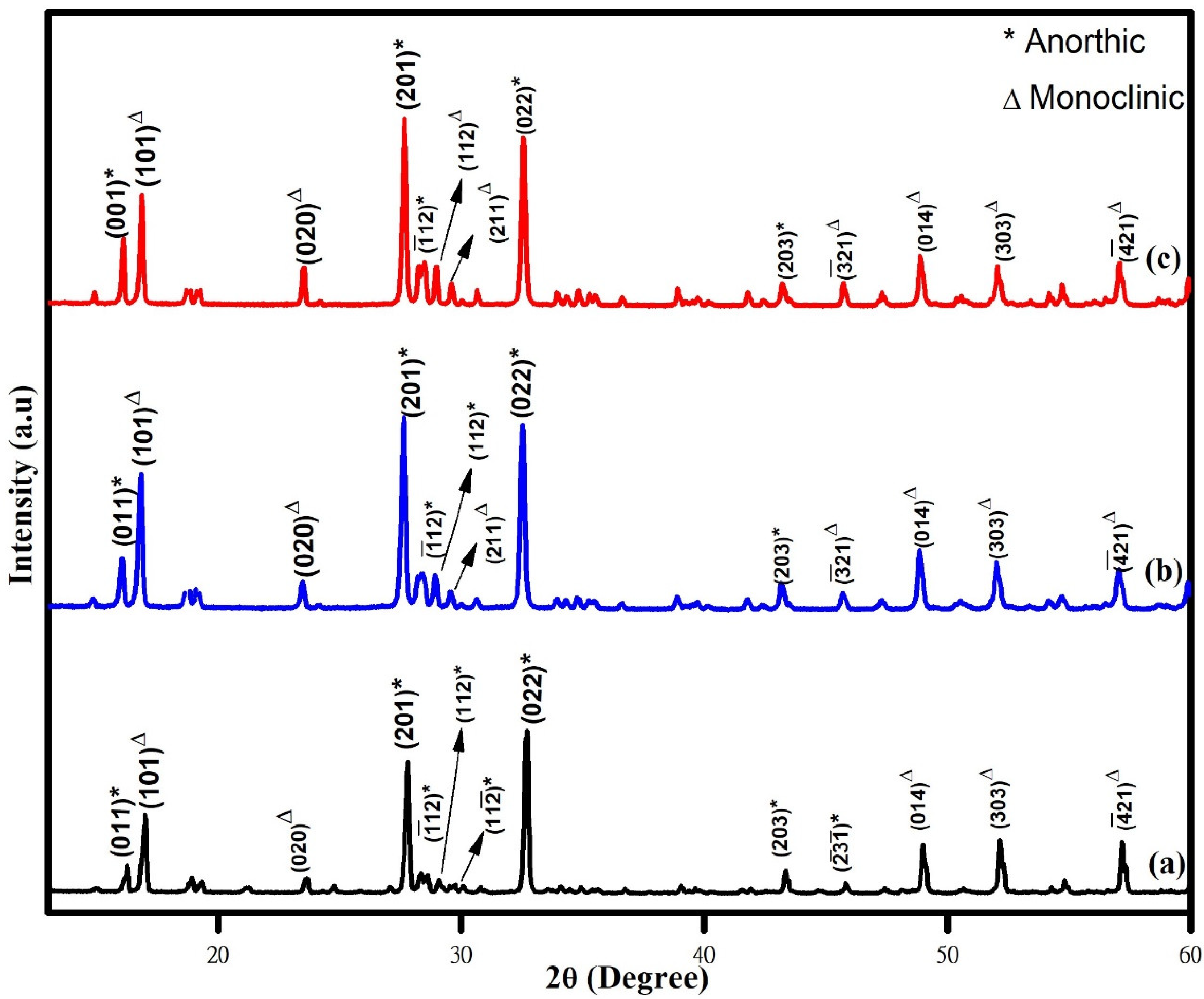
The XRD patterns of the WO3 nanoparticles synthesized with the aid of different chelating agents (phthalic acid, citric acid, and oxalic acid) are shown in Figure 1. The obtained XRD patterns of the synthesized nanoparticles are matched well with both anorthic/triclinic (JCPDS Card No: 83-0947) and monoclinic (JCPDS Card No: 83-09507) phases, which indicates that the synthesized nanoparticles are in mixed phases with good crystalline quality. On observing the XRD pattern, it was found that the peaks related to monoclinic phase of WO3 nanoparticles increases in the order of phthalic acid < citric acid < oxalic acid. Many studies highlighted the advantages of mixed-phase WO3 nanoparticles in enhancing the photocatalytic activity. The formation of mixed phases has been previously reported for WO3 nanostructures synthesized using a microwave-aided wet-chemical process [23], nano-crystalline WO3 obtained using a colloidal polymer crystal template [24]. The average crystallite sizes of the WO3 samples are estimated from the Scherer equation. The average crystallite size values were found to be 84 nm, 72 nm, and 63 nm for the samples synthesized using phthalic acid, citric acid, and oxalic acid, respectively, as the chelating agents. The variation in the crystallite size might be due to the influence of different chelating agents.
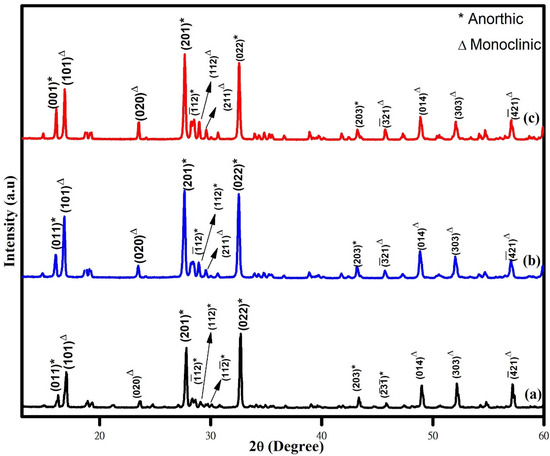
Figure 1.
XRD pattern of (a) phthalic-acid-, (b) citric-acid-, and (c) oxalic-acid-assisted WO3 nanoparticles.
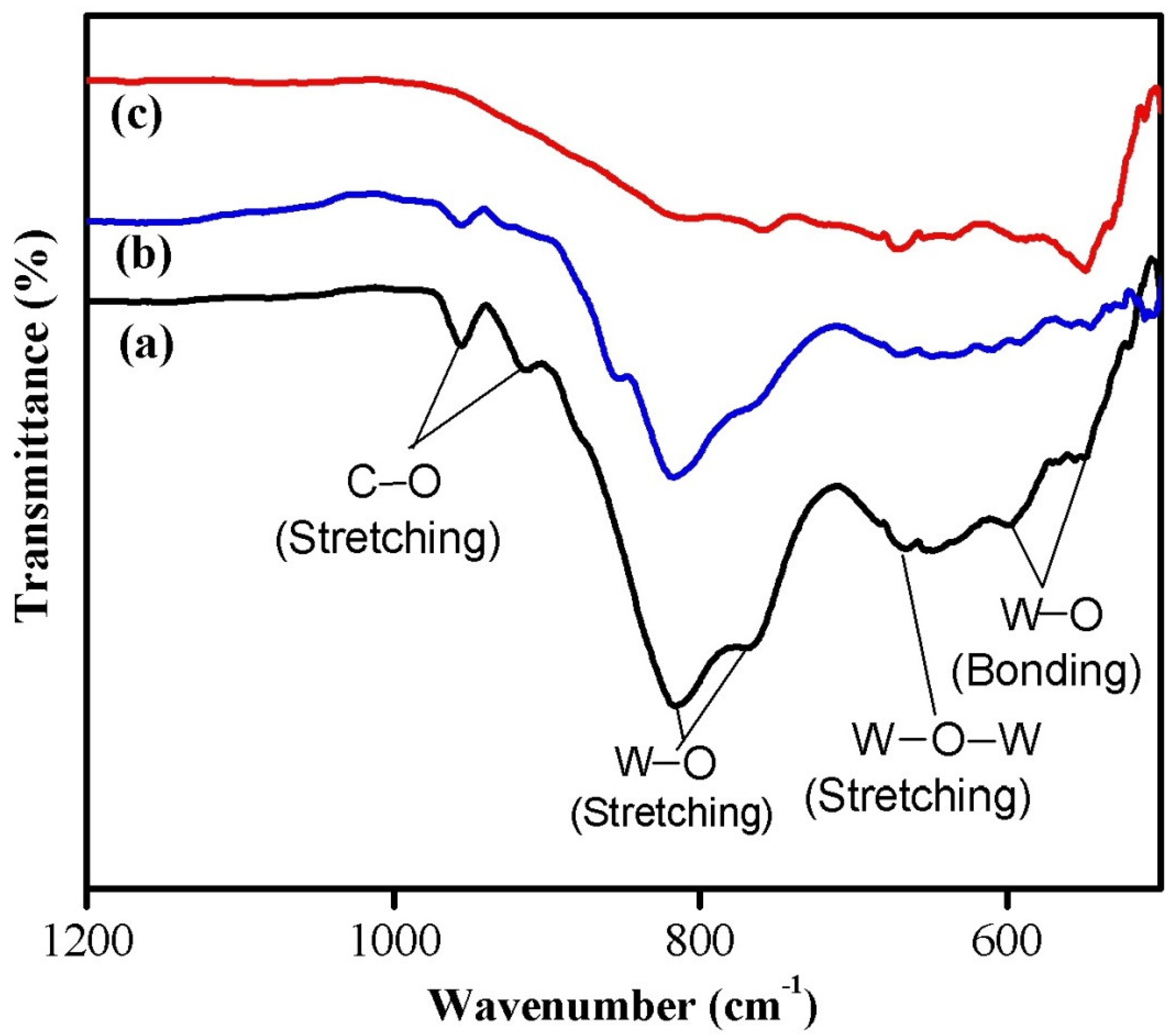
The FTIR spectroscopy measurements were carried out at room temperature to understand the chemical bonding configuration of the synthesized nanoparticles. The FTIR spectra of WO3 nanoparticles synthesized using various chelating agents are shown in Figure 2. The observed FTIR peaks are in good agreement with the previously reported spectra for WO3 nanostructures [21,25]. The formation of crystalline WO3 structure for the synthesized nanoparticles was confirmed from the appearance of broad transmittance peak in the wavenumber range of 500–1000 cm−1, which is commonly observed for tungsten oxide compounds due to vibrations associated with the W–O vibration mode. The FTIR spectra showed characteristic peaks of WO3 associated with W–O bonding (bending) mode (543–550 cm−1 and 595–600 cm−1), W–O–W stretching mode (650–670 cm−1), and W–O stretching mode (760–766 cm−1 and 815–818 cm−1) vibrations. The peaks observed at 914 cm−1 and 956 cm−1 are related to the C–O stretching mode vibrations. The observed variations in the FTIR peak positions for the samples might be attributed to the different size of the synthesized nanoparticles.
