Influence of Different Isolation Methods on Chemical Composition and Bioactivities of the Fruit Peel Oil of Citrus medica L. var. sarcodactylis (Noot.) Swingle
Abstract
:1. Introduction
2. Materials and Methods
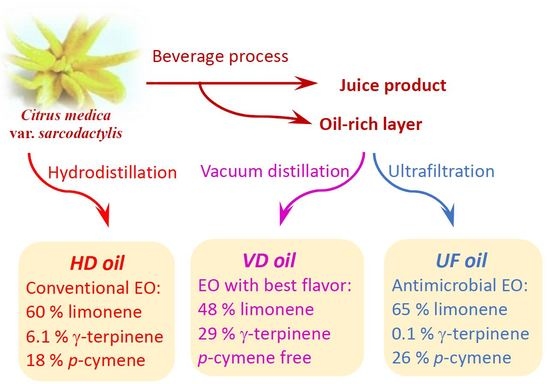
2.1. Materials
2.2. Gas Chromatographic/Mass Spectral Analysis
2.3. Antimicrobial Screening
2.4. Antioxidant Activity Screening
3. Results
3.1. Essential Oil Compositions
3.2. Antimicrobial Activity of Fingered Citron Oils
3.3. Antioxidant Activity
4. Discussions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Pharmacopoeia Commission of the Ministry of Health of the People’s Republic of China. The Pharmacopoeia of the People’s Republic of China (PPRC), 4th ed.China Medical Science Press: Beijing, China, 2010; Volume 1.
- Chen, W.R.; Zhang, Z.Z.; Xin, D.D.; Guo, W.D. Identification and expression analysis of cold-regulated genes in fingered citron (Citrus medica L. var sarcodactylis Swingle). In Vitro Cell. Dev. Biol-Anim. 2010, 46, 120–121. [Google Scholar]
- Jin, X.L.; Xu, L.S.; Zheng, X.H. Chemical compositions of essential oil from fingered citron. J. Instrum. Anal. 2000, 19, 71–73. [Google Scholar]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, M.; Brahim, N.B.; Sebei, H. Composition of peel essential oils from four selected tunisian citrus species: Evidence for the genotypic influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Peng, C.H.; Ker, Y.B.; Weng, C.F.; Peng, C.C.; Huang, C.N.; Lin, L.Y.; Peng, R.Y. Insulin secretagogue bioactivity of finger citron fruit (Citrus medica L. var. sarcodactylis Hort, Rutaceae). J. Agric. Food Chem. 2009, 57, 8812–8819. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N. Essential oils and anxiolytic aromatherapy. Nat. Prod. Commun. 2009, 4, 1305–1316. [Google Scholar] [PubMed]
- Liang, S.J.; Wu, H.; Lun, X.; Lu, D.W. Secretory cavity development and its relationship with the accumulation of essential oil in fruits of Citrus medica L. var. Sarcodactylis (Noot.) Swingle. J. Integr. Plant Biol. 2006, 48, 573–583. [Google Scholar] [CrossRef]
- Zhao, X.J.; Ji, B.P.; Zhao, L.; Li, J.H. Comparison of extraction methods of volatile oil from citrus medica L. var. sarcodactylis(Noot) Swingle. Food Sci. 2007, 28, 167–170. [Google Scholar]
- Wu, Z.; Li, H.; Yang, Y.; Zhan, Y.; Tu, D.W. Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stages of maturity. Ind. Crops Prod. 2013, 46, 311–316. [Google Scholar]
- Mitiku, S.B.; Sawamura, M.; Itoh, T.; Ukeda, H. Volatile components of peel cold-pressed oils of two cultivars of sweet orange (Citrus sinensis (L.) Osbeck) from Ethiopia. Flavour Fragr. J. 2000, 15, 240–244. [Google Scholar] [CrossRef]
- Kamadia, V.V.; Yoon, Y.; Schilling, M.W.; Marshall, D.L. Relationships between odorant concentration and aroma intensity. J. Food Sci. 2006, 71, S193–S197. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, B.P.; Zhou, F.; Zhao, X.J.; Li, B. Study on chemical compositions of 12 volatile oils from bergamots. Food Sci. 2006, 27, 179–184. [Google Scholar]
