Vital Signs Monitoring Based on Interferometric Fiber Optic Sensors
Abstract
:1. Introduction
2. Structures and Principles of Interferometric FOS
2.1. Optical Fiber Mach–Zehnder Interferometer
2.2. Optical Fiber Sagnac Interferometer
2.3. Optical Fiber Michelson Interferometer
2.4. Optical Fiber Fabry-Perot Interferometers
2.5. The Comparison of Different Interferometers for Vital Signs Monitoring
3. Demodulation Schemes
3.1. PGC Demodulation Scheme
3.2. Moving-Coil Phase Compensation Scheme
3.3. Coupler Demodulation Scheme
3.4. The Comparison of Three Demodulation Schemes
4. Application of Vital Signs Monitoring
4.1. Heart Rate
4.2. Respiration
4.3. Sleeping
4.4. Myocardial Contractility
4.5. Body Temperature
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.; Yu, Y.; Zhong, J. Research status and development prospects of human vital signs monitoring clothing. IOP Conf. Ser. Earth Environ. Sci. 2019, 233, 042031. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Sheikh, N.; Biagini, E.; Papadakis, M.; Sinagra, G.; Pelliccia, A.; Rapezzi, C.; Sharma, S.; Olivotto, I. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm 2020, 17, 142–151. [Google Scholar] [CrossRef]
- Liu, H.; Allen, J.; Zheng, D.; Chen, F. Recent development of respiratory rate measurement technologies. Physiol. Meas. 2019, 40, 07TR01. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinci, G.; Lindner, S.; Barbon, F.; Mann, S.; Hofmann, M.; Duda, A.; Weigel, R.; Koelpin, A. Six-port radar sensor for remote respiration rate and heartbeat vital-sign monitoring. IEEE Trans. Microw. Theory Tech. 2013, 61, 2093–2100. [Google Scholar] [CrossRef]
- Shao, D.; Tsow, F.; Liu, C.; Yang, Y.; Tao, N. Simultaneous monitoring of ballistocardiogram and photoplethysmogram using a camera. IEEE Trans. Biomed. Eng. 2016, 64, 1003–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.A.; Malik, A.S.; Fofi, D.; Saad, N.M.; Ali, Y.S.; Meriaudeau, F. Video-based heartbeat rate measuring method using ballistocardiography. IEEE Sens. J. 2017, 17, 4544–4557. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Rajan, G.; Semenova, Y.; Farrell, G. Overview of Fiber Optic Sensor Technologies for Strain/Temperature Sensing Applications in Composite Materials. Sensors 2016, 16, 99. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Lau, D.; Teo, J.T.; Ng, S.H.; Yang, X.; Kei, P.L. Simultaneous measurement of breathing rate and heart rate using a microbend multimode fiber optic sensor. J. Biomed. Opt. 2014, 19, 057001. [Google Scholar] [CrossRef]