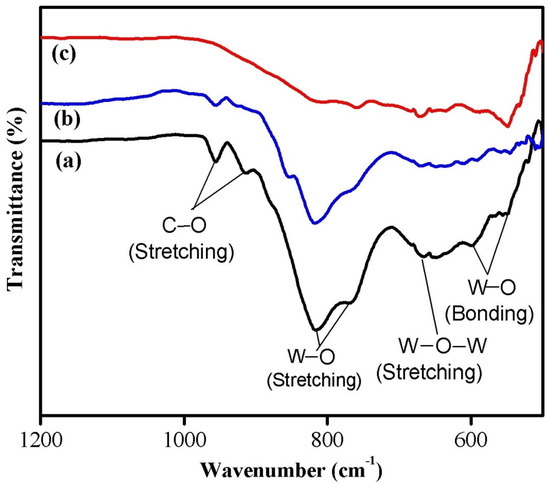
Figure 2.
FTIR spectra of (a) phthalic-acid-, (b) citric-acid-, and (c) oxalic-acid-assisted WO3 nanoparticles.
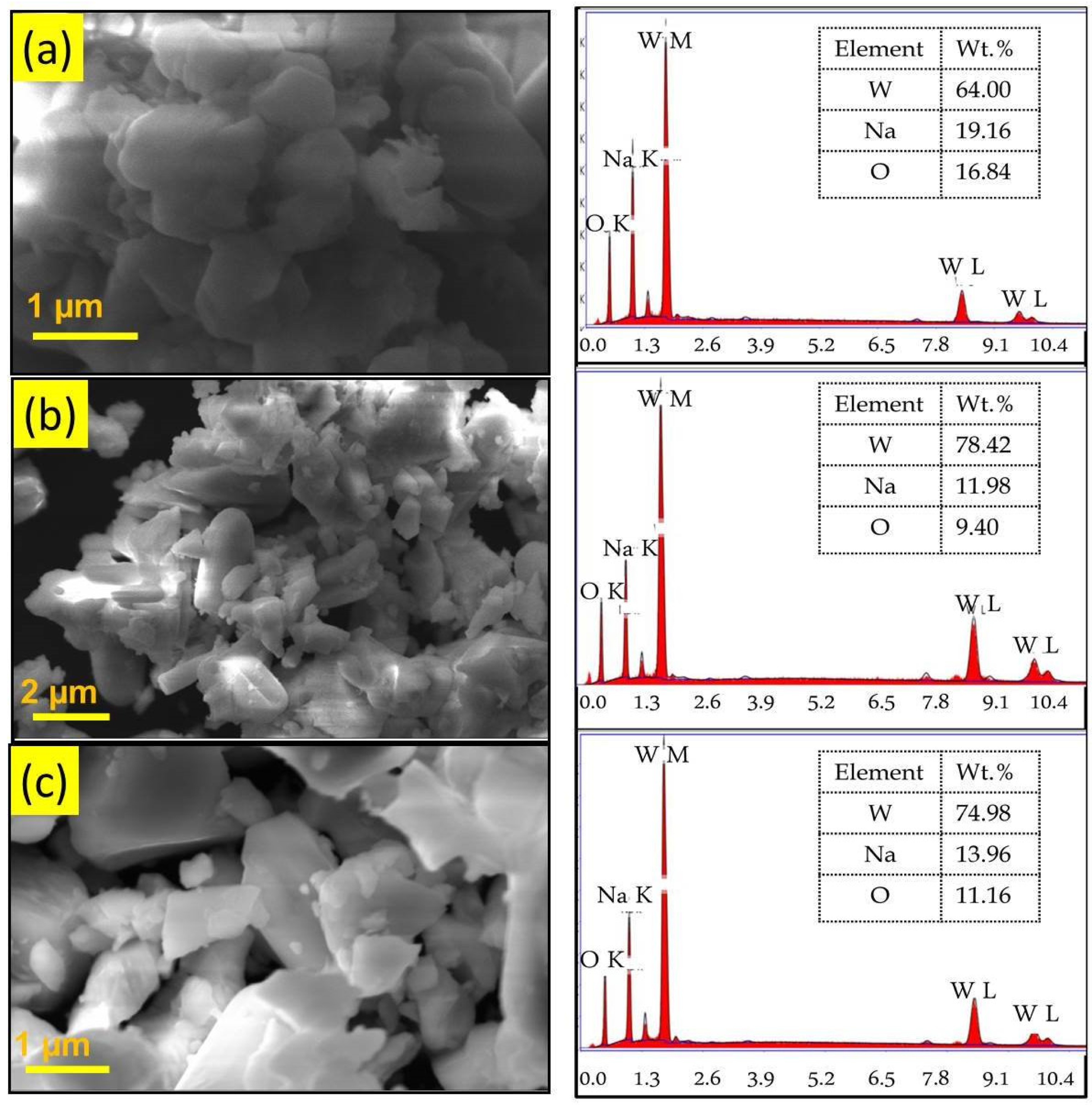
The morphological studies and the elemental composition of the WO3 samples were analyzed by SEM and EDX analyses. Figure 3 shows the SEM and EDX images of the WO3 nanoparticles synthesized using different chelating agents. SEM images of all the samples showed large, agglomerated particles having irregular shape, and this might be due to the high surface energy of nanoparticles. This result indicates that the chelating agent has no significant effect on the morphology of nanoparticles. The EDX pattern confirms the presence of W and O elements. Furthermore, the presence of a sodium (Na) peak due to the use of sodium tungstate as precursor in the synthesis process was observed [26]. The peaks representing the impurity elements are absent in all the samples.
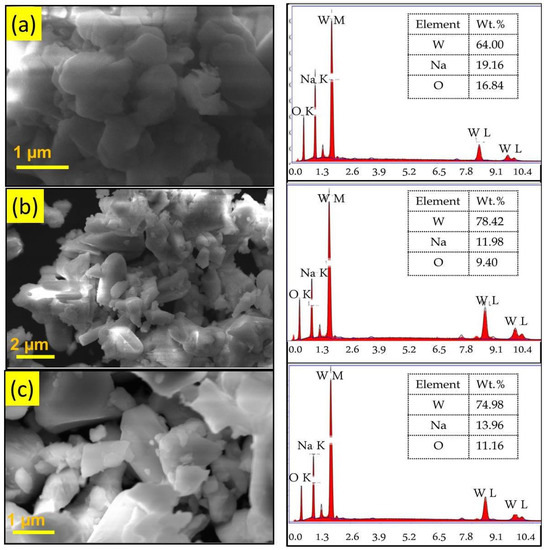
Figure 3.
