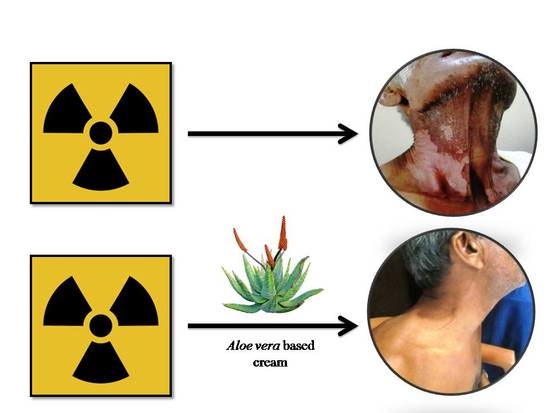
An Aloe Vera-Based Cosmeceutical Cream Delays and Mitigates Ionizing Radiation-Induced Dermatitis in Head and Neck Cancer Patients Undergoing Curative Radiotherapy: A Clinical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Radiation Therapy Treatment
2.3. Application of JBO and AVC
2.4. Patient Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Oral Health Programme: Global Data on Incidence of Oral Cancer; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- De Vita, V.T.; Lawrence, T.S.; Rosenberg, S.A. Cancer: Principles & Practice of Oncology, 8th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2004. [Google Scholar]
- Mantovani, G.; Proto, E.; Massa, E.; Mulas, C.; Madeddu, C.; Mura, L. Induction chemotherapy followed by concomitant chemoradiation therapy in advanced head and neck cancer: A phase II study for organ-sparing purposes evaluating feasibility, effectiveness and toxicity. Int. J. Oncol. 2002, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; Bourhis, J.; Domenge, C.; Designe, L. Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: Three meta analyses of updated individual data. MACHNC Collaborative Group. Meta Analysis of Chemotherapy on Head and Neck Cancer. Lancet 2000, 355, 949–955. [Google Scholar] [CrossRef]
- Salvo, N.; Barnes, E.; van Draanen, J.; Stacey, E.; Mitera, G.; Breen, D.; Giotis, A.; Czarnota, G.; Pang, J.; De Angelis, C. Prophylaxis and management of acute radiation-induced skin reactions: A systematic review of the literature. Curr. Oncol. 2010, 17, 94–112. [Google Scholar] [PubMed]
- Glean, E.; Edwards, S.; Faithfull, S.; Meredith, C.; Richards, C.; Smith, M.; Colyer, H. Intervention for acute radiotherapy induced skin reactions in cancer patients: The development of a clinical guideline recommended for use by the college of radiographers. J. Radiother Pract. 2001, 2, 75–84. [Google Scholar] [CrossRef]
- Palatty, P.L.; Azmidah, A.; Rao, S.; Jayachander, D.; Thilakchand, K.R.; Rai, M.P.; Haniadka, R.; Simon, P.; Ravi, R.; Jimmy, R.; et al. Topical application of a sandal wood oil and turmeric based cream prevents radiodermatitis in head and neck cancer patients undergoing external beam radiotherapy: A pilot study. Br. J. Radiol. 2014, 87, 20130490. [Google Scholar] [CrossRef] [PubMed]
- Hymes, S.R.; Strom, E.A.; Fife, C. Radiation dermatitis: Clinical presentation, pathophysiology, and treatment 2006. J. Am. Acad. Dermatol. 2006, 54, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Noble-Adams, R. Radiation-induced reactions 1: An examination of the phenomenon. Br. J. Nurs. 1999, 8, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Noble-Adams, R. Radiation-induced reactions 2: Development of a measurement tool. Br. J. Nurs. 1999, 8, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Bolderston, A.; Lloyd, N.; Wong, R.; Holden, L.; Robb-Blenderman, L. Supportive Care Guidelines Group of Cancer Care Ontario Program in Evidence-Based Care. The prevention and management of acute skin reactions related to radiation therapy: A systematic review and practice guideline. Support. Care Cancer 2006, 14, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Radvansky, L.J.; Pace, M.B.; Siddiqui, A. Prevention and management of radiation-induced dermatitis, mucositis, and xerostomia. Am. J. Health Syst. Pharm. 2013, 70, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Scott, C.; Stevens, R.; Marconi, B.; Champion, L.; Freedman, G.M.; Asrari, F.; Pilepich, M.V.; Gagnon, J.D.; Wong, G. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG). Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1307–1310. [Google Scholar] [PubMed]
- Olsen, D.L.; Raub, W.J.; Bradley, C.; Johnson, M.; Macias, J.L.; Love, V.; Markoem, A. The effect of Aloe vera gel/mild soap versus mild soap alone in preventing skin reactions in patients undergoing radiation therapy. Oncol. Nurs. Forum. 2001, 28, 543–547. [Google Scholar] [PubMed]
- Heggie, S.; Bryant, G.P.; Tripcony, L.; Keller, J.; Rose, P.; Glendenning, M.; Heath, J. A Phase III study on the efficacy of topical Aloe vera gel on irradiated breast tissue. Cancer Nurs. 2002, 25, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Becker-Schiebe, M.; Mengs, U.; Schaefer, M.; Bulitta, M.; Hoffmann, W. Topical use of a silymarin-based preparation to prevent radiodermatitis: Results of a prospective study in breast cancer patients. Strahlenther Onkol. 2011, 187, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Pommier, P.; Gomez, F.; Sunyach, M.P.; D’Hombres, A.; Carrie, C.; Montbarbon, X. Phase III randomized trial of Calendula officinalis compared with trolamine for the prevention of acute dermatitis during irradiation for breast cancer. J. Clin. Oncol. 2004, 22, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Haniadka, R.; Kamble, P.S.; Azmidha, A.; Mane, P.P.; Geevarughese, N.M.; Palatty, P.L.; Baliga, M.S. Review on the Use of Aloe vera (Aloe) in Dermatology; Watson, R.R., Zibadi, S., Eds.; Bioactive Dietary Factors and Plant Extracts in Dermatology; Springer: New York, NY, USA, 2013; pp. 125–136. [Google Scholar]
- Reuter, J.; Jocher, A.; Stump, J.; Grossjohann, B.; Franke, