Choroidal Melanoma: A Mini Review
Abstract
:1. Introduction
2. Epidemiology
3. Pathophysiology
4. Genetics
5. Risk Factors
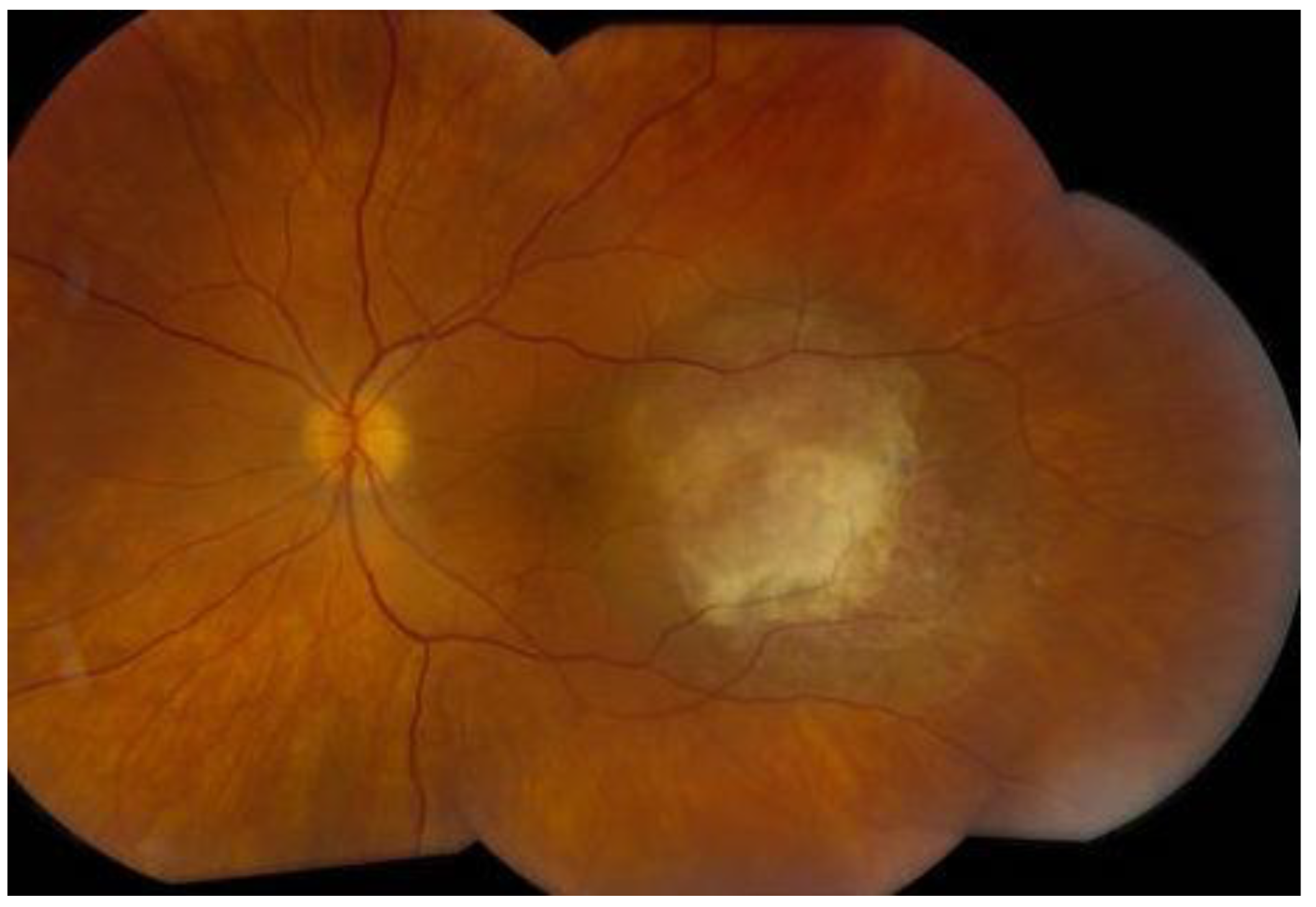
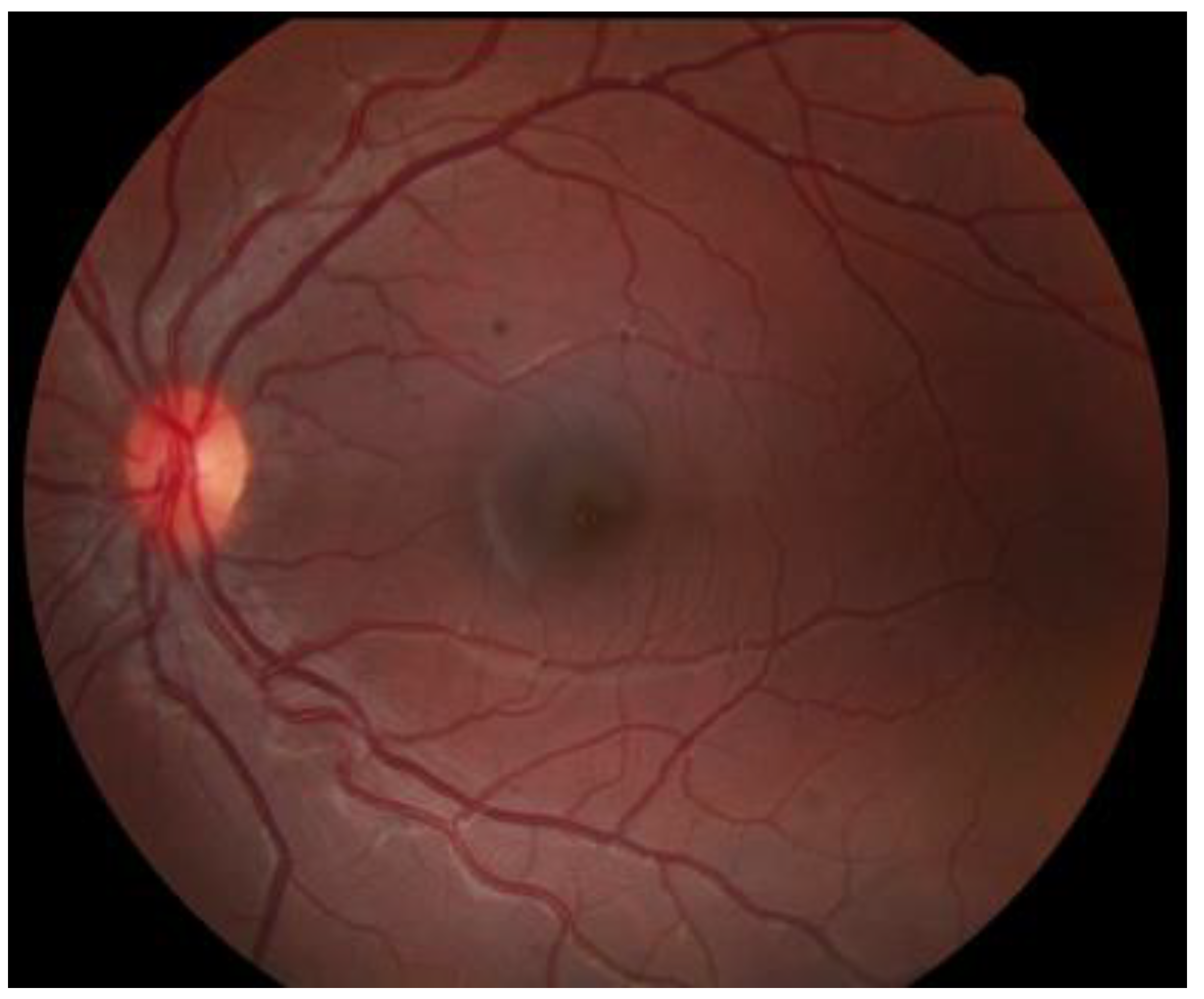
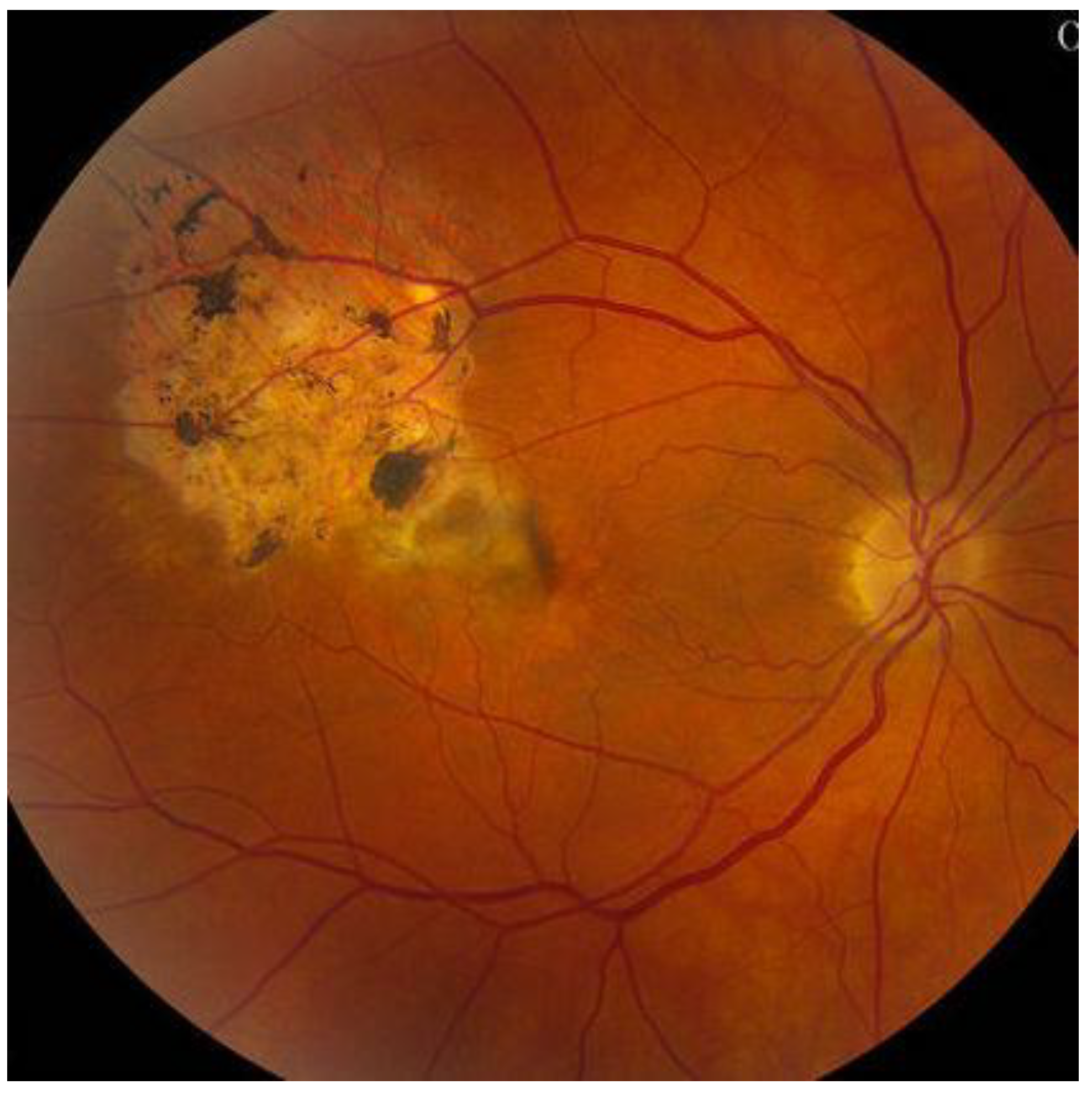
6. History