SEM and EDX images of (a) phthalic-acid-, (b) citric-acid-, and (c) oxalic-acid-assisted WO3 nanoparticles.
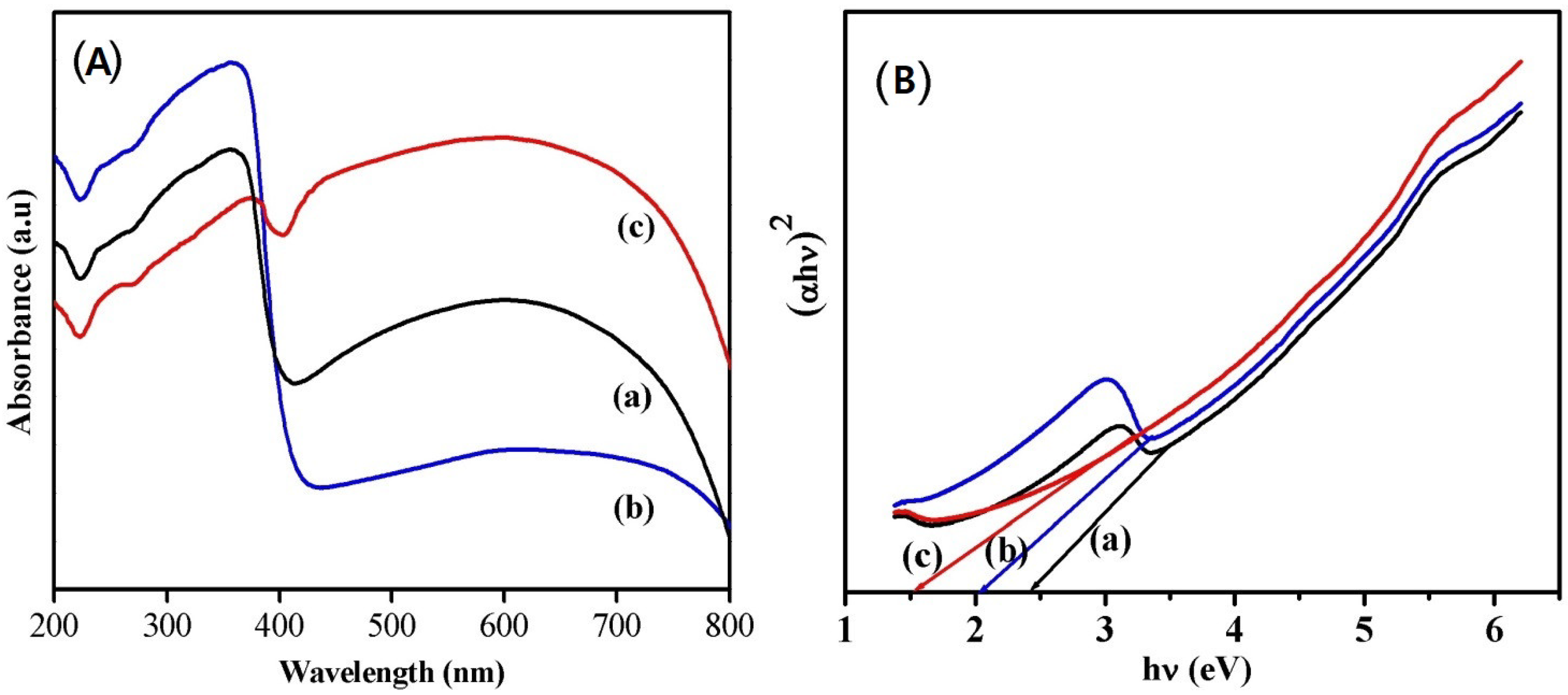
In order to understand the potential of the synthesized nanoparticles for catalytic applications, the optical properties were investigated using UV–visible absorption spectroscopy measurements. The UV–visible absorption spectra recorded at room temperature are shown in Figure 4A. It is observed that the nanoparticles synthesized using phthalic acid (Figure 4A-a) and citric acid (Figure 4A-b) have strong absorption peaks around 360 nm and a broad absorption peak in the visible region with less intensity, which is in accordance with the earlier-reported literature [11]. Notably, the sample synthesized using oxalic acid (Figure 4A-c) showed a strong and broad absorption in the whole UV–visible region, with higher intensity indicating its better photocatalytic property than the other samples. A similar absorption in the visible region was reported for Ir-doped WO3 nanoparticles synthesized by hydrothermal method [2]. The band gap energy values of the sample were evaluated using the Tauc’s equation from the absorption spectra by extrapolating the linear portion of hν in the (αhν)2 versus hν plot (Figure 4B). The calculated band gap energy values were found to be 2.32, 1.99, and 1.55 eV for the samples synthesized using phthalic acid, citric acid, and oxalic acid, respectively.
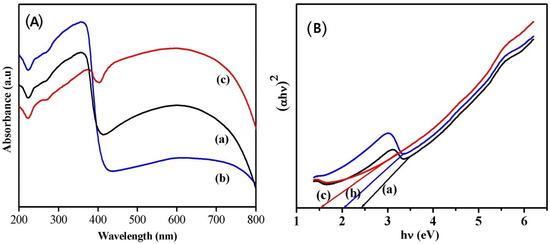
Figure 4.
(A) UV–vis absorption spectra and Tauc’s plot (B) of phthalic acid (a), (b) citric acid, and (c) oxalic acid assisted WO3 nanoparticles.
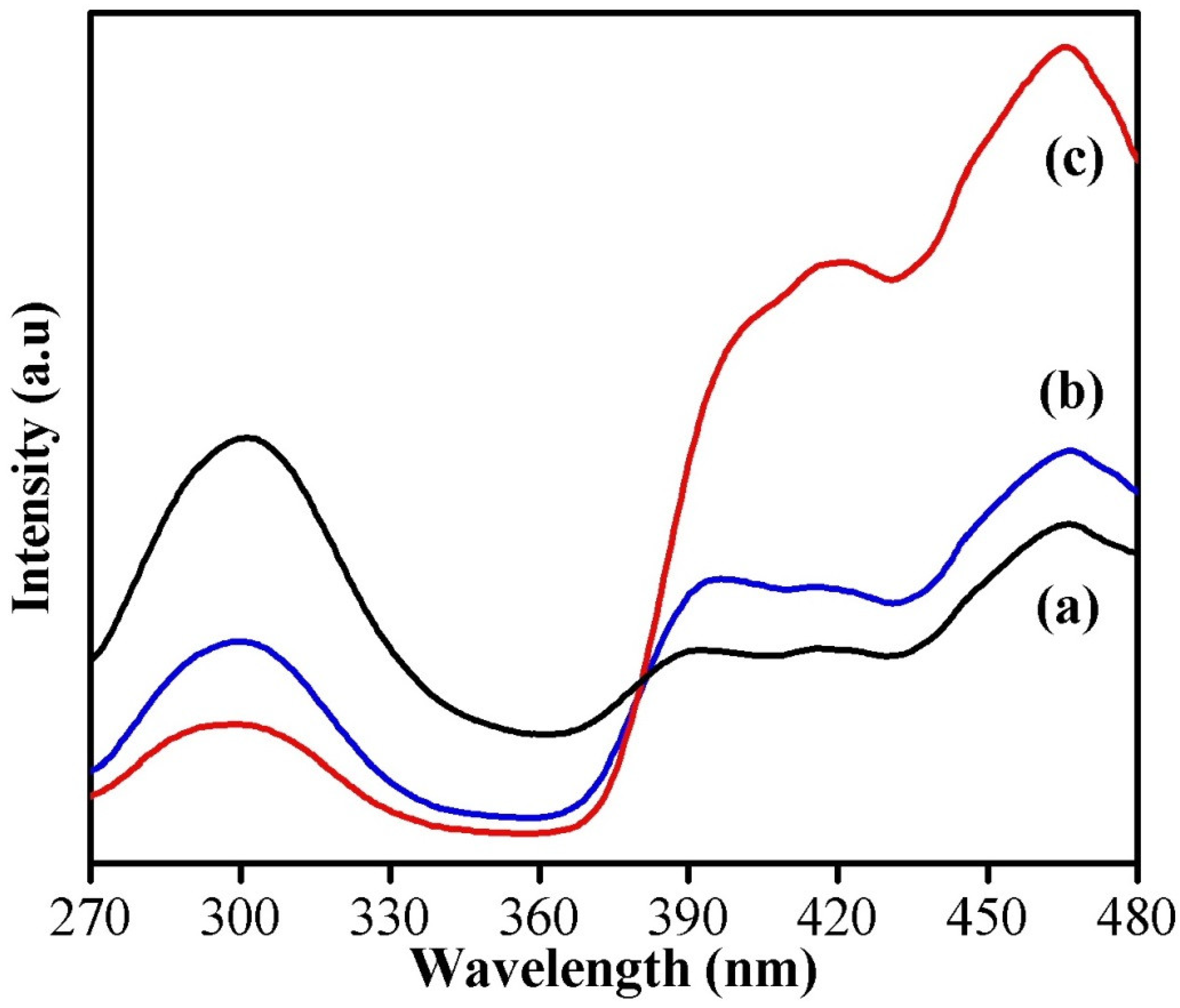
The room temperature PL spectra of the samples shown in Figure 5 were obtained using an excitation wavelength of 250 nm with 150 W Xenon lamp as the source. It was observed from the spectra that all three samples have broad-level emissions approximately around 300 nm (ultraviolet band), which originates from the exciton recombination and is usually referred to as near-band-edge emission (NBE). A weak shoulder peak seen as a bluish green emission around 466 nm is due to the presence of defects or oxygen vacancies in the nanostructures [27]. The peak corresponding to NBE seems to be decreased for oxalic-acid-assisted WO3 nanoparticles, but the intensity of the peak arising from defects or oxygen vacancies seems to be higher, which indicates the presence of more defects in it. It has been reported that the oxygen vacancies/interstitials on the surface of metal oxide nanoparticles can act as a scavenger of electrons and holes, which will reduce the recombination of photogenerated electrons and holes, and it is the prime factor responsible for enhancing the degradation efficiency of a catalyst [28]. Hence, it is expected that the WO3 nanoparticles synthesized using oxalic acid will exhibit better catalytic dye-degradation efficiency compared to the others.
