Transient Thermal Response of Blood Vessels during Laser Irradiation Monitored by Laser Speckle Contrast Imaging
Abstract
:1. Introduction
2. Materials and Methods
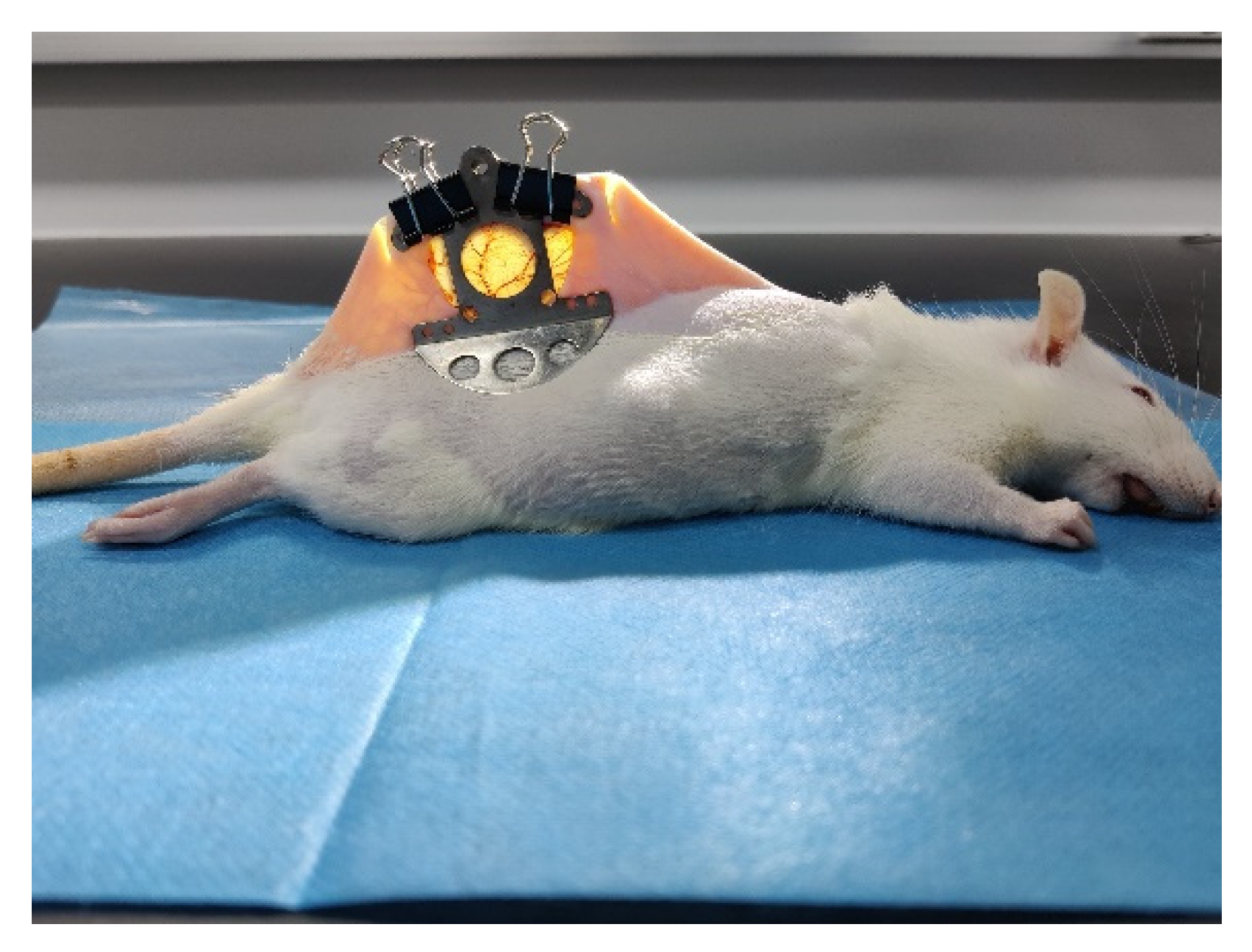
2.1. Animal Preparation
2.2. LSCI System
2.3. Theory of the LSCI and the Anisotropic Diffusion Filter
3. Results
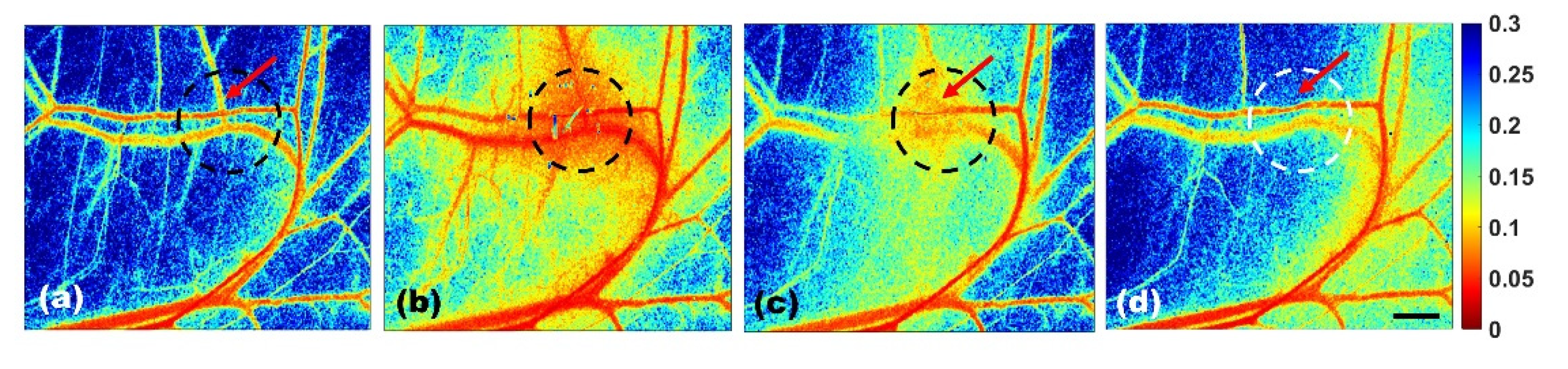
3.1. Transient Thermal Response of Blood Vessels by the sLSCI Algorithm
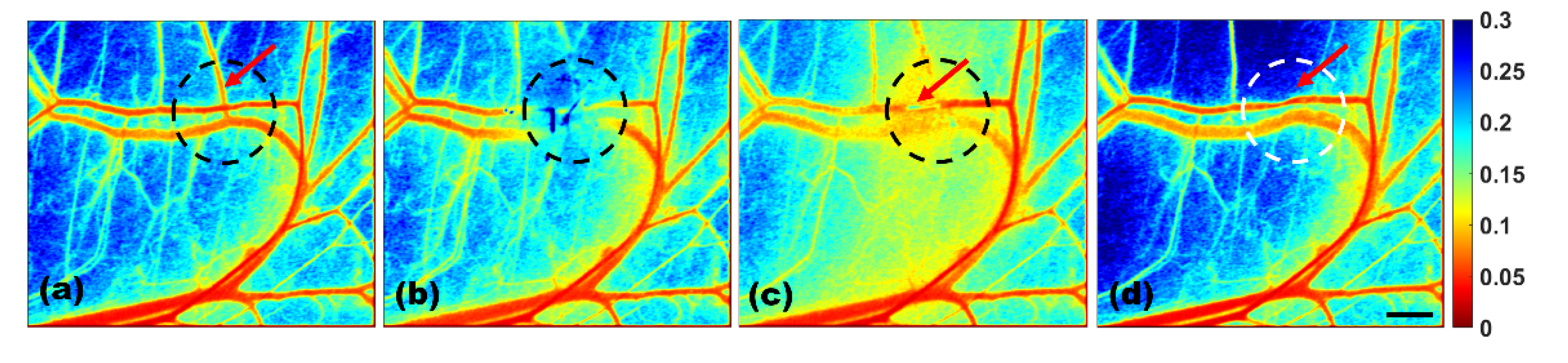
3.2. Transient Thermal Response of Blood Vessels by the tLSCI Algorithm
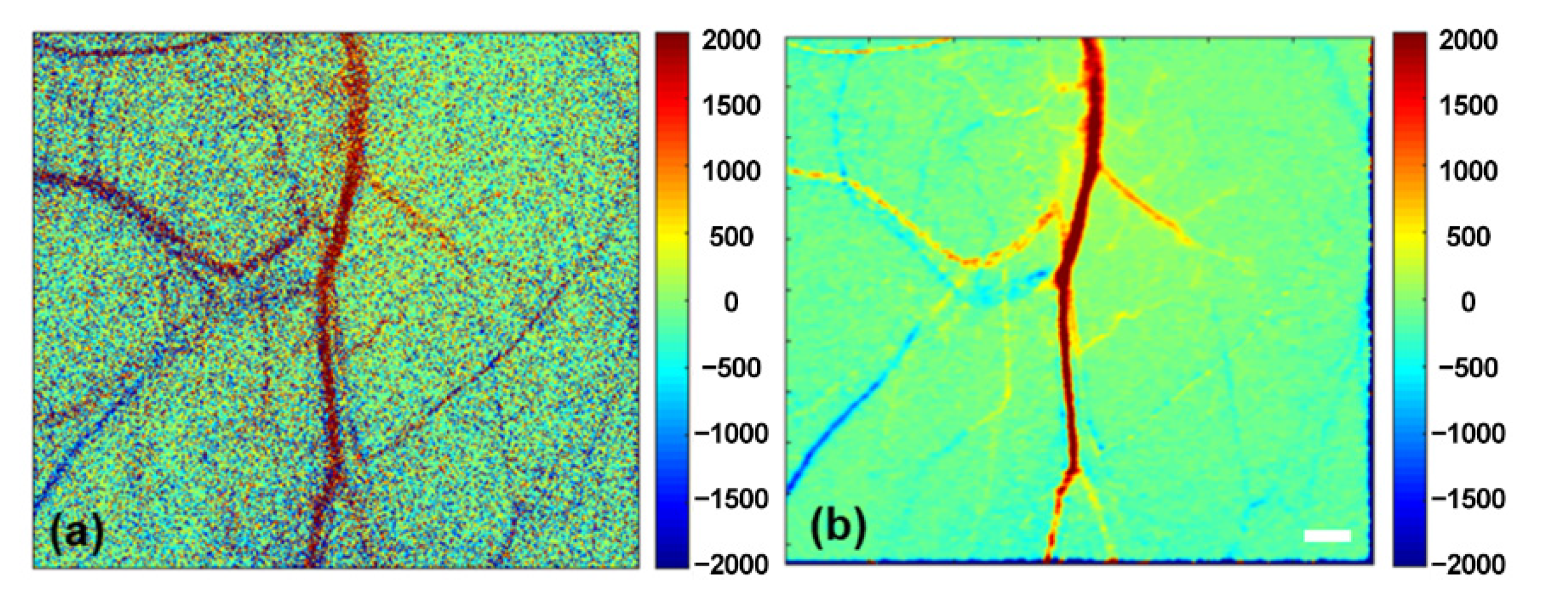
3.3. SNR and Temporal-Resolution Improvement by Introducing the ADF Algorithm
3.4. Label-Free High-Resolution Thrombosis Monitoring by the tLSCI-ADF Algorithm
3.5. Speckle Flow Index
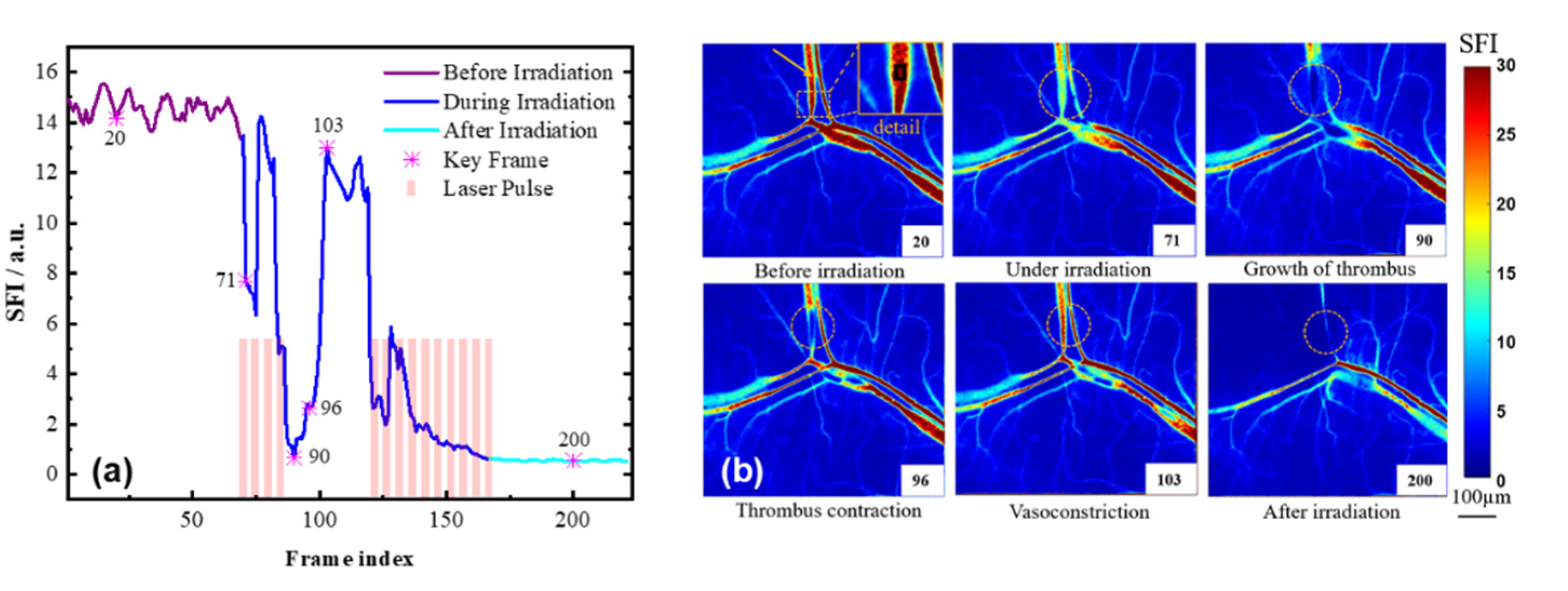
3.6. Blood-Flow Monitoring during Laser Irradiation Using the SFI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alster, T.S.; Wilson, F. Treatment of port-wine stains WITH the flashlamp-pumped pulsed dye-laser-extended clinical-experience in children and adults. Ann. Plast. Surg. 1994, 32, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. Microvasculature can be selectively damaged using dye lasers: A basic theory and experimental evidence in human skin. Lasers Surg. Med. 1981, 1, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Choi, B.; McFarlane, S.; Motosue, A.; Jung, B.J.; Khan, M.H.; Ramirez-San-Juan, J.C.; Nelson, J.S. Description and analysis of treatments for port-wine stain birthmarks. Arch. Facial Plast. Surg. 2005, 7, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Li, R.; Jia, H.; Chen, B.; Wu, W.; Ying, Z. Experimental and Numerical Investigation on the Transient Vascular Thermal Response to Multi-Pulse Nd:YAG Laser. Lasers Surg. Med. 2017, 49, 852–865. [Google Scholar] [CrossRef]