- Yang, H.; Zhou, A.M.; Lin, M.H.; Xie, W.; Chen, S.X.; Chen, H.M. Research progress of extraction methods and pharmacological effects of bergamot essential oil. J. Food Saf. Qual. 2013, 4, 1347–1352. [Google Scholar]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.Y.; Yang, K.M.; Cheng, M.C.; Cheng, G.E.; Lin, L.Y. Determination of volatile components and antioxidative capacity of essential oils extracted from the medicinal citron and fingered citron. Taiwan. J. Agric. Chem. Food Sci. 2011, 49, 74–81. [Google Scholar]
- Shiota, H. Volatile components in the peel oil from fingered citron (Citrus medica L. var. sarcodactylis Swingle). Flavour Fragr. J. 1990, 5, 33–37. [Google Scholar] [CrossRef]
- Yang, J.; Gao, H.Y.; Chu, G.H.; Li, Z.H.; Niu, Y.W.; Cai, M.; Hu, A.F.; Jiang, J. Characterization of volatile constitutes and odorous compounds in essential oil of finger citron (Citrus medica L. var. sarcodactylis Swingle) by GC-MS and GC-O. Food Sci. 2015, 36, 194–197. [Google Scholar]
- Satyal, P.; Paudel, P.; Kafle, A.; Pokharel, S.K.; Lamichhane, B.; Dosoky, N.S.; Moriarity, D.M.; Setzer, W.N. Bioactivities of volatile components from Nepalese Artemisia species. Nat. Prod. Commun. 2012, 7, 1651–1658. [Google Scholar] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Sahm, D.F. Antibacterial Susceptibility Tests: Dilution Methods; American Society for Microbiology: Washington, DC, USA, 1991; pp. 1105–1116. [Google Scholar]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Fernandes, C.; Catrinescu, C.; Castilho, P.; Russo, P.; Carrott, M.; Breen, C. Catalytic conversion of limonene over acid activated serra de dentro (SD) bentonite. Appl. Catal. A Gen. 2007, 318, 108–120. [Google Scholar] [CrossRef]
- Belsito, E.L.; Carbone, C.; di Gioia, M.L.; Leggio, A.; Liguoiri, A.; Perri, F.; Siciliano, C.; Viscomi, M.C. Comparison of the volatile constituents in cold-pressed bergamot oil and a volatile oil isolated by vacuum distillation. J. Agric. Food Chem. 2007, 55, 7847–7851. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.D.; Zheng, J.S.; Deng, G.; Chen, W.R.; Sun, J. Antibacterial effects of essential oil from fingered citrons. J. Chin. Cereals Oils Assoc. 2009, 24, 103–107. [Google Scholar]
- Delgado, B.; Fernández, P.S.; Palop, A.; Periago, P.M. Effect of thymol and cymene on Bacillus cereus vegetative cells evaluated through the use of frequency distributions. Food Microbiol. 2004, 21, 327–334. [Google Scholar] [CrossRef]
- Kiskó, G.; Roller, S. Carvacrol and p-cymene inactivate Escherichia coli O157:H7 in apple juice. BMC Microbiol. 2005, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pina-Vaz, C.; Gonçalves Rodrigues, A.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Goncalves, M.; Martinez-de-Oliveira, J. Antifungal activity of thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]



| Entry | Compounds | KI a | VD Oil c | UF Oil d | HD Oil e | Ref Oil f | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| RI b | Area (%) | RI | Area (%) | RI | Area (%) | RI | Area (%) | |||
| (I) cyclic monoterpene hydrocarbons | ||||||||||
| 1 | α-thujene | 930 | 931 | 0.649 | 932 | 0.192 | 933 | 0.552 | 924 | 1.34 |
| 2 | α-pinene | 939 | 936 | 3.317 | 938 | 2.440 | 938 | 3.721 | 934 | 2.92 |
| 3 | camphene | 954 | 944 | 0.03 | ||||||
| 4 | sabinene | 975 | 974 | 0.143 | 974 | 0.065 | 967 | 0.40 | ||
| 5 | β-pinene | 979 | 976 | 1.887 | 978 | 1.793 | 976 | 1.952 | 975 | 2.70 |