- Leal-Junior, A.G.; Diaz, C.R.; Leitão, C.; Pontes, M.J.; Marques, C.; Frizera, A. Polymer optical fiber-based sensor for simultaneous measurement of breath and heart rate under dynamic movements. Opt. Laser Technol. 2019, 109, 429–436. [Google Scholar] [CrossRef]
- Dziuda, Ł.; Krej, M.; Skibniewski, F.W. Fiber Bragg grating strain sensor incorporated to monitor patient vital signs during MRI. IEEE Sens. J. 2013, 13, 4986–4991. [Google Scholar] [CrossRef]
- Koyama, S.; Haseda, Y.; Ishizawa, H.; Okazaki, F.; Bonefacino, J.; Tam, H.-Y. Measurement of Pulsation Strain at the Fingertip Using a Plastic FBG Sensor. IEEE Sens. J. 2021, 21, 21537–21545. [Google Scholar] [CrossRef]
- Lyu, W.; Xu, W.; Yang, F.; Chen, S.; Tan, F.; Yu, C. Non-invasive measurement for cardiac variations using a fiber optic sensor. IEEE Photonics Technol. Lett. 2021, 33, 990–993. [Google Scholar] [CrossRef]
- Lyu, W.; Tan, F.; Chen, S.; Yu, C. Myocardial contractility assessment using fiber optic sensors. In Proceedings of the Asia Communications and Photonics Conference, Chengdu, China, 2–5 November 2019; p. M4A-152. [Google Scholar]
- Lyu, W.; Chen, S.; Tan, F.; Yu, C. Non-invasive heart rate variability measurement during sleep based on fiber optic sensor. In Proceedings of the 2021 26th Optoelectronics and Communications Conference, Hong Kong, China, 3–7 July 2021; p. JS3F-2. [Google Scholar]
- Tan, F.; Lyu, W.; Chen, S.; Liu, Z.; Yu, C. Contactless vital signs monitoring based on few-mode and multi-core fibers. Opto-Electron. Adv. 2020, 3, 190034. [Google Scholar] [CrossRef]
- Ke, C.; Cai, Y.; Zhao, T.; Li, Z. Research on intelligent mattress based on improved SMS structure sensing fiber. J. Phys. Conf. Ser. 2021, 1082, 022023. [Google Scholar] [CrossRef]
- Qu, J.; Shen, Y.; Xu, W.; Tan, F.; Yu, C.; Yu, C. Non-invasive vital signs monitoring based on polarization maintaining fiber and Sagnac interferometer. In Proceedings of the 24th OptoElectronics and Communications Conference/International Conference Photonics in Switching and Computing, OECC/PSC 2019, Fukuoka, Japan, 7–11 July 2019; p. 8817846. [Google Scholar]