- Jia, H.; Chen, B.; Li, D. Dynamic optical absorption characteristics of blood after slow and fast heating. Lasers Med. Sci. 2017, 32, 513–525. [Google Scholar] [CrossRef]
- Fercher, A.F.; Briers, J.D. flow visualization by means of single-exposure speckle photography. Opt. Commun. 1981, 37, 326–330. [Google Scholar] [CrossRef]
- Jia, W.; Sun, V.; Tran, N.; Choi, B.; Liu, S.-W.; Mihm, M.C., Jr.; Phung, T.L.; Nelson, J.S. Long-Term Blood Vessel Removal With Combined Laser and Topical Rapamycin Antiangiogenic Therapy: Implications for Effective Port Wine Stain Treatment. Lasers Surg. Med. 2010, 42, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Heger, M.; Salles, I.I.; Bezemer, R.; Cloos, M.A.; Mordon, S.R.; Begu, S.; Deckmyn, H.; Beek, J.F. Laser-induced primary and secondary hemostasis dynamics and mechanisms in relation to selective photothermolysis of port wine stains. J. Dermatol. Sci. 2011, 63, 139–147. [Google Scholar] [CrossRef]
- Ma, J.; Chen, B.; Zhang, Y.; Li, D.; Xing, Z.L. Multiple laser pulses in conjunction with an optical clearing agent to improve the curative effect of cutaneous vascular lesions. Lasers Med. Sci. 2017, 32, 1321–1335. [Google Scholar] [CrossRef]
- Nebritova, O.A. A study of hemodynamics and the structure of capillary blood flow using the method of laser speckle contrast analysis LASCA. J. Phys. Conf. Ser. 2019, 1348, 012065. [Google Scholar] [CrossRef]