| 6 | α-phellandrene | 1002 | 1003 | 0.264 | 999 | 0.09 | ||||
| 7 | α-terpinene | 1017 | 1016 | 0.688 | 1012 | 0.68 | ||||
| 8 | p-cymene | 1024 | 1023 | 26.099 | 1026 | 17.561 | 1015 | 0.13 | ||
| 9 | limonene | 1029 | 1035 | 47.599 | 1029 | 64.667 | 1031 | 60.129 | 1022 | 47.79 |
| 10 | γ-terpinene | 1059 | 1064 | 29.455 | 1058 | 0.050 | 1058 | 6.172 | 1049 | 32.08 |
| 11 | terpinolene | 1088 | 1089 | 1.651 | 1087 | 0.154 | 1084 | 1.37 | ||
| 12 | p-cymenene | 1091 | 1090 | 0.072 | ||||||
| (II) acyclic monoterpene hydrocarbons | ||||||||||
| 13 | β-myrcene | 990 | 992 | 1.551 | 991 | 0.653 | 991 | 1.786 | 982 | 1.67 |
| 14 | (Z)-β-ocimene | 1037 | 1040 | 5.914 | 1038 | 5.098 | 1031 | 0.36 | ||
| 15 | (E)-β-ocimene | 1050 | 1050 | 1.377 | 1048 | 0.601 | 1039 | 0.52 | ||
| 16 | allo-ocimene | 1132 | 1130 | 0.345 | ||||||
| (III) acyclic oxygenated monoterpenoids | ||||||||||
| 17 | linalool | 1096 | 1101 | 0.113 | 1087 | 0.15 | ||||
| 18 | citronellal | 1153 | 1153 | 0.055 | 1132 | 0.17 | ||||
| 19 | nerol | 1229 | 1227 | 0.123 | ||||||
| 20 | (Z)-ocimenone | 1229 | 1228 | 0.273 | ||||||
| 21 | neral | 1238 | 1240 | 0.706 | 1216 | 0.38 | ||||
| 22 | geraniol | 1252 | 1253 | 0.093 | 1219 | 1.56 | ||||
| 23 | linalyl acetate | 1257 | 1210 | 0.10 | ||||||
| 24 | geranial | 1267 | 1269 | 0.945 | 1238 | 0.45 | ||||
| 25 | neryl acetate | 1361 | 1364 | 0.018 | 1364 | 0.022 | 1364 | 0.059 | 1341 | 0.04 |
| 26 | geranyl acetate | 1381 | 1384 | 0.018 | 1385 | 0.029 | 1385 | 0.103 | 1359 | 0.05 |
| (IV) aldehydes | ||||||||||
| 27 | nonanal | 1150 | 1083 | 0.02 | ||||||
| 28 | decanal | 1201 | 1200 | 0.014 | 1187 | 0.00 | ||||
| (V) cyclic oxygenated monoterpenoids | ||||||||||
| 29 | cis-limonene oxide | 1136 | 1119 | 0.02 | ||||||
| 30 | trans-limonene oxide | 1142 | ||||||||
| 31 | terpinen-4-ol | 1177 | 1173 | 0.121 | 1177 | 0.185 | 1176 | 0.071 | 1166 | 0.13 |
| 32 | p-cymen-8-ol | 1182 | 1185 | 0.266 | 1184 | 0.077 | ||||
| 33 | α-terpineol | 1188 | 1185 | 0.091 | 1190 | 0.076 | 1190 | 0.055 | 1177 | 0.67 |
| 34 | trans-carveol | 1216 | 1217 | 0.284 | 1217 | 0.129 | ||||
| 35 | cis-carveol | 1229 | 1228 | 0.099 | ||||||
| 36 | carvone | 1243 | 1240 | 0.786 | 1241 | 0.063 | ||||
| 37 | carvyl acetate | 1282 | 1279 | 0.234 | ||||||
| (VI) sesquiterpene hydrocarbons | ||||||||||
| 38 | δ-elemene | 1338 | 1335 | 0.026 | ||||||
| 39 | α-cubebene | 1348 | 1347 | 0.014 | ||||||
| 40 | β-cubebene | 1388 | 1388 | 0.007 | ||||||
| 41 | β-elemene | 1390 | 1390 | 0.010 | ||||||
| 42 | α-cis-bergamotene | 1412 | 1413 | 0.027 | 1414 | 0.016 | 1413 | 0.020 | ||
| 43 | β-caryophyllene | 1419 | 1416 | 0.488 | 1416 | 0.012 | 1425 | 0.13 | ||
| 44 | α-trans-bergamotene | 1434 | 1434 | 0.453 | 1434 | 0.300 | 1433 | 0.387 | 1436 | 0.13 |
| 45 | γ-elemene | 1436 | 1498 | 0.08 | ||||||
| 46 | α-humulene | 1454 | 1451 | 0.041 | ||||||
| 47 | β-trans-farnesene | 1456 | 1457 | 0.044 | 1455 | 0.030 | ||||
| 48 | germacrene D | 1485 | 1479 | 0.519 | 1480 | 0.012 | 1481 | 0.017 | 1484 | 0.28 |
| 49 | β-ionone | 1488 | 1468 | 0.03 | ||||||
| 50 | bicyclogermacrene | 1500 | 1495 | 0.108 | ||||||
| 51 | α-bisabolene | 1503 | 1503 | 0.044 | 1501 | 0.020 | ||||
| 52 | β-bisabolene | 1505 | 1507 | 0.510 | 1503 | 0.429 | 1507 | 0.556 | 1503 | 0.21 |
| 52 | δ-cadinene | 1523 | 1523 | 0.016 | ||||||