- Ke, C.; Cai, Y.; Zhao, T.; Li, Z. Research on Smart Mattress Based on Fiber Unbalanced Sagnac Loop. IOP Conf. Ser. Earth Environ. Sci. 2021, 769, 042039. [Google Scholar] [CrossRef]
- Zazula, D.; Đonlagić, D.; Šprager, S. Application of fibre-optic interferometry to detection of human vital signs. J. Laser Health Acad. 2012, 2012, 27–32. [Google Scholar]
- Šprager, S.; Đonlagić, D.; Zazula, D. Heartbeat detection applying activity index on optical interferometric signal. In Proceedings of the 11th WSEAS international conference on Instrumentation, Measurement, Circuits and Systems, and Proceedings of the 12th WSEAS International Conference on Robotics, Control and Manufacturing Technology, and Proceedings of the 12th WSEAS International Conference on Multimedia Systems & Signal Processing, Rovaniemi, Finland, 18–20 April 2012; pp. 77–82. [Google Scholar]
- Pullteap, S.; Samartkit, P. A High Sensitivity of Vital Signs Detector using Fiber Optic-based Fabry-Perot Interferometer. ECTI Trans. Electr. Eng. Electron. Commun. 2020, 18, 98–106. [Google Scholar] [CrossRef]
- Samartkit, P.; Pullteap, S.; Seat, H.C. Validation of Fiber Optic-Based Fabry–Perot Interferometer for Simultaneous Heart Rate and Pulse Pressure Measurements. IEEE Sens. J. 2020, 21, 6195–6201. [Google Scholar] [CrossRef]
- Li, Y.; Dong, B.; Chen, E.; Wang, X.; Zhao, Y. Heart-rate monitoring with an ethyl alpha-cyanoacrylate based fiber fabry-perot sensor. IEEE J. Sel. Top. Quantum Electron. 2020, 27, 1–6. [Google Scholar] [CrossRef]
- Priest, R.G. Analysis of fiber interferometer utilizing 3 × 3 fiber coupler. IEEE Trans. Microw. Theory Tech. 1982, 30, 1589–1591. [Google Scholar] [CrossRef] [Green Version]
- Jackson, D.; Priest, R.; Dandridge, A.; Tveten, A.B. Elimination of drift in a single-mode optical fiber interferometer using a piezoelectrically stretched coiled fiber. Appl. Opt. 1980, 19, 2926–2929. [Google Scholar] [CrossRef]
- Dandridge, A.; Tveten, A.B.; Giallorenzi, T.G. Homodyne demodulation scheme for fiber optic sensors using phase generated carrier. IEEE Trans. Microw. Theory Tech. 1982, 30, 1635–1641. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Lin, J.; Liu, H.; Ma, Y.; Yan, L.; Jin, P. Diaphragm based long cavity Fabry–Perot fiber acoustic sensor using phase generated carrier. Opt. Commun. 2017, 382, 514–518. [Google Scholar] [CrossRef]