- Hu, Z.; Li, D.; Zhong, X.; Li, Y.; Xuan, A.; Yu, T.; Zhu, J.; Zhu, D. In vivo tissue optical clearing assisted through-skull targeted photothrombotic ischemic stroke model in mice. J. Biomed. Opt. 2022, 27, 065001. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, X.; Zhang, R.; Zhang, K.; Zhou, Z.; Elson, D.S. Improving temporal resolution and speed sensitivity of laser speckle contrast analysis imaging based on noise reduction with an anisotropic diffusion filter. J. Opt. 2018, 20, 075301. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, M.M.; Hajjarian, Z.; Van Cott, E.M.; Nadkarni, S.K. Assessing blood coagulation status with laser speckle rheology. Biomed. Opt. Express 2014, 5, 817–831. [Google Scholar] [CrossRef]
- Briers, J.D.; Webster, S. Laser speckle contrast analysis (LASCA): A nonscanning, full-field technique for monitoring capillary blood flow. J. Biomed. Opt. 1996, 1, 174–179. [Google Scholar] [CrossRef]
- Li, P.C.; Ni, S.L.; Zhang, L.; Zeng, S.Q.; Luo, Q.M. Imaging cerebral blood flow through the intact rat skull with temporal laser speckle imaging. Opt. Lett. 2006, 31, 1824–1826. [Google Scholar] [CrossRef]
- Qiu, J.; Li, P.; Luo, W.; Wang, J.; Zhang, H.; Luo, Q. Spatiotemporal laser speckle contrast analysis for blood flow imaging with maximized speckle contrast. J. Biomed. Opt. 2010, 15, 016003. [Google Scholar] [CrossRef]
- Cheng, W.; Zhu, X.; Chen, X.; Li, M.; Lu, J.; Li, P. Manhattan Distance-Based Adaptive 3D Transform-Domain Collaborative Filtering for Laser Speckle Imaging of Blood Flow. IEEE Trans. Med. Imaging 2019, 38, 1726–1735. [Google Scholar] [CrossRef]