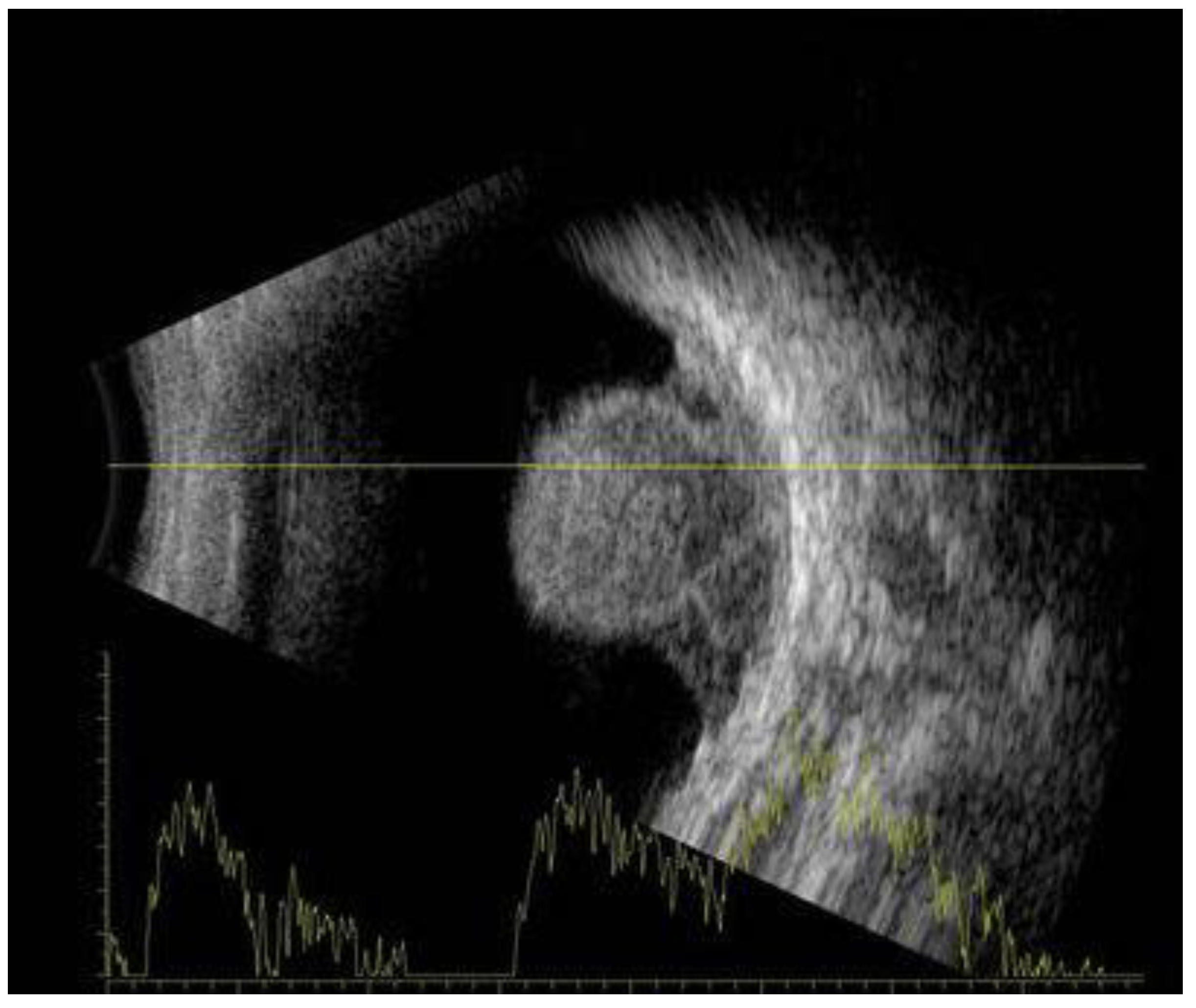
7. Physical Examination and Evaluation
8. Differential Diagnoses
9. Classifications
- Size according to COMS:
- -
- Small: 4.0–8.0 mm diameter and/or 1.0–2.4 mm height;
- -
- Medium: 6–16 mm diameter and/or 2.5–10.0 mm height;
- -
- Large: >16 mm diameter and/or >10.0 mm height [35].
- Based on the cell type: epithelioid, spindle, mixed [34].
- The tumour, Node, Metastasis (TNM) staging system has several limitations for the classification and prognostication of uveal melanomas, such as the lack of lymph node dissemination, unlike other tumours including conjunctival melanoma [36]. In fact, Cai and colleagues [36] reported that gene-expression profiling is superior in choroidal melanoma classification compared with the TNM classification system.