- Chang, T.; Lang, J.; Sun, W.; Chen, J.; Yu, M.; Gao, W.; Cui, H.-L. Phase compensation scheme for fiber-optic interferometric vibration demodulation. IEEE Sens. J. 2017, 17, 7448–7454. [Google Scholar] [CrossRef]
- Udd, E.; Spillman, W.B., Jr. Fiber Optic Sensors: An Introduction for Engineers and Scientists; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Sheem, S.K. Optical fiber interferometers with [3 × 3] directional couplers: Analysis. J. Appl. Phys. 1981, 52, 3865–3872. [Google Scholar] [CrossRef]
- Kamenev, O.T.; Petrov, Y.S.; Podlesnykh, A. Use of 3 × 3 Coupler in the Fiber Optic Strainmeter Based on Mach-Zehnder Interferometer. In Proceedings of the KnE Energy, VII International Conference on Photonics and Information Optics (PhIO), Moscow, Russia, 24–26 January 2018; pp. 388–392. [Google Scholar]
- Xu, D.; Yang, F.; Chen, D.; Cai, H.; Qu, R. Laser phase noise measurement by using an adjustment-free Michelson interferometer based on 3×3 optical coupler. In Proceedings of the Asia Communications and Photonics Conference, Hong Kong, China, 19–23 November 2015; p. ASu3C.4. [Google Scholar]
- Park, S.; Lee, J.; Kim, Y.; Lee, B.H. Nanometer-scale vibration measurement using an optical quadrature interferometer based on 3× 3 fiber-optic coupler. Sensors 2020, 20, 2665. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Rim, S.; Kim, Y.; Lee, B.H. Noncontact photoacoustic imaging based on optical quadrature detection with a multiport interferometer. Opt. Lett. 2019, 44, 2590–2593. [Google Scholar] [CrossRef]
- Chen, S.; Tan, F.; Lyu, W.; Yu, C. Ballistocardiography monitoring system based on optical fiber interferometer aided with heartbeat segmentation algorithm. Biomed. Opt. Express 2020, 11, 5458–5469. [Google Scholar] [CrossRef]
- Yang, F.; Lyu, W.; Pan, C.; Yang, S.; Tan, F.; Chen, S.; Yu, C. Contactless vital signs monitoring based on optical fiber Mach-Zehnder interferometer aided with passive homodyne demodulation methods. In Proceedings of the Asia Communications and Photonics Conference, Beijing, China, 24–27 October 2020; p. M4A-79. [Google Scholar]
- Wang, R.; Zhao, J.; Sun, Y.; Yu, H.; Zhou, N.; Zhang, H.; Jia, D. Wearable respiration monitoring using an in-line few-mode fiber Mach-Zehnder interferometric sensor. Biomed. Opt. Express 2020, 11, 316–329. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Yang, H.; Qiu, Y.; Li, H.; Chen, Z.; Yu, C. Non-invasive measurement of vital signs based on seven-core fiber interferometer. IEEE Sens. J. 2021, 21, 10703–10710. [Google Scholar] [CrossRef]