- Song, X.M.; Pogue, B.W.; Jiang, S.D.; Doyley, M.M.; Dehghani, H.; Tosteson, T.D.; Paulsen, K.D. Automated region detection based on the contrast-to-noise ratio in near-infrared tomography. Appl. Opt. 2004, 43, 1053–1062. [Google Scholar] [CrossRef] [Green Version]
- Sang, X.; Li, D.; Chen, B. Improving imaging depth by dynamic laser speckle imaging and topical optical clearing for in vivo blood flow monitoring. Lasers Med. Sci. 2021, 36, 387–399. [Google Scholar] [CrossRef]
- Murari, K.; Li, N.; Rege, A.; Jia, X.; All, A.; Thakor, N. Contrast-enhanced imaging of cerebral vasculature with laser speckle. Appl. Opt. 2007, 46, 5340–5346. [Google Scholar] [CrossRef]
- Ramirez-San-Juan, J.C.; Ramos-Garcia, R.; Guizar-Iturbide, I.; Martinez-Niconoff, G.; Choi, B. Impact of velocity distribution assumption on simplified laser speckle imaging equation. Opt. Express 2008, 16, 3197–3203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Lee, Y.; Lee, S.; Kim, J.G. Changes in Breast-tumor Blood Flow in Response to Hypercapnia during Chemotherapy with Laser Speckle Flowmetry. Curr. Opt. Photonics 2019, 3, 555–565. [Google Scholar]
- Owen, Y.; David, C.; Bernard, C. Real-time blood flow visualization using the graphics processing unit. J. Biomed. Opt. 2011, 16. [Google Scholar]
- Kazmi, S.M.S.; Richards, L.M.; Schrandt, C.J.; Davis, M.A.; Dunn, A.K. Expanding applications, accuracy, and interpretation of laser speckle contrast imaging of cerebral blood flow. J. Cereb. Blood Flow Metab. 2015, 35, 1076–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecht, N.; Woitzik, J.; Koenig, S.; Horn, P.; Vajkoczy, P. Laser speckle imaging allows real-time intraoperative blood flow assessment during neurosurgical procedures. J. Cereb. Blood Flow Metab. 