10. Management
11. Radiation Therapy
12. Enucleation
13. Laser Therapy
14. Choroidal Melanoma Treatment
15. Management for Metastatic Disease
16. Surveillance
17. Prognosis and Survival
18. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, P.; Singh, A. Choroidal melanoma. Oman J. Ophthalmol. 2012, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef] [PubMed]
- Violanti, S.S.; Bononi, I.; Gallenga, C.E.; Martini, F.; Tognon, M.; Perri, P. New insights into molecular oncogenesis and therapy of uveal melanoma. Cancers 2019, 11, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krantz, B.A.; Dave, N.; Komatsubara, K.M.; Marr, B.P.; Carvajal, R.D. Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin. Ophthalmol. 2017, 11, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzidou, C.; Trivli, A.; Dalianis, G.; Apessou, D.; Spandidos, D.A.; Goulielmos, G.N. Advanced choroidal melanoma with a desirable aesthetic outcome after enucleation: A case report. Oncol. Lett. 2018, 16, 511–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadowsky, D.; Delijani, K.; Lim, J.; Cabrera, M. Uveal melanoma. Georget. Med. Rev. 2022, 6, 24. [Google Scholar] [CrossRef]
- Vasalaki, M.; Fabian, I.D.; Reddy, M.A.; Cohen, V.M.; Sagoo, M.S. Ocular oncology: Advances in retinoblastoma, uveal melanoma and conjunctival melanoma. Br. Med. Bull. 2017, 121, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Xia, F.; Yu, Z.; Deng, A.; Gao, G. Identification of molecular subtyping system and four-gene prognostic signature with immune-related genes for uveal melanoma. Exp. Biol. Med. 2022, 247, 246–262. [Google Scholar] [CrossRef]
- Bornfeld, N.; Prescher, G.; Becher, R.; Hirche, H.; Jöckel, K.; Horsthemke, B. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996, 347, 1222–1225. [Google Scholar] [CrossRef]
- Damato, B.; Dopierala, J.A.; Coupland, S.E. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin. Cancer Res. 2010, 16, 6083–6092. [Google Scholar] [CrossRef]
- Cassoux, N.; Rodrigues, M.J.; Plancher, C.; Asselain, B.; Levy-Gabriel, C.; Lumbroso-Le Rouic, L.; Piperno-Neumann, S.; Dendale, R.; Sastre, X.; Desjardins, L.; et al. Genome-wide profiling is a clinically relevant and affordable prognostic test in posterior uveal melanoma. Br. J. Ophthalmol. 2014, 98, 769–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Laurin Council, M.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavuzyigitoglu, S.; Koopmans, A.E.; Verdijk, R.M.; Vaarwater, J.; Eussen, B.; Van Bodegom, A.; Paridaens, D.; Kiliç, E.; de Klein, A.; Rotterdam Ocular Melanoma Study Group. Uveal melanomas with SF3B1 mutations: A distinct subclass associated with late-onset metastases. Ophthalmology 2016, 123, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Maßhöfer, L.; Temming, P.; Rahmann, S.; Metz, C.; Bornfeld, N.; van de Nes, J.; Klein-Hitpass, L.; Hinnebusch, A.G.; Horsthemke, B.; et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat. Genet. 2013, 45, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbour, J.W. A prognostic test to predict the risk of metastasis in uveal melanoma based on a 15-gene expression profile. In Molecular Diagnostics for Melanoma; Springer: Berlin/Heidelberg, Germany, 2014; pp. 427–440. [Google Scholar]
- McDonald, K.A.; Krema, H.; Chan, A.-W. Cutaneous signs and risk factors for ocular melanoma. J. Am. Acad. Dermatol. 2021, 84, 1732–1734. [Google Scholar] [CrossRef]
- Smith, J.H.; Padnick-Silver, L.; Newlin, A.; Rhodes, K.; Rubinstein, W.S. Genetic study of familial uveal melanoma: Association of uveal and cutaneous melanoma with cutaneous and ocular nevi. Ophthalmology 2007, 114, 774–779. [Google Scholar] [CrossRef]