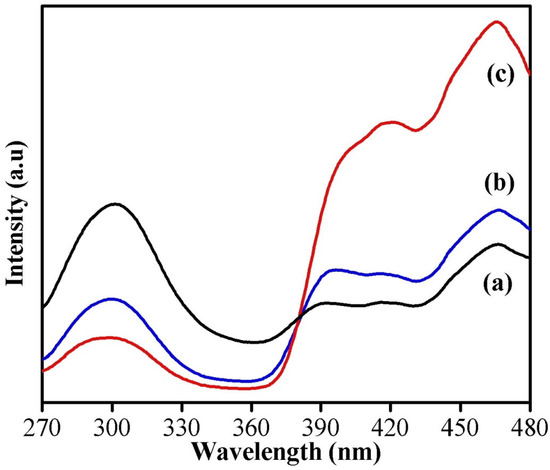
Figure 5.
PL spectra of (a) phthalic-acid-, (b) citric-acid-, and (c) oxalic-acid-assisted WO3 nanoparticles.
3.2. Dye-Degradation Activity
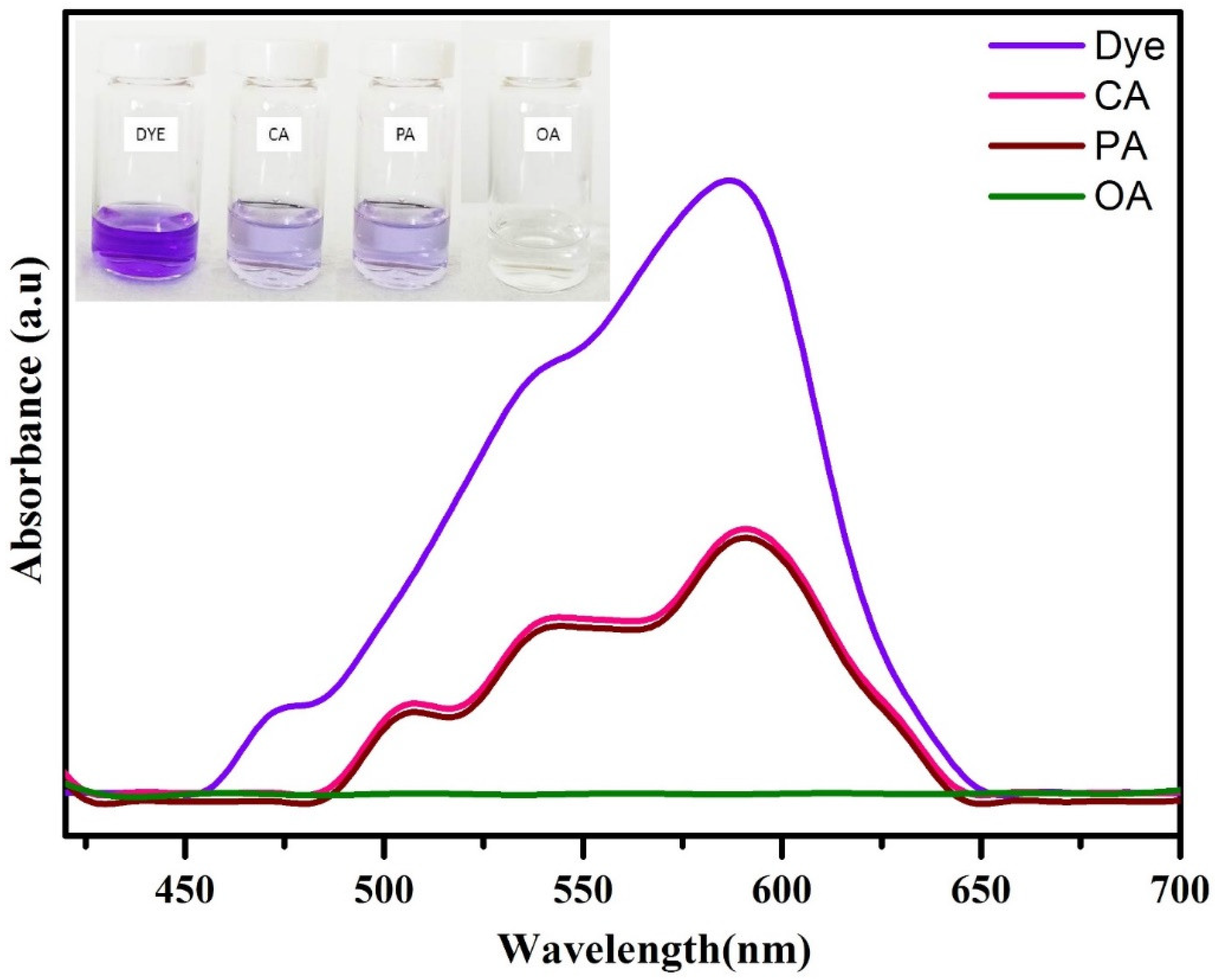
Figure 6 depicts the comparison of degradation efficiency of WO3 nanoparticles synthesized using phthalic acid, citric acid, and oxalic acid. From the results, it is clear that the oxalic-acid-assisted WO3 nanoparticles exhibited better dye-degradation efficiency compared to citric and phthalic acid. This enhancement in the catalytic activity is due to the presence of large surface area, better absorption in the visible region, and presence of oxygen vacancies/interstitials. In addition to these properties, it is well-known that the catalytic behavior of WO3 nanoparticles is greatly influenced by its phase structure. It has been recognized that the monoclinic WO3 phase is the most stable phase at room temperature and is expected to exhibit higher catalytic activity when compared with other WO3 phases [18].
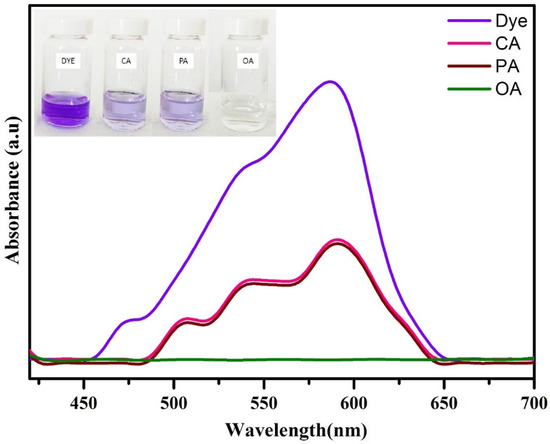
Figure 6.
CV dye-degradation efficiency of WO3 nanoparticles synthesized by using different chelating agents.
In order to confirm the stability of dye solution and the role of the catalyst in the degradation process, a blank test was carried out, i.e., in the absence of catalyst by taking other parameters such as H2O2 concentration and dye concentration as 0.5 mL and 20 ppm, respectively, and the results are depicted in Figure S1. Only a slight decrease in the intensity of dye was observed even after 240 min of reaction. This highlights the stability and dye-degradation ability of the synthesized WO3 nanoparticles.