- Nishyama, M.; Miyamoto, M.; Watanabe, K. Respiration and body movement analysis during sleep in bed using hetero-core fiber optic pressure sensors without constraint to human activity. J. Biomed. Opt. 2011, 16, 017002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, D.; Kumar, R.; Ng, W.P.; Fu, Y.-q.; Yuan, J.; Yu, C.; Wu, Y.; Zhou, G.; Farrell, G. A simple optical fiber interferometer based breathing sensor. Meas. Sci. Technol. 2017, 28, 035105. [Google Scholar] [CrossRef] [Green Version]
- Favero, F.C.; Pruneri, V.; Villatoro, J. Microstructured optical fiber interferometric breathing sensor. J. Biomed. Opt. 2012, 17, 037006. [Google Scholar] [CrossRef]
- Mathew, J.; Semenova, Y.; Farrell, G. A miniature optical breathing sensor. Biomed. Opt. Express 2012, 3, 3325–3331. [Google Scholar] [CrossRef] [Green Version]
- Nurmi, S.; Saaresranta, T.; Koivisto, T.; Meriheinä, U.; Palva, L. Validation of an Accelerometer Based BCG Method for Sleep Analysis; Department of Electrical Engineering and Automation, Aalto University: Espoo, Finland, 2016. [Google Scholar]
- Cao, K.; Liu, Y.; Qu, S. Compact fiber biocompatible temperature sensor based on a hermetically-sealed liquid-filling structure. Opt. Express 2017, 25, 29597–29604. [Google Scholar] [CrossRef]
- Wang, S.; Lv, R.; Zhao, Y.; Qian, J. A Mach-Zehnder interferometer-based High Sensitivity Temperature sensor for human body monitoring. Opt. Fiber Technol. 2018, 45, 93–97. [Google Scholar] [CrossRef]
- Tan, F.; Chen, S.; Lyu, W.; Liu, Z.; Yu, C.; Lu, C.; Tam, H.Y. Non-invasive human vital signs monitoring based on twin-core optical fiber sensors. Biomed. Opt. Express 2019, 10, 5940–5951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, Y.; Tan, F.; Chen, S.; Lyu, W.; Yang, F.; Yu, C. IJK complex detection within BCG signal based on multi-core fiber sensors. In Proceedings of the Asia Communications and Photonics Conference/International Conference on Information Photonics and Optical Communications 2020 (ACP/IPOC), Beijing, China, 24–27 October 2020; p. S3G-3. [Google Scholar]
- Ang, K.H.; Chong, G.; Li, Y. PID control system analysis, design, and technology. IEEE Trans. Control Syst. Technol. 2005, 13, 559–576. [Google Scholar]