- Singh, A.D.; Kalyani, P.; Topham, A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology 2005, 112, 1784–1789. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Livesey, M.; Walker, B.; Garoon, R.; Bucci, M.; Feinstein, E.; Pesch, A.; Gonzalez, C.; Lally, S.E.; et al. Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis: Analysis of 7872 consecutive eyes. JAMA Ophthalmol. 2013, 131, 993–1003. [Google Scholar] [CrossRef] [Green Version]
- Shah, C.P.; Weis, E.; Lajous, M.; Shields, J.A.; Shields, C.L. Intermittent and chronic ultraviolet light exposure and uveal melanoma: A meta-analysis. Ophthalmology 2005, 112, 1599–1607. [Google Scholar] [CrossRef]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.-H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Prim. 2020, 6, 24. [Google Scholar] [CrossRef]
- Kaler, C.J.; Dollar, J.J.; Cruz, A.M.; Kuznetsoff, J.N.; Sanchez, M.I.; Decatur, C.L.; Licht, J.D.; Smalley, K.S.; Correa, Z.M.; Kurtenbach, S.; et al. BAP1 loss promotes suppressive tumor immune microenvironment via upregulation of PROS1 in class 2 uveal melanomas. Cancers 2022, 14, 3678. [Google Scholar] [CrossRef] [PubMed]
- Damato, E.M.; Damato, B.E. Detection and time to treatment of uveal melanoma in the United Kingdom: An evaluation of 2384 patients. Ophthalmology 2012, 119, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Ebert, J.J.; Di Nicola, M.; Williams, B.K., Jr. Operative complications of posterior uveal melanoma surgery. Int. Ophthalmol. Clin. 2022, 62, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Solnik, M.; Paduszyńska, N.; Czarnecka, A.M.; Synoradzki, K.J.; Yousef, Y.A.; Chorągiewicz, T.; Rejdak, R.; Toro, M.D.; Zweifel, S.; Dyndor, K.; et al. Imaging of uveal melanoma—Current standard and methods in development. Cancers 2022, 14, 3147. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.M.; Egan, K.M.; Kim, I.K.; Gragoudas, E.S. Mortality after diagnosis of small melanocytic lesions of the choroid. Arch. Ophthalmol. 2010, 128, 996–1000. [Google Scholar] [CrossRef] [Green Version]
- Harbour, J.W.; Paez-Escamilla, M.; Cai, L.; Walter, S.D.; Augsburger, J.J.; Correa, Z.M. Are risk factors for growth of choroidal nevi associated with malignant transformation? Assessment with a validated genomic biomarker. Am. J. Ophthalmol. 2019, 197, 168–179. [Google Scholar] [CrossRef]
- Dalvin, L.A.; Shields, C.L.; Ancona-Lezama, D.A.; Michael, D.Y.; Di Nicola, M.; Williams, B.K., Jr.; Lucio-Alvarez, J.A.; Ang, S.M.; Maloney, S.M. Combination of multimodal imaging features predictive of choroidal nevus transformation into melanoma. Br. J. Ophthalmol. 2019, 103, 1441–1447. [Google Scholar] [CrossRef]
- Shields, C.L.; Sioufi, K.; Srinivasan, A.; Di Nicola, M.; Masoomian, B.; Barna, L.E.; Bekerman, V.P.; Say, E.A.; Mashayekhi, A.; Emrich, J.; et al. Visual outcome and millimeter incremental risk of metastasis in 1780 patients with small choroidal melanoma managed by plaque radiotherapy. JAMA Ophthalmol. 2018, 136, 1325–1333. [Google Scholar] [CrossRef] [Green Version]
- Negretti, G.S.; Kalafatis, N.E.; Shields, J.A.; Shields, C.L. Choroidal melanoma masquerading as central serous chorioretinopathy. Ophthalmol. Retina 2022, in press. [Google Scholar] [CrossRef]
- Obuchowska, I.; Konopińska, J. Importance of optical coherence tomography and optical coherence tomography angiography in the imaging and differentiation of choroidal melanoma: A review. Cancers 2022, 14, 3354. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhang, L.; Tang, F.; Wei, X. Application of multimodal and molecular imaging techniques in the detection of choroidal melanomas. Front. Oncol. 2021, 10, 617868. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Augsburger, J.J.; Brown, G.C.; Stephens, R.F. The differential diagnosis of posterior uveal melanoma. Ophthalmology 1980, 87, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Choroidal Melenoma. 2016. Available online: https://www.aao.org/topic-detail/choroidal-melanoma-europe (accessed on 15 May 2020).