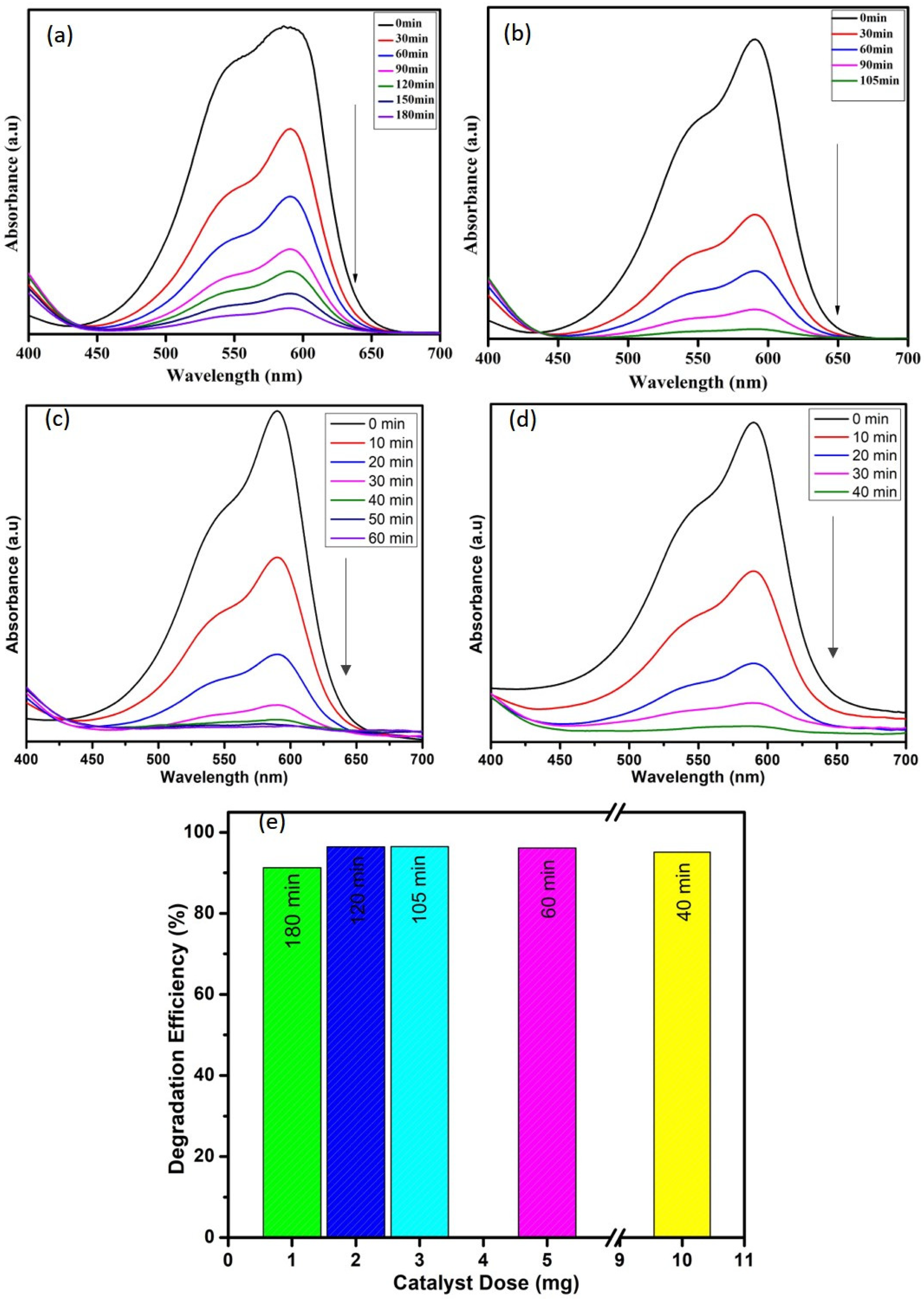
The effect of catalyst dose (1, 3, 5, and 10 mg) on the degradation of crystal violet dye (10 ppm) in the presence of H2O2 (0.5 mL) was investigated, and the obtained results are depicted in Figure 7. From the results, it is clear that the degradation efficiency increases with increasing the catalyst dose, and the degradation time also decreases with the dose. The 10 mg of WO3 nanoparticles degraded 95% of crystal violet dye in 40 min, whereas the lesser quantity took more time. Hence, the catalyst dosage positively affects the degradation process, which might be due to the presence of more adsorption sites and creation of numerous free electrons in the conduction band [2].
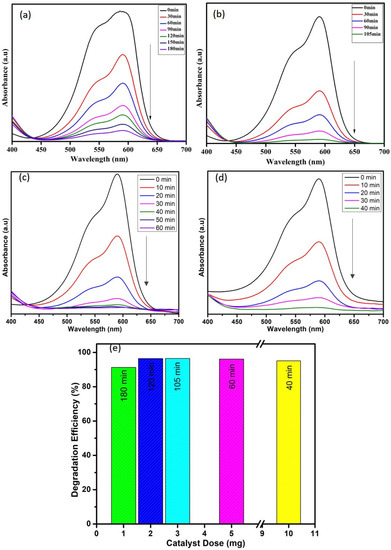
Figure 7.
Effect of catalyst dose (a) 1 mg, (b) 3 mg, (c) 5 mg, and (d) 10 mg and (e) comparison among the efficiencies of all concentrations on degradation of CV dye.
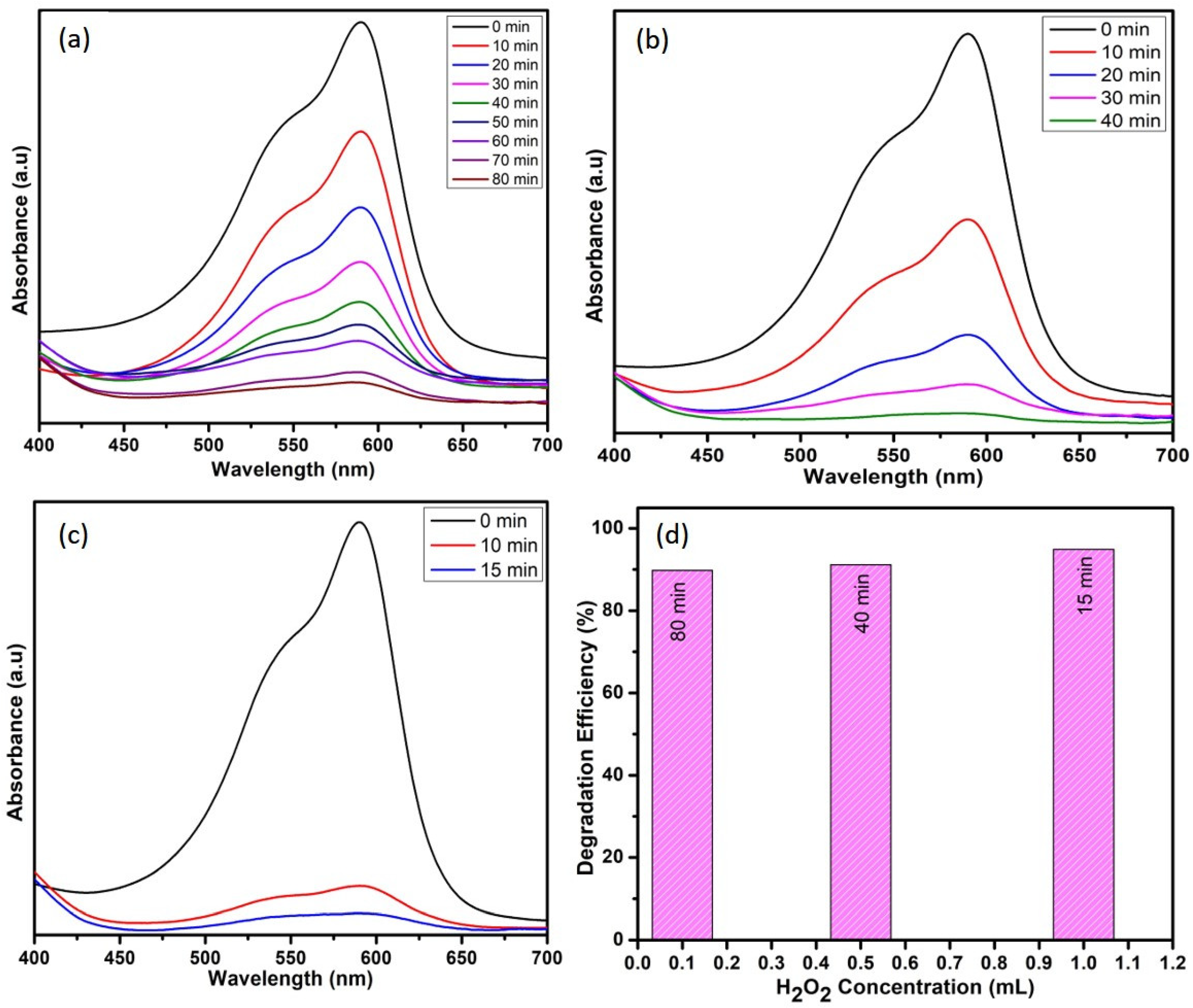
The degradation activity of crystal violet can be effective by the appropriate addition of hydrogen peroxide. The electron–hole recombination represents the main cause for low efficiencies and is limited by the use of oxidant systems such as hydrogen peroxide. By adding H2O2, higher efficiencies are obtained, confirming that the electron–hole pair recombination is reduced [29]. Thus, the effect of hydrogen peroxide oxidant on CV removal by WO3 nanoparticles was studied at different hydrogen peroxide concentrations by maintaining the catalyst dosage (10 mg) and dye concentration (10 ppm) as constant. Figure 8 indicates that the dye-degradation efficiency is directly proportional to H2O2 concentration. The degradation time seems to be reduced from 80 to 15 min while using 1 mL of H2O2. This enhanced degradation efficiency is due to the efficient separation of electron–hole pairs, which might be due to the electron accepting nature of H2O2 [30,31]. In aqueous solution, to generate ●OH radicals, the holes at the surface are scavenged by surface hydroxyl groups and water molecules. Now, the dye molecules are oxidized to the final products, i.e., H2O and CO2, because of the very strong oxidizing agent ●OH radicals [2].