- Cheshire, W.P., Jr. Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci. 2016, 196, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Kovoor, P.; Ricciardello, M.; Collins, L.; Uther, J.B.; Ross, D.L. Risk to patients from radiation associated with radiofrequency ablation for supraventricular tachycardia. Circulation 1998, 98, 1534–1540. [Google Scholar] [CrossRef]
- Hajar, R. Risk factors for coronary artery disease: Historical perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Julius, S.; Palatini, P.; Nesbitt, S.D. Tachycardia: An important determinant of coronary risk in hypertension. J. Hypertens. Suppl. 1998, 16, S9–S15. [Google Scholar]
- Stein, P.K.; Rich, M.W.; Rottman, J.N.; Kleiger, R.E. Stability of index of heart rate variability in patients with congestive heart failure. Am. Heart J. 1995, 129, 975–981. [Google Scholar] [CrossRef]
- Burger, A.J.; Charlamb, M.; Weinrauch, L.A.; D’Elia, J.A. Short-and long-term reproducibility of heart rate variability in patients with long-standing type I diabetes mellitus. Am. J. Cardiol. 1997, 80, 1198–1202. [Google Scholar] [CrossRef]
- Ewing, D.J.; Neilson, J.M.; Shapiro, C.M.; Stewart, J.A.; Reid, W. Twenty four hour heart rate variability: Effects of posture, sleep, and time of day in healthy controls and 5 Ballistocardiography 153 comparison with bedside tests of autonomic function in diabetic patients. Br. Heart J. 1991, 65, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Konrady, A.O.; Rudomanov, O.G.; Yacovleva, O.I.; Shlyakhto, E.V. Power spectral components of heart rate variability in different types of cardiac remodelling in hypertensive patients. Med. Sci. Monit. 2001, 7, 58–63. [Google Scholar] [PubMed]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T., Jr.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef]
- Życzkowski, M.; Szustakowski, M.; Ciurapiński, W.; Uziębło-Życzkowska, B. Interferometric fiber optics based sensor for monitoring of the heart activity. Acta Phys. Pol. A 2011, 120, 782–784. [Google Scholar] [CrossRef]
- Sprager, S.; Donlagic, D.; Zazula, D. Estimation of heart rate, respiratory rate and motion by using optical interferometer as body sensor. In Proceedings of the Proceedings of the IASTED International Conference on Signal and Image Processing, Dallas, TX, USA, 14–16 December 2011; pp. 280–287. [Google Scholar]