2013, 33, 1000–1007. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Jeong, Y. In vivo imaging for neurovascular disease research. Arch. Pharmacal Res. 2019, 42, 263–273. [Google Scholar] [CrossRef]
- Tarantini, S.; Fulop, G.A.; Kiss, T.; Farkas, E.; Zolei-Szenasi, D.; Galvan, V.; Toth, P.; Csiszar, A.; Ungvari, Z.; Yabluchanskiy, A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience 2017, 39, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Briers, J.D.; Fercher, A.F. Retinal blood-flow visualization by means of laser speckle photography. Investig. Ophthalmol. Vis. Sci. 1982, 22, 255–259. [Google Scholar]
- Patel, D.D.; Lipinski, D.M. Validating a low-cost laser speckle contrast imaging system as a quantitative tool for assessing retinal vascular function. Sci. Rep. 2020, 10, 7177. [Google Scholar] [CrossRef]
- Asano, T.; Kunikata, H.; Yasuda, M.; Nishiguchi, K.M.; Abe, T.; Nakazawa, T. Ocular microcirculation changes, measured with laser speckle flowgraphy and optical coherence tomography angiography, in branch retinal vein occlusion with macular edema treated by ranibizumab. Int. Ophthalmol. 2021, 41, 151–162. [Google Scholar] [CrossRef]
- Forrester, K.R.; Tulip, J.; Leonard, C.; Stewart, C.; Bray, R.C. A laser speckle imaging technique for measuring tissue perfusion. IEEE Trans. Biomed. Eng. 2004, 51, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Tran, N.; Nelson, J.S.; Choi, B. Noninvasive blood flow imaging for real-time feedback during laser therapy of port wine stain birthmarks. In Proceedings of the 3rd Frontiers in Biomedical Devices Conference and Exposition, Irvine, CA, USA, 18–20 June 2008; pp. 111–112. [Google Scholar]