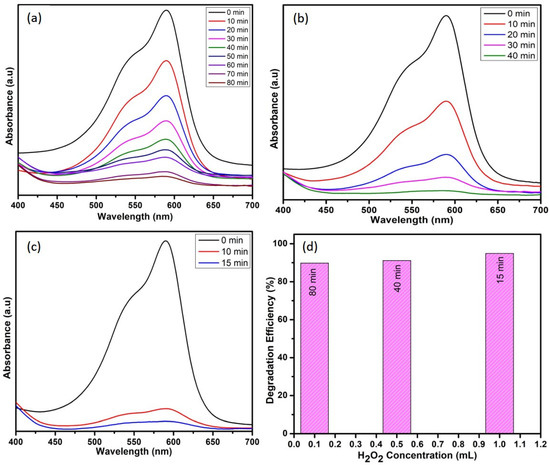
Figure 8.
Effect of H2O2 (a) 0.1 mL, (b) 0.5 mL, and (c) 1 mL and (d) comparison among the efficiencies of H2O2 concentrations on degradation of CV dye.
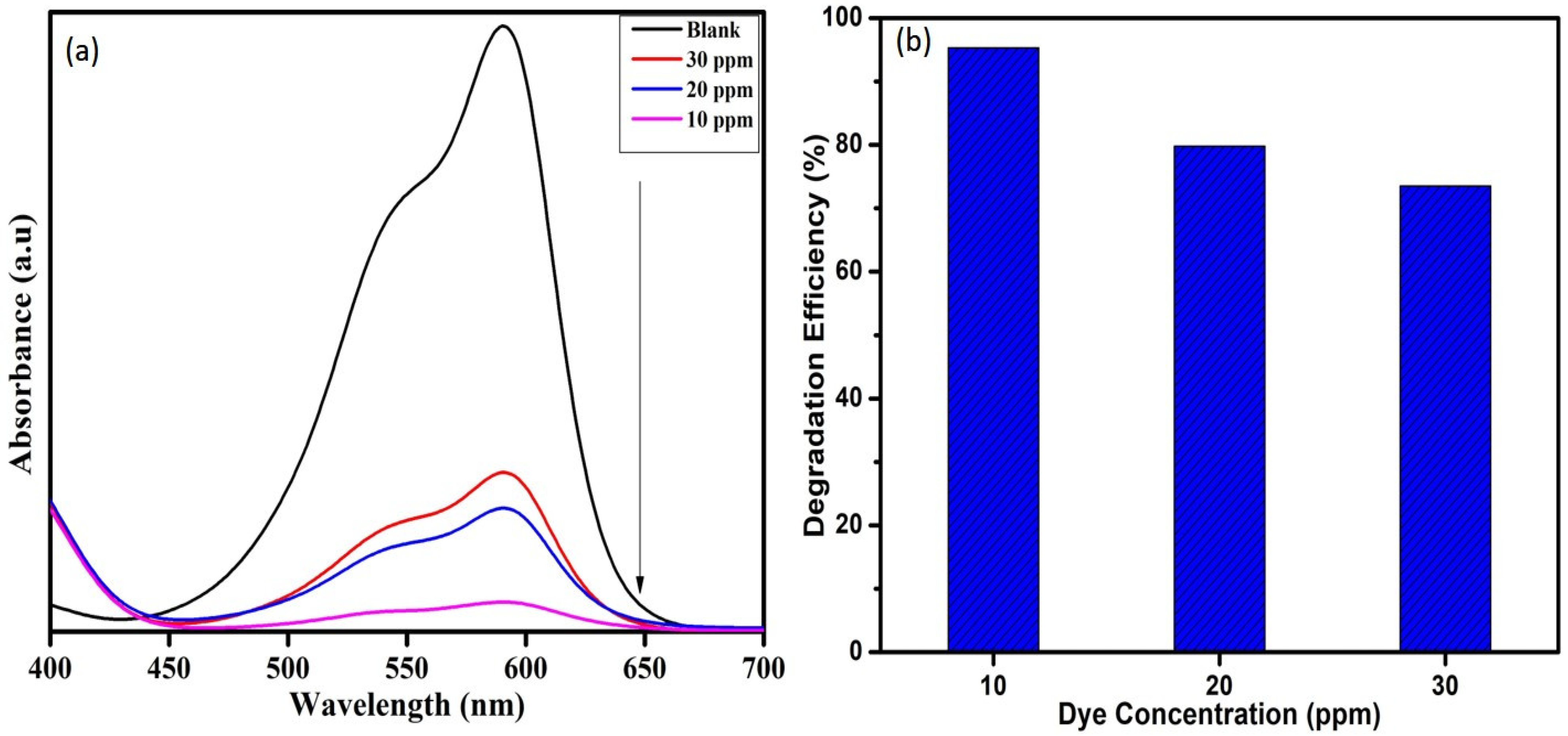
To study the effect of dye concentration on degradation of crystal violet dye, the concentration was varied as 10, 20, and 30 ppm while keeping the other parameters such as catalyst dose (1 mg) and H2O2 (0.5 mL) as a constant. It was found that 10 ppm dye degraded 95%, whereas the 20 and 30 ppm dyes degraded 79 and 73% in 135 min, as shown in Figure 9. Degradation time increases with increasing dye concentration. The dye solution at higher concentration hinders the penetration of photons to the catalyst surface, which in turn suppresses the effectiveness of the catalyst in degrading dye molecules [32,33].
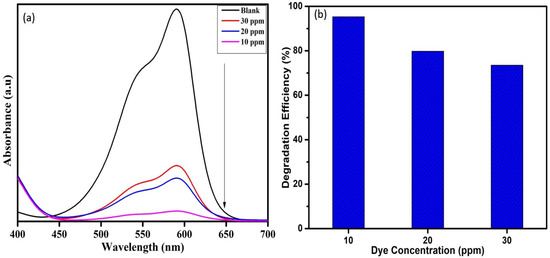
Figure 9.
(a) UV-Visible absorption spectra and (b) Degradation efficiency of WO3 nanoparticles on varying CV dye concentration.