- Margo, C.E. The collaborative ocular melanoma study: An overview. Cancer Control 2004, 11, 304–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Paez-Escamilla, M.; Walter, S.D.; Tarlan, B.; Decatur, C.L.; Perez, B.M.; Harbour, J.W. Gene expression profiling and PRAME status versus tumor-node-metastasis staging for prognostication in uveal melanoma. Am. J. Ophthalmol. 2018, 195, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L. Management of posterior uveal melanoma: Past, present, and future: The 2014 Charles L. Schepens lecture. Ophthalmology 2015, 122, 414–428. [Google Scholar] [CrossRef]
- Caminal, J.; Padrón-Pérez, N.; Arias, L.; Masuet-Aumatell, C.; Gutiérrez, C.; Piulats, J.; Pera, J.; Català, J.; Rubio, M.J.; Arruga, J. Transscleral resection without hypotensive anaesthesia vs iodine-125 plaque brachytherapy in the treatment of choroidal melanoma. Eye 2016, 30, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Bechrakis, N.E.; Bornfeld, N.; Zöller, I.; Foerster, M.H. Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology 2002, 109, 1855–1861. [Google Scholar] [CrossRef]
- Force, T.; Simpson, E.R.; Gallie, B.; Laperrierre, N.; Beiki-Ardakani, A.; Kivelä, T.; Raivio, V.; Heikkonen, J.; Desjardins, L.; Dendale, R.; et al. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy 2014, 13, 1–14. [Google Scholar]
- Seibel, I.; Cordini, D.; Rehak, M.; Hager, A.; Riechardt, A.I.; Böker, A.; Heufelder, J.; Weber, A.; Gollrad, J.; Besserer, A.; et al. Local recurrence after primary proton beam therapy in uveal melanoma: Risk factors, retreatment approaches, and outcome. Am. J. Ophthalmol. 2015, 160, 628–636. [Google Scholar] [CrossRef]
- Willerding, G.D.; Cordini, D.; Moser, L.; Krause, L.; Foerster, M.H.; Bechrakis, N.E. Neoadjuvant proton beam irradiation followed by transscleral resection of uveal melanoma in 106 cases. Br. J. Ophthalmol. 2016, 100, 463–467. [Google Scholar] [CrossRef]
- Caminal, J.M.; Lorenzo, D.; Gutierrez, C.; Slocker, A.; Piulats, J.M.; Cobos, E.; Garcia-Bru, P.; Morwani, R.; Santamaria, J.F.; Arias, L. Local resection in choroidal melanoma: A review. J. Clin. Med. 2022, 11, 7156. [Google Scholar] [CrossRef] [PubMed]
- Puusaari, I.; Damato, B.; Kivelä, T. Transscleral local resection versus iodine brachytherapy for uveal melanomas that are large because of tumour height. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Augsburger, J.J.; Lauritzen, K.; Gamel, J.W.; DeBrakeleer, D.J.; Lowry, J.C.; Eisenman, R. Matched Group Study of Surgical Resection versus Cobalt-60 Plaque Radiotherapy for Primary Choroidal or Ciliary Body Melanoma; SLACK Incorporated: West Deptford, NJ, USA, 1990; pp. 682–688. [Google Scholar]
- Foulds, W.S.; Damato, B.E.; Burton, R.L. Local resection versus enucleation in the management of choroidal melanoma. Eye 1987, 1, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Badiyan, S.N.; Rao, R.C.; Apicelli, A.J.; Acharya, S.; Verma, V.; Garsa, A.A.; DeWees, T.; Speirs, C.K.; Garcia-Ramirez, J.; Esthappan, J.; et al. Outcomes of iodine-125 plaque brachytherapy for uveal melanoma with intraoperative ultrasonography and supplemental transpupillary thermotherapy. Int. J. Radiat. Oncol.*Biol.*Phys. 2014, 88, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Saakian, S.; Val’skiĭ, V.; Semenova, E.; Amirian, A. Transpupillary thermotherapy in the treatment of recurrent and residual choroidal melanomas: Preliminary results. Vestn. Oftalmol. 2009, 125, 11–15. [Google Scholar]
- Harbour, J.W.; Meredith, T.A.; Thompson, P.A.; Gordon, M.E. Transpupillary thermotherapy versus plaque radiotherapy for suspected choroidal melanomas. Ophthalmology 2003, 110, 2207–2214. [Google Scholar] [CrossRef]
- Tarmann, L.; Wackernagel, W.; Avian, A.; Mayer, C.; Schneider, M.; Winkler, P.; Langmann, G. Ruthenium-106 plaque brachytherapy for uveal melanoma. Br. J. Ophthalmol. 2015, 99, 1644–1649. [Google Scholar] [CrossRef]
- Turcotte, S.; Bergeron, D.; Rousseau, A.P.; Mouriaux, F. Primary transpupillary thermotherapy for choroidal indeterminate melanocytic lesions. Can. J. Ophthalmol. 2014, 49, 464–467. [Google Scholar] [CrossRef]