- Tran, V.P.; Al-Jumaily, A.A. Non-contact real-time estimation of intrapulmonary pressure and tidal volume for chronic heart failure patients. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 3564–3567. [Google Scholar]
- Voscopoulos, C.; Ladd, D.; Campana, L.; George, E. Non-invasive respiratory volume monitoring to detect apnea in post-operative patients: Case series. J. Clin. Med. Res. 2014, 6, 209. [Google Scholar] [CrossRef] [Green Version]
- Kermit, M.; Eide, Å.J.; Lindblad, T.; Waldemark, K. Treatment of obstructive sleep apnea syndrome by monitoring patients airflow signals. Pattern Recognit. Lett. 2000, 21, 277–281. [Google Scholar] [CrossRef]
- Leonard, P.; Beattie, T.; Addison, P.; Watson, J. Standard pulse oximeters can be used to monitor respiratory rate. Emerg. Med. J. 2003, 20, 524–525. [Google Scholar] [CrossRef] [Green Version]
- Sayadi, O.; Weiss, E.H.; Merchant, F.M.; Puppala, D.; Armoundas, A.A. An optimized method for estimating the tidal volume from intracardiac or body surface electrocardiographic signals: Implications for estimating minute ventilation. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H426–H436. [Google Scholar] [CrossRef] [PubMed]
- Lafortuna, C.L.; Passerini, L. A new instrument for the measurement of rib cage and abdomen circumference variation in respiration at rest and during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 259–265. [Google Scholar] [CrossRef]
- Reyes, B.A.; Reljin, N.; Kong, Y.; Nam, Y.; Chon, K.H. Tidal volume and instantaneous respiration rate estimation using a volumetric surrogate signal acquired via a smartphone camera. IEEE J. Biomed. Health Inform. 2016, 21, 764–777. [Google Scholar] [CrossRef]
- Fraser, A.G.; Angelini, G.D.; Ikram, S.; Butchart, E.G. Left atrial ball thrombus: Echocardiographic features and clinical implications. Eur. Heart J. 1988, 9, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.P.; Rittogers, S.E.; Froester, W.F.; Boudoulas, H. A critical review of the systolic time intervals. Circulation 1977, 56, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Forouzanfar, M.; Baker, F.C.; Colrain, I.M.; Goldstone, A.; de Zambotti, M. Automatic analysis of pre-ejection period during sleep using impedance cardiogram. Psychophysiology 2019, 56, e13355. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, M.; Inan, O.T.; Giovangrandi, L.; Kovacs, G.T. Rapid assessment of cardiac contractility on a home bathroom scale. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 864–869. [Google Scholar] [CrossRef]