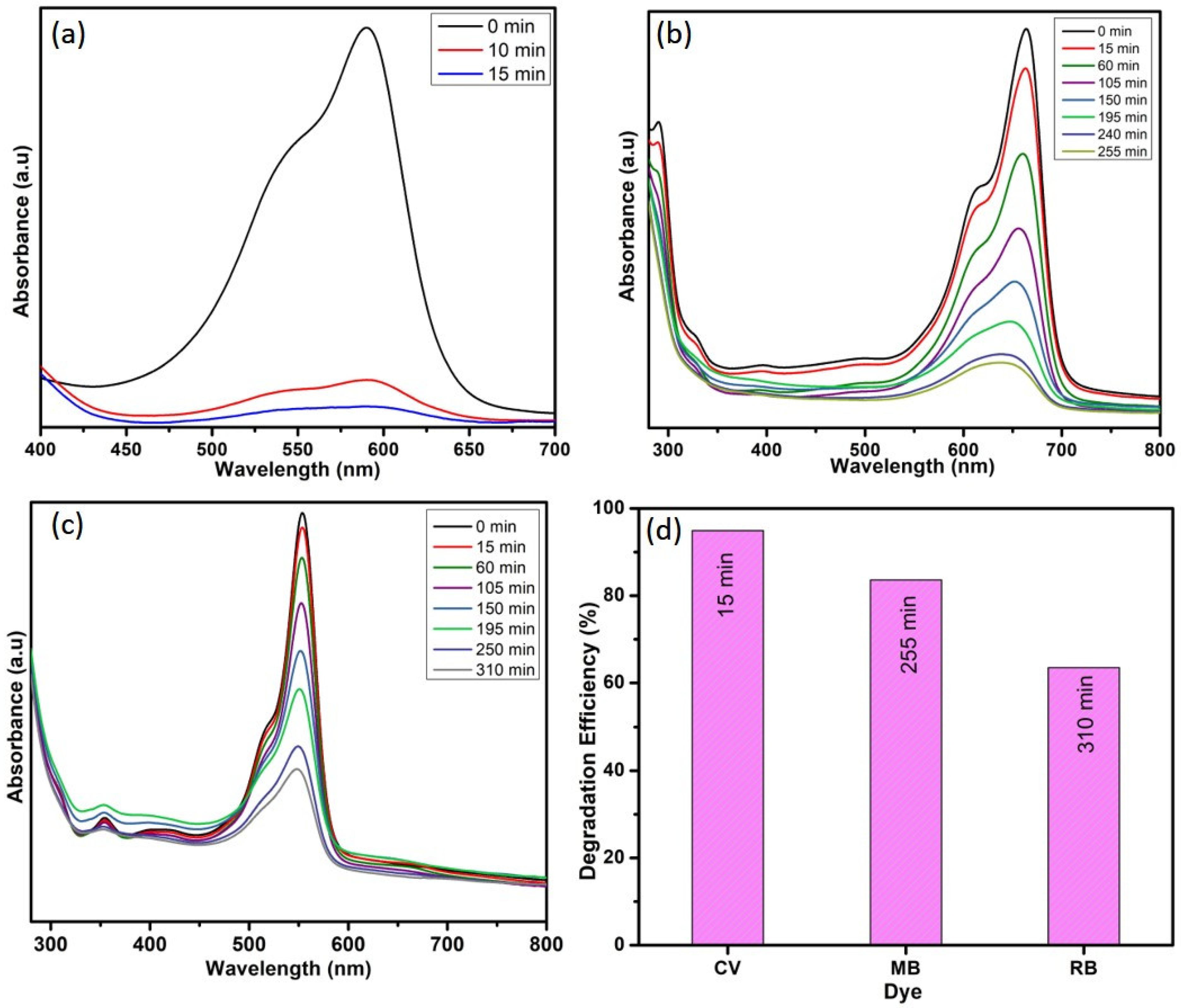
The obtained results indicate that the optimal conditions for obtaining better degradation efficiency towards CV dye are 10 ppm dye concentration, 10 mg catalyst dose, and 1 mL H2O2 concentration. The dye-degradation efficiency of WO3 nanoparticles towards MB and RB dye was carried out in the above-mentioned conditions. Figure 10 depicts that the 95% of CV dye was degraded in 15 min, whereas only 83% of MB dye and 63% of RB dye were degraded in 255 min and 310 min, respectively. These results are compared with the available literature, and the same is listed in Table 1. The comparison table reveals that the as-synthesized WO3 nanoparticles have better dye-degradation ability than the other WO3 nanoparticles and their composites. This might be due to its lower band gap value and lower electron–hole recombination rate, as evidenced from UV and PL analysis.
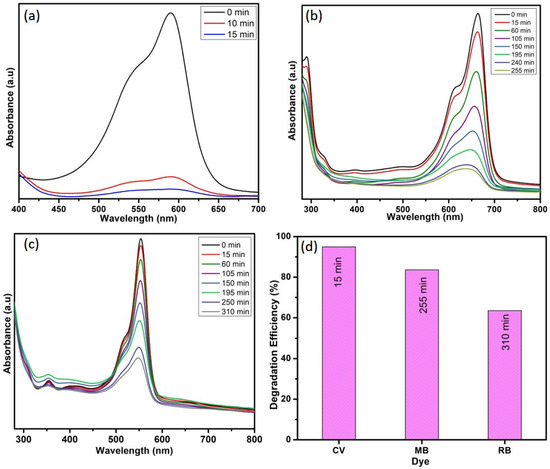
Figure 10.
Photocatalytic degradation of (a) CV, (b) MB, and (c) RB and (d) dyes-degradation efficiency.

Table 1.
Catalytic efficiencies of different WO3 materials for different dye molecules.
4. Conclusions
In summary, WO3 nanoparticles are prepared by the simple, cost-effective co-precipitation method. This work mainly focused on studying the effect of different chelating agents (phthalic, citric, and oxalic acid) on structural, morphological, optical, and catalytic dye-degradation properties of WO3 nanoparticles. The existence of a mixed-phase (monoclinic–triclinic) nature of the synthesized nanoparticles was confirmed by XRD analysis. The vibrational modes corresponding to WO3 nanoparticles were observed in the FTIR spectra. The large, agglomerated particles were obtained in SEM images, and the purity of the synthesized samples was confirmed from EDX analysis. The band gap energy of WO3 nanoparticles was found to be in the range of 2.32–1.55 eV. The presence of a greater number of defects in oxalic-acid-assisted WO3 nanoparticles was identified from the high, intense defect emission peak obtained in PL spectra. The optimum parameters for the degradation of crystal violet dye are 10 mg catalyst, 1 mL of H2O2, and 10 ppm dye concentration. Further, the degradation efficiency of WO3 towards MB and RB dye was also studied. Thus, the prepared WO3 nanoparticles using oxalic acid proved to be an effective candidate for the treatment of organic pollutants in wastewater.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/photonics9110849/s1, Figure S1: CV dye degradation in the absence of catalyst.
Author Contributions
Conceptualization, S.K.; validation, P.M.P., A.S. and G.N.; investigation, P.T. and G.N.; resources, S.B.; writing—original draft preparation, P.T.; writing—review and editing, P.M.P., S.K. and S.B.; supervision, S.K., P.M.P. and S.B.; project administration, S.K. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by UGC-DAE-Consortium for Scientific Research (Project Sanction No: CSR-KN/CRS-107/2018–19/1046).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request by the corresponding author.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Rashad, S.; Zaki, A.; Farghali, A. Morphological effect of titanate nanostructures on the photocatalytic degradation of crystal violet. Nanomater. Nanotechnol. 2019, 9, 1847980418821778. [Google Scholar] [CrossRef]
- Rahmat, M.; Rehman, A.; Rahmat, S.; Bhatti, H.N.; Iqbal, M.; Khan, W.S.; Bajwa, S.Z.; Rahmat, R.; Nazir, A. Highly efficient removal of crystal violet dye from water by MnO2 based nanofibrous mesh/photocatalytic process. J. Mater. Res. Technol. 2019, 8, 5149–5159. [Google Scholar] [CrossRef]
- Kim, D.; Kim, G.; Bae, H.; Kim, E.; Moon, B.; Cheon, D.; Tarte, N.H. An Energy Independent WO3/MoCl5 Nano-Sized Catalyst for the Superior Degradation of Crystal Violet and Rhodamine B Dye. Catalysts 2019, 9, 642. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Weldegebrieal, G.K.; Garg, S.; Oh, W.C.; Hamizi, N.A.; Johan, M.R. Enhanced Photocatalytic Activity of rGO-CuO Nanocomposites for the Degradation of Organic Pollutants. Catalysts 2021, 11, 1008. [Google Scholar] [CrossRef]
- Miyazaki, H.; Ishigaki, T.; Suzuki, H.; Ota, T. Effect of Film Thickness and Air Atmosphere on Photochromic Properties of WO3-Based Composite Films. Bull. Chem. Soc. Jpn. 2016, 89, 20–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, W.; Li, Y. New insight into gas sensing performance of nanorods assembled and nanosheets assembled hierarchical WO3•H2O structures. Mater. Lett. 2018, 235, 49–52. [Google Scholar] [CrossRef]
- Zhao, Q.; Fang, Y.; Qiao, K.; Wei, W.; Yao, Y.; Gao, Y. Printing of WO3/ITO nanocomposite electrochromic smart windows. Sol. Energy Mater. Sol. Cells 2019, 194, 95–102. [Google Scholar] [CrossRef]
- Song, K.; Liu, X.; Tian, C.; Deng, H.; Wang, J.; Su, X. Oxygen defect-rich WO3-x nanostructures with high photocatalytic activity for dehydration of isopropyl alcohol to propylene. Surf. Interfaces 2018, 14, 245–250. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, G.-L.; Kuang, Q.; Sun, S.-G.; Yang, S. Hierarchical WO3 flowers comprising porous single-crystalline nanoplates show enhanced lithium storage and photocatalysis. Nano Res. 2012, 5, 826–832. [Google Scholar] [CrossRef]
- Zheng, H.; Tachibana, Y.; Kalantar-zadeh, K. Dye-sensitized solar cells based on WO3. Langmuir 2010, 26, 9148–19152. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Prabavathi, S.L.; Saravanakumar, K.; Jones, B.F.; Muthuraj, V. Iridium nanoparticles anchored WO3 nanocubes as an efficient photocatalyst for removal of refractory contaminants (crystal violet and methylene blue). Chem. Phys. Lett. 2020, 745, 137285. [Google Scholar] [CrossRef]
- Deepa, B.; Rajendran, V. Pure and Cu metal doped WO3 prepared via co-precipitation method and studies on their structural, morphological, electrochemical and optical properties. Nano-Struct. Nano-Objects 2018, 16, 185–192. [Google Scholar] [CrossRef]
- Yao, S.; Qu, F.; Wang, G.; Wu, X. Facile hydrothermal synthesis of WO3 nanorods for photocatalysts and supercapacitors. J. Alloys Compd. 2017, 724, 695–702. [Google Scholar] [CrossRef]