- Allan, D.; Chockalingam, N.; Naemi, R. Validation of a non-invasive imaging photoplethysmography device to assess plantar skin perfusion, a comparison with laser speckle contrast analysis. J. Med. Eng. Technol. 2021, 45, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Gnyawali, S.C.; Blum, K.; Pal, D.; Ghatak, S.; Khanna, S.; Roy, S.; Sen, C.K. Retooling Laser Speckle Contrast Analysis Algorithm to Enhance Non-Invasive High Resolution Laser Speckle Functional Imaging of Cutaneous Microcirculation. Sci. Rep. 2017, 7, 41048. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, X.; Chen, B.; Li, D.; Pan, D.; Sang, X. Transient Thermal Response of Blood Vessels during Laser Irradiation Monitored by Laser Speckle Contrast Imaging. Photonics 2022, 9, 520. https://doi.org/10.3390/photonics9080520
Sang X, Chen B, Li D, Pan D, Sang X. Transient Thermal Response of Blood Vessels during Laser Irradiation Monitored by Laser Speckle Contrast Imaging. Photonics. 2022; 9(8):520. https://doi.org/10.3390/photonics9080520
Chicago/Turabian StyleSang, Xu, Bin Chen, Dong Li, Deqing Pan, and Xuehao Sang. 2022. "Transient Thermal Response of Blood Vessels during Laser Irradiation Monitored by Laser Speckle Contrast Imaging" Photonics 9, no. 8: 520. https://doi.org/10.3390/photonics9080520
APA StyleSang, X., Chen, B., Li, D., Pan, D., & Sang, X. (2022). Transient Thermal Response of Blood Vessels during Laser Irradiation Monitored by Laser Speckle Contrast Imaging. Photonics, 9(8), 520. https://doi.org/10.3390/photonics9080520