| (VII) oxygenated sesquiterpenoids | ||||||||||
| 54 | caryophyllene oxide | 1583 | 1585 | 0.132 | 1585 | 0.260 | ||||
| (VIII) coumarins | ||||||||||
| 55 | 5,7-dimethoxycoumarin | 1937 | 1934 | 0.27 | ||||||
| Total | 99.442 | 99.011 | 99.749 | 96.951 | ||||||
| Sample | Antimicrobial Activity (MIC, μg/mL) | |||||
|---|---|---|---|---|---|---|
| S. aureus (G+) a | B. cereus (G+) | P. aeruginosa (G−) b | S. marcescens (G−) | C. albicans (Fungus) | A. niger (Fungus) | |
| Vacuum distillation oil | 625 | 313 | 625 | 1250 | 2500 | 156 |
| Ultrafiltration oil | 156 | 156 | 39 | 625 | 1250 | 20 |
| Hydrodistillation oil | 1250 | 1250 | 1250 | 1250 | 2500 | 625 |
| No. | Compounds | VD Oil | UF Oil | HD Oil | Ref Oil | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count a | STC b (%) | RR c | Count | STC (%) | RR | Count | STC (%) | RR | Count | STC (%) | RR | ||
| (I) | cyclic monoterpene hydrocarbons | 9 | 85.654 | 1 | 7 | 95.313 | 1.113 | 8 | 90.305 | 1.054 | 11 | 89.53 | 1.045 |
| (II) | acyclic monoterpene hydrocarbons | 4 | 9.187 | 1 | 1 | 0.653 | 0.071 | 3 | 7.485 | 0.815 | 3 | 2.55 | 0.278 |
| (III) | acyclic oxygenated monoterpenoids | 8 | 2.071 | 1 | 3 | 0.324 | 0.156 | 2 | 0.162 | 0.078 | 8 | 2.90 | 1.400 |
| (IV) | aldehydes | 1 | 0.014 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.02 | 1.492 |
| (V) | cyclic oxygenated monoterpenoids | 2 | 0.211 | 1 | 6 | 1.832 | 8.682 | 6 | 0.493 | 2.335 | 3 | 0.82 | 3.886 |
| (VI) | Sesquiterpene hydrocarbons | 14 | 2.306 | 1 | 4 | 0.757 | 0.328 | 7 | 1.044 | 0.453 | 6 | 0.86 | 0.373 |
| (VII) | Oxygenated sesquiterpenoids | 0 | 0 | - | 1 | 0.132 | - | 1 | 0.260 | - | 0 | 0 | - |
| (VIII) | coumarins | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | 1 | 0.27 | - |
| Sum of Identified Compounds | 38 | 22 | 27 | 34 | |||||||||
| Featuring Aroma Intensity | very intense | light | medium intense | not given | |||||||||
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, G.; Craft, J.D.; Steinberg, K.M.; Li, P.L.; Pokharel, S.K.; Setzer, W.N. Influence of Different Isolation Methods on Chemical Composition and Bioactivities of the Fruit Peel Oil of Citrus medica L. var. sarcodactylis (Noot.) Swingle. Medicines 2017, 4, 1. https://doi.org/10.3390/medicines4010001
Deng G, Craft JD, Steinberg KM, Li PL, Pokharel SK, Setzer WN. Influence of Different Isolation Methods on Chemical Composition and Bioactivities of the Fruit Peel Oil of Citrus medica L. var. sarcodactylis (Noot.) Swingle. Medicines. 2017; 4(1):1. https://doi.org/10.3390/medicines4010001
Chicago/Turabian StyleDeng, Gang, Jonathan D. Craft, Kelly Marie Steinberg, Pei Lei Li, Suraj Kumar Pokharel, and William N. Setzer. 2017. "Influence of Different Isolation Methods on Chemical Composition and Bioactivities of the Fruit Peel Oil of Citrus medica L. var. sarcodactylis (Noot.) Swingle" Medicines 4, no. 1: 1. https://doi.org/10.3390/medicines4010001
APA StyleDeng, G., Craft, J. D., Steinberg, K. M., Li, P. L., Pokharel, S. K., & Setzer, W. N. (2017). Influence of Different Isolation Methods on Chemical Composition and Bioactivities of the Fruit Peel Oil of Citrus medica L. var. sarcodactylis (Noot.) Swingle. Medicines, 4(1), 1. https://doi.org/10.3390/medicines4010001