- Huang, K.; Shen, Y.; Zhang, F.; Wang, S.; Wei, X. Evaluation of effects of thymidylate synthase and excision repair cross-complementing 1 polymorphisms on chemotherapy outcome in patients with gastrointestinal tumors using peripheral venous blood. Oncol. Lett. 2016, 11, 3477–3482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onseng, K.; Johns, N.P.; Khuayjarernpanishk, T.; Subongkot, S.; Priprem, A.; Hurst, C.; Johns, J. Beneficial effects of adjuvant melatonin in minimizing oral mucositis complications in head and neck cancer patients receiving concurrent chemoradiation. J. Altern. Complement. Med. 2017, 23, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Young, J.S.; Simmons, J.W. Chemotherapeutic medications and their emergent complications. Emerg. Med. Clin. 2014, 32, 563–578. [Google Scholar] [CrossRef] [PubMed]

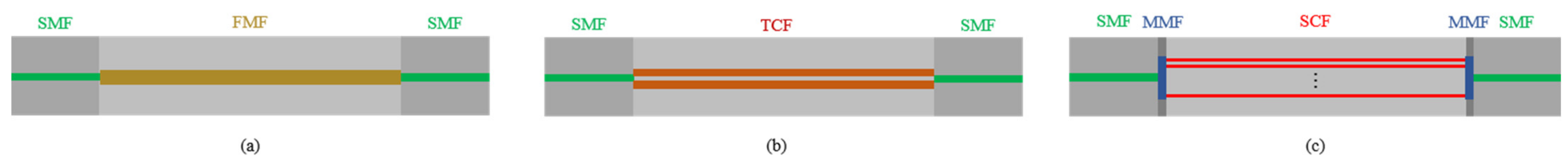
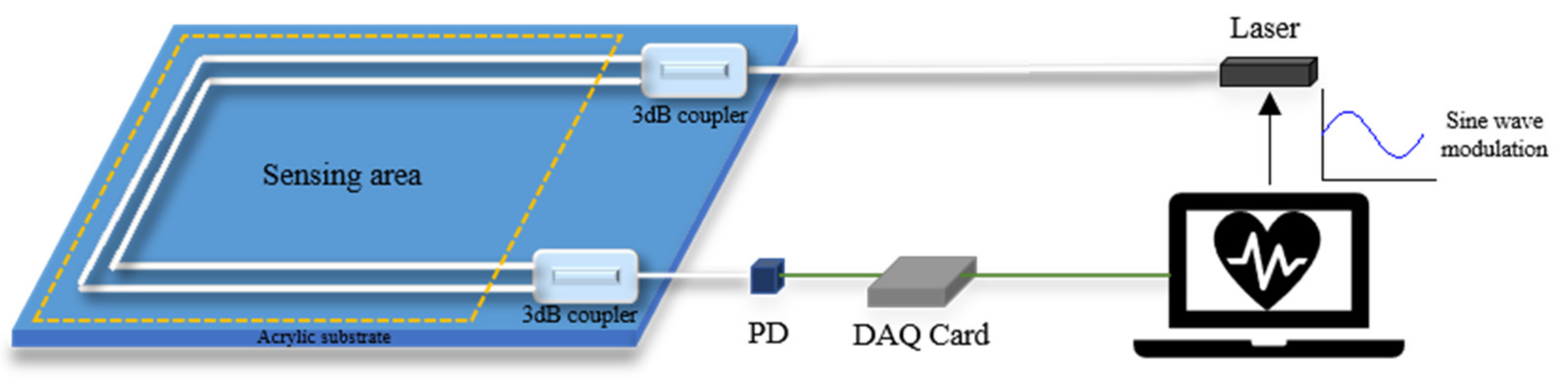

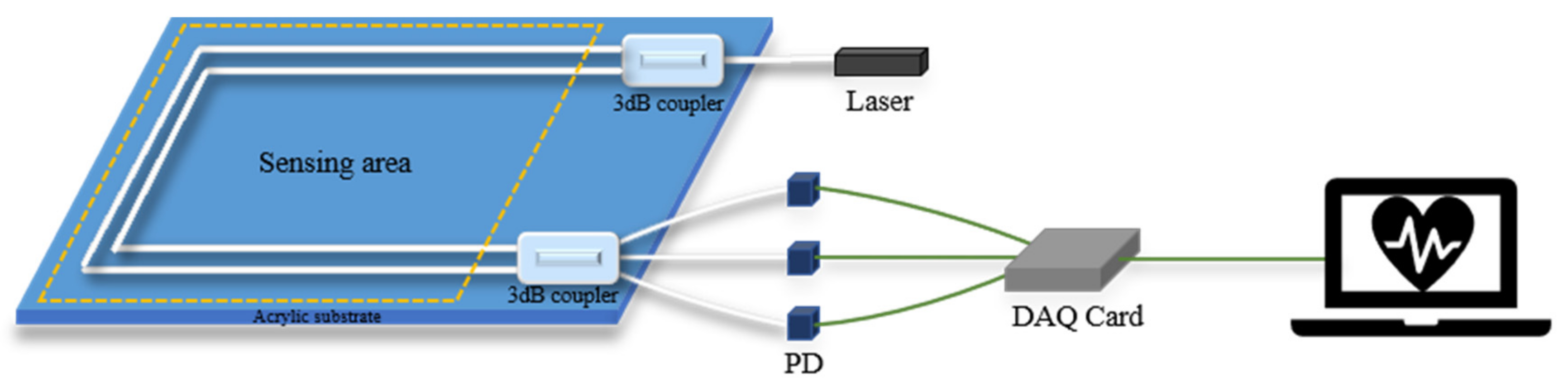
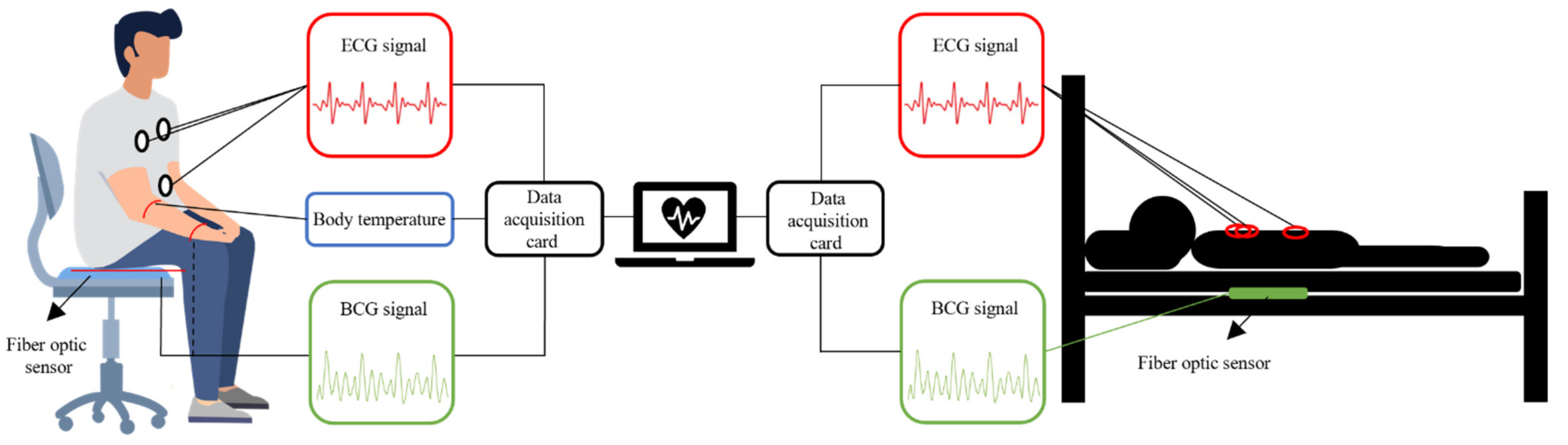
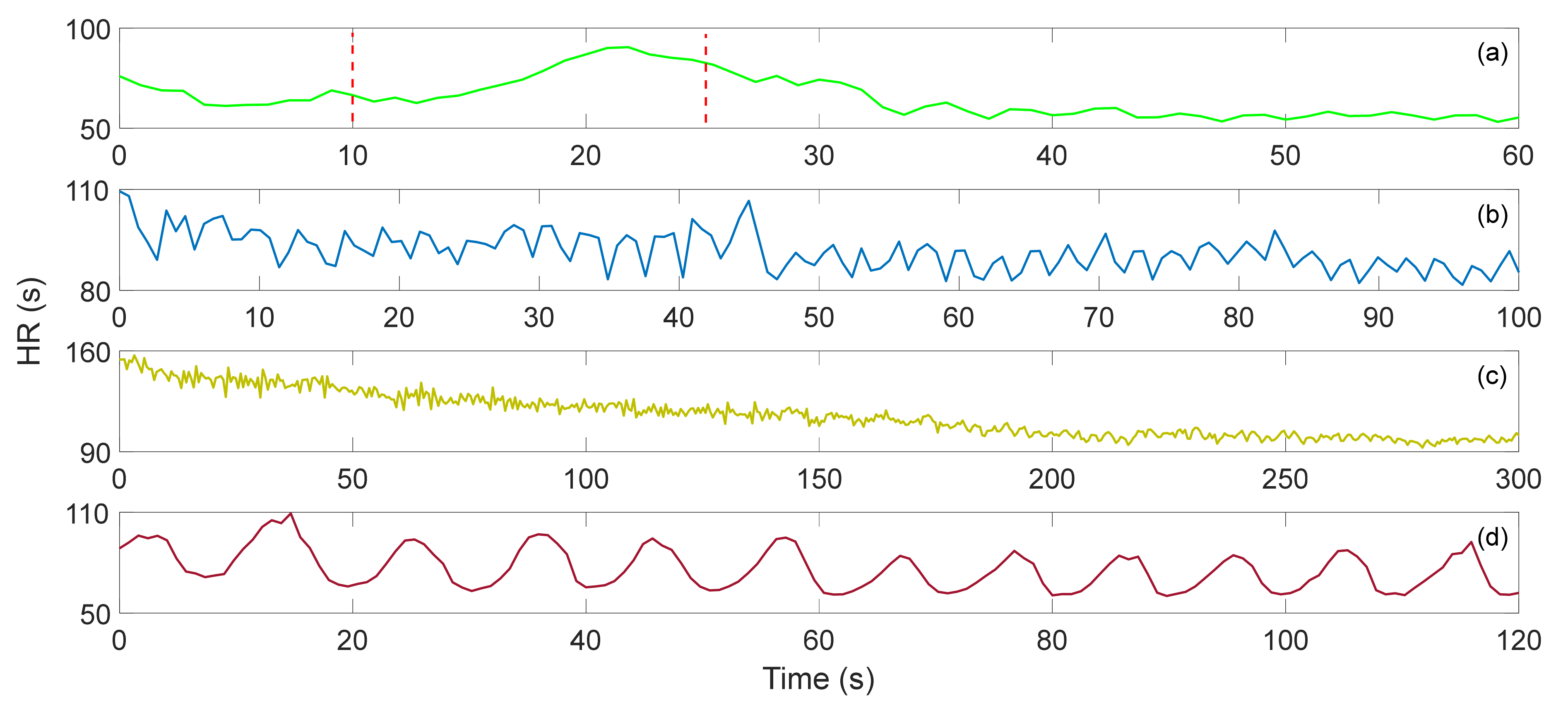
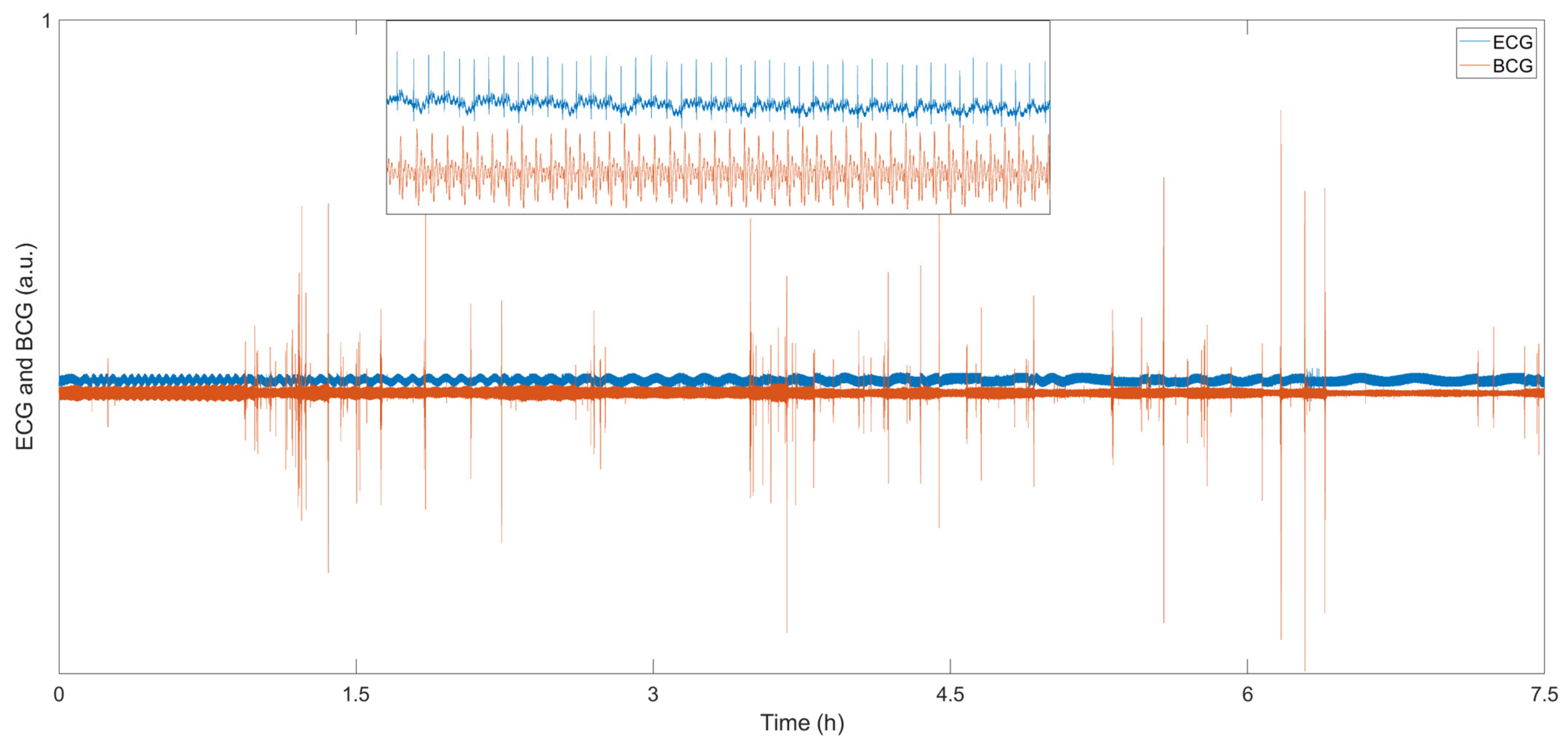
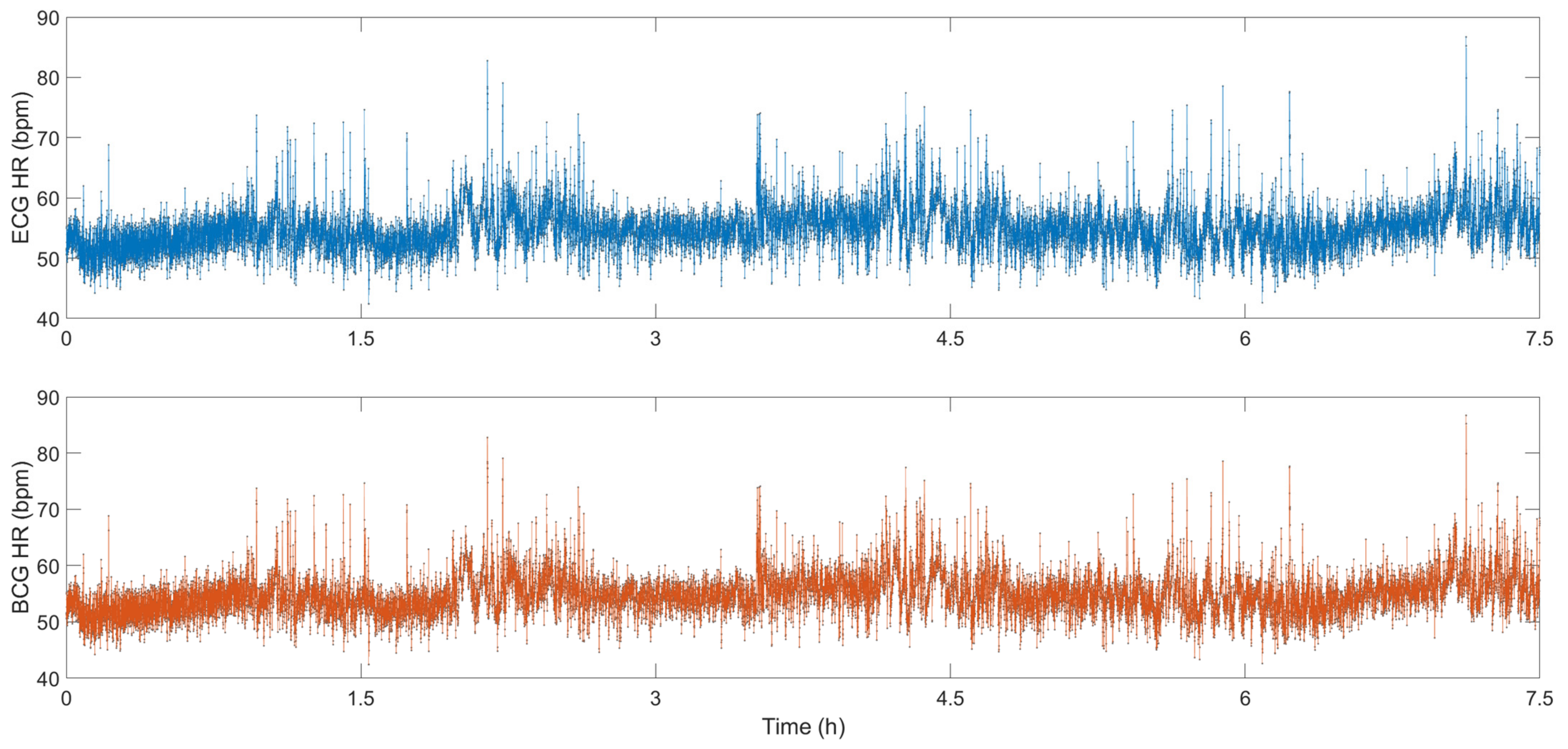
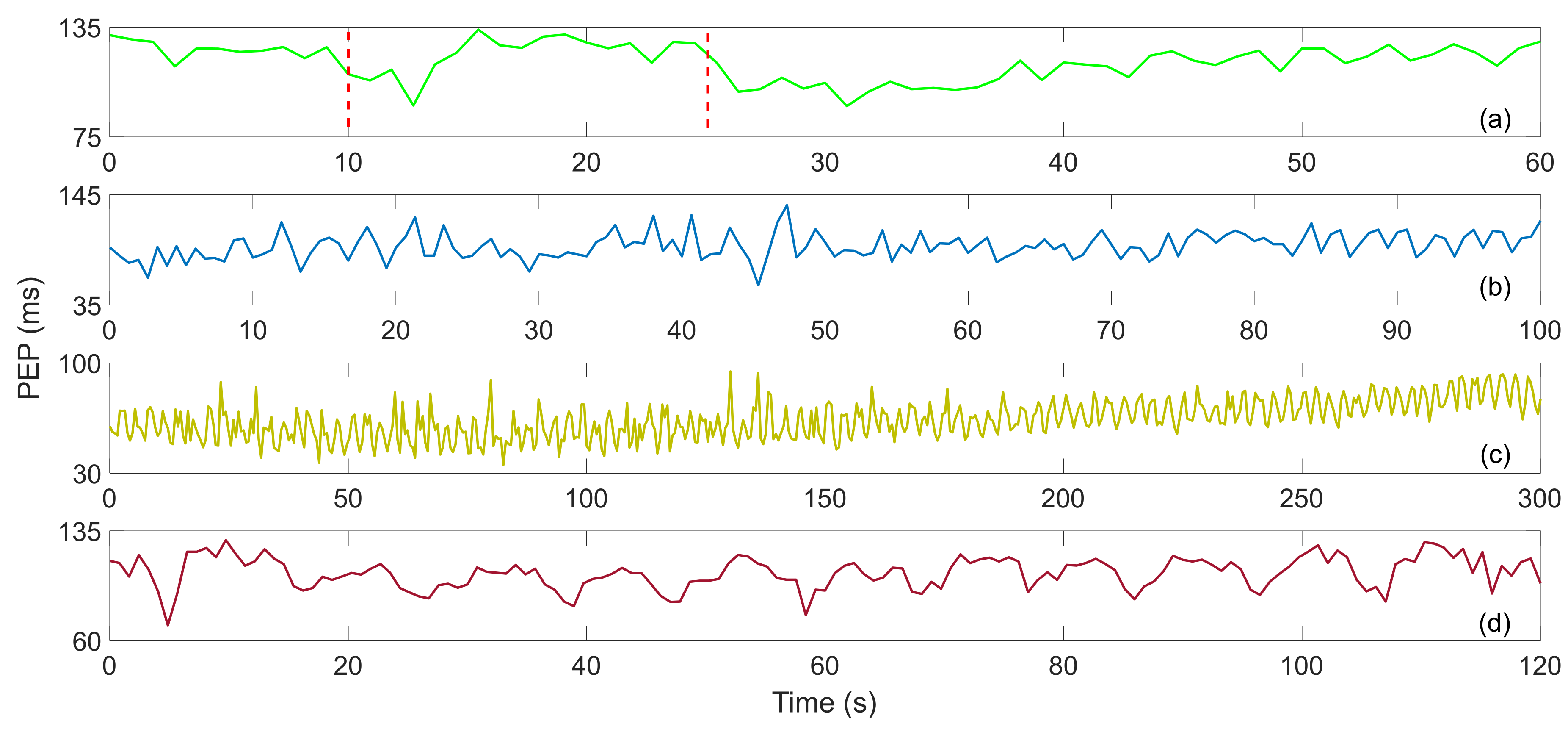

| Interferometers | Manufacturing | Size | Cost | Feasibility |
|---|---|---|---|---|
| Mach–Zehnder interferometer | Easy | Large | Low | Feasible |
| Sagnac interferometer | Medium | Large | Low | Feasible |
| Michelson interferometer | Medium | Large | Low | Feasible |
| Fabry–Perot interferometers | Difficult | Small | High | Feasible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, W.; Chen, S.; Tan, F.; Yu, C. Vital Signs Monitoring Based on Interferometric Fiber Optic Sensors. Photonics 2022, 9, 50. https://doi.org/10.3390/photonics9020050
Lyu W, Chen S, Tan F, Yu C. Vital Signs Monitoring Based on Interferometric Fiber Optic Sensors. Photonics. 2022; 9(2):50. https://doi.org/10.3390/photonics9020050
Chicago/Turabian StyleLyu, Weimin, Shuyang Chen, Fengze Tan, and Changyuan Yu. 2022. "Vital Signs Monitoring Based on Interferometric Fiber Optic Sensors" Photonics 9, no. 2: 50. https://doi.org/10.3390/photonics9020050