- Nagarjuna, R.; Challagulla, S.; Sahu, P.; Roy, S.; Ganesan, R. Polymerizable sol–gel synthesis of nano-crystalline WO3 and its photocatalytic Cr(VI) reduction under visible light. Adv. Powder Technol. 2017, 28, 3265–3273. [Google Scholar] [CrossRef]
- Kafizas, A.; Francàs, L.; Vazquez, C.S.; Ling, M.; Li, Y.; Glover, E.; Cafferty, L.M.; Blackman, C.S.; Darr, J.A.; Parkin, I.P. Optimizing the Activity of Nano-needle Structured WO3 Photoanodes for Solar Water Splitting—Direct Synthesis Via Chemical Vapor Deposition. J. Phys. Chem. C 2017, 121, 5983–5993. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of Inorganic Nanomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 121–139. [Google Scholar]
- Lin, J.; Hu, P.; Zhang, Y.; Fan, M.; He, Z.; Ngaw, C.K.; Loo, J.S.C.; Liao, D.; Tan, T.T.Y. Understanding the photoelectrochemical properties of a reduced graphene oxide–WO3 heterojunction photoanode for efficient solar-light-driven overall water splitting. RSC Adv. 2013, 3, 9330–9336. [Google Scholar] [CrossRef]
- Liu, H.; Peng, T.; Ke, D.; Peng, Z.; Yan, C. Preparation and photocatalytic activity of dysprosium doped tungsten trioxide nanoparticles. Mater. Chem. Phys. 2007, 104, 377–383. [Google Scholar] [CrossRef]
- Ahmadian, H.; Tehrani, F.S.; Aliannezhadi, M. Hydrothermal synthesis and characterization of WO3 nanostructures: Effects of capping agent and pH. Mater. Res. Express 2019, 6, 105024. [Google Scholar] [CrossRef]
- Ghasemi, L.; Jafari, H. Morphological Characterization of Tungsten Trioxide Nanopowders Synthesized by Sol-Gel Modified Pechini’s Method. Mater. Res. 2017, 20, 1713–1721. [Google Scholar] [CrossRef]
- Asghari, H.A.; Arabi, A.; Haratizadeh, H. A novel approach for solution combustion synthesis of tungsten oxide nanoparticles for photocatalytic and electrochromic applications. Ceram. Int. 2019, 46, 403–414. [Google Scholar] [CrossRef]
- Preethi, T.; Senthil, K.; Pachamuthu, M.P.; Balakrishnaraja, R.; Sundaravel, B.; Geetha, N.; Bellucci, S. Effect of Fe Doping on Photocatalytic Dye-Degradation and Antibacterial Activity of SnO2 Nanoparticles. Adsorpt. Sci. Technol. 2022, 2022, 9334079. [Google Scholar] [CrossRef]
- Periasamy, P.; Krishnakumar, T.; Sathish, M.; Chavali, M.; Siril, P.F.; Devarajan, V.P. Structural and electrochemical studies of tungsten oxide (WO3) nanostructures prepared by microwave assisted wet-chemical technique for supercapacitor. J. Mater. Sci. Mater. Electron. 2018, 29, 6157–6166. [Google Scholar]
- Sadakane, M.; Sasaki, K.; Kunioku, H.; Ohtani, B.; Abe, R.; Ueda, W. Preparation of 3-D ordered macroporous tungsten oxides and nano-crystalline particulate tungsten oxides using a colloidal crystal template method, and their structural characterization and application as photocatalysts under visible light irradiation. J. Mater. Chem. 2010, 20, 1811–1818. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Dang, D.V.; Nguyen, D.C. Hydrothermal synthesis and NH3 gas sensing property of WO3 nanorods at low temperature. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 035006. [Google Scholar] [CrossRef]
- Kolhe, P.S.; Mutadak, P.; Maiti, N.; Sonawane, K.M. Synthesis of WO3 nanoflakes by hydrothermal route and its gas sensing application. Sens. Actuators A Phys. 2020, 304, 111877. [Google Scholar] [CrossRef]
- Sivakarthik, P.; Thangaraj, V.; Parthibavarman, M. A facile and one-pot synthesis of pure and transition metals (M = Co & Ni) doped WO3 nanoparticles for enhanced photocatalytic performance. J. Mater. Sci. Mater. Electron. 2017, 28, 5990–5996. [Google Scholar]
- Baradaran, M.; Ghodsi, F.E. Highly efficient visible photocatalytic degradation of MB organic dye by heteromorphic ZnO/AZO/ZnO nanocatalysts: Effect of AZO thickness. J. Sol-Gel Sci. Technol. 2019, 92, 25–39. [Google Scholar] [CrossRef]
- Carcel, R.A.; Andronic, L.; Duta, A. Photocatalytic Degradation of Methyl orange Using TiO2, WO3 and Mixed Thin Films Under Controlled pH and H2O2. J. Nanosci. Nanotechnol. 2011, 11, 9095–9101. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A. Photocatalytic degradation of azo dye acid red 14 in water: Investigation of the effect of operational parameters. J. Photochem. Photobiol. A: Chem. 2003, 157, 111–116. [Google Scholar] [CrossRef]
- Farrokhi, M.; Hosseini, S.-C.; Yang, J.-K.; Shirzad-Siboni, M. Application of ZnO–Fe3O4 Nanocomposite on the Removal of Azo Dye from Aqueous Solutions: Kinetics and Equilibrium Studies. Water Air Soil Pollut. 2014, 225, 2113. [Google Scholar] [CrossRef]
- Vasić, M.B.; Randjelovic, M.; Momčilović, M.Z.; Matović, B.Z.; Zarubica, A.R. Degradation of crystal violet over heterogeneous TiO2-based catalysts: The effect of process parameters. Process. Appl. Ceram. 2016, 10, 189–198. [Google Scholar] [CrossRef]
- Kumar, R.; Rashid, J.; Barakat, M. Zero valent Ag deposited TiO2 for the efficient photocatalysis of methylene blue under UV-C light irradiation. Colloids Interface Sci. Commun. 2015, 5, 1–4. [Google Scholar] [CrossRef]
- Govindaraj, T.; Mahendran, C.; Marnadu, R.; Shkir, M.; Manikandan, V. The remarkably enhanced visible-light-photocatalytic activity of hydrothermally synthesized WO3 nanorods: An effect of Gd doping. Ceram. Int. 2020, 47, 4267–4278. [Google Scholar] [CrossRef]
- Warsi, A.-Z.; Ahmad, T.; Aziz, F.; Warsi, M.F.; Munir, S.; Agboola, P.O.; Shakir, I. Synthesis and characterisation of WO3 and Ag2O nanoparticles and their nanocomposite for photocatalytic degradation of dyes. Int. J. Environ. Anal. Chem. 2022. [Google Scholar] [CrossRef]
- Antony, A.J.; Kala, S.M.J.; Joel, C.; Bennie, R.B.; Praveendaniel, S. Enhancing the visible light induced photocatalytic properties of WO3 nanoparticles by doping with vanadium. J. Phys. Chem. Solids 2021, 157, 110169. [Google Scholar] [CrossRef]
- Eroi, S.N.; Ello, A.S.; Diabate, D.; Ossonon, D.B. Heterogeneous WO3/H2O2 system for degradation of Indigo Carmin dye from aqueous solution. S. Afr. J. Chem. Eng. 2021, 37, 53–60. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).