- Mashayekhi, A.; Shields, C.L.; Rishi, P.; Atalay, H.T.; Pellegrini, M.; McLaughlin, J.P.; Patrick, K.A.; Morton, S.J.; Remmer, M.H.; Parendo, A.; et al. Primary transpupillary thermotherapy for choroidal melanoma in 391 cases: Importance of risk factors in tumor control. Ophthalmology 2015, 122, 600–609. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Berman, E.L.; Zahler, J.D.; Hoberman, D.M.; Dinh, D.H.; Mashayekhi, A.; Shields, J.A. Choroidal nevus transformation into melanoma: Analysis of 2514 consecutive cases. Arch. Ophthalmol. 2009, 127, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, P.; Mihajlovic, M.; Djordjevic-Jocic, J.; Vlajkovic, S.; Cekic, S.; Stefanovic, V. Ocular melanoma: An overview of the current status. Int. J. Clin. Exp. Pathol. 2013, 6, 1230. [Google Scholar] [PubMed]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Burr, J.M.; Mitry, E.; Rachet, B.; Coleman, M. Survival from uveal melanoma in England and Wales 1986 to 2001. Ophthalmic Epidemiol. 2007, 14, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Sosman, J.A.; Quevedo, J.F.; Milhem, M.M.; Joshua, A.M.; Kudchadkar, R.R.; Linette, G.P.; Gajewski, T.F.; Lutzky, J.; Lawson, D.H.; et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: A randomized clinical trial. JAMA 2014, 311, 2397–2405. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Wang, H.; Ma, F.; Qian, H.; Yi, Z.; Xu, B. The efficacy and safety of programmed cell death 1 and programmed cell death 1 ligand inhibitors for advanced melanoma: A meta-analysis of clinical trials following the PRISMA guidelines. Medicine 2016, 95, e3134. [Google Scholar] [CrossRef]
- Martinez-Perez, D.; Viñal, D.; Solares, I.; Espinosa, E.; Feliu, J. Gp-100 as a novel therapeutic target in uveal melanoma. Cancers 2021, 13, 5968. [Google Scholar] [CrossRef]
- Chen, L.N.; Carvajal, R.D. Tebentafusp for the treatment of HLA-A* 02: 01–positive adult patients with unresectable or metastatic uveal melanoma. Expert Rev. Anticancer Ther. 2022, 22, 1017–1027. [Google Scholar] [CrossRef]
- Willson, J.K.; Albert, D.M.; Diener-West, M.; McCaffrey, L.; Moy, C.S.; Scully, R.E. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the collaborative ocular melanoma study (COMS). COMS report no. 15. Arch. Ophthalmol. 2001, 119, 670–676. [Google Scholar]
- Diener-West, M.; Reynolds, S.M.; Agugliaro, D.J.; Caldwell, R.; Cumming, K.; Earle, J.D.; Green, D.L.; Hawkins, B.S.; Hayman, J.; Jaiyesimi, I.; et al. Screening for metastasis from choroidal melanoma: The collaborative ocular melanoma study group report 23. J. Clin. Oncol. 2004, 22, 2438–2444. [Google Scholar] [CrossRef]
- Nathan, P.; Cohen, V.; Coupland, S.; Curtis, K.; Damato, B.; Evans, J.; Fenwick, S.; Kirkpatrick, L.; Li, O.; Marshall, E.; et al. Uveal melanoma UK national guidelines. Eur. J. Cancer 2015, 51, 2404–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Shields, C.; Shields, J. Prognostic factors in uveal melanoma. Melanoma Res. 2001, 11, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.; Duke, C.; Coupland, S.E.; Hiscott, P.; Smith, P.A.; Campbell, I.; Douglas, A.; Howard, P. Cytogenetics of uveal melanoma: A 7-year clinical experience. Ophthalmology 2007, 114, 1925–1931.e1. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Zimak, D.M.; Ivanković, M.; Marković, I.; Antunica, A.G. Liver metastasis in uveal melanoma—Treatment options and clinical outcome. Front. Biosci.-Landmark 2022, 27, 72. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, N.; Mamdouh, D.; Elkordi, A. Choroidal Melanoma: A Mini Review. Medicines 2023, 10, 11. https://doi.org/10.3390/medicines10010011
Soliman N, Mamdouh D, Elkordi A. Choroidal Melanoma: A Mini Review. Medicines. 2023; 10(1):11. https://doi.org/10.3390/medicines10010011
Chicago/Turabian StyleSoliman, Noha, Diaa Mamdouh, and Aisha Elkordi. 2023. "Choroidal Melanoma: A Mini Review" Medicines 10, no. 1: 11. https://doi.org/10.3390/medicines10010011
APA StyleSoliman, N., Mamdouh, D., & Elkordi, A. (2023). Choroidal Melanoma: A Mini Review. Medicines, 10(1), 11. https://doi.org/10.3390/medicines10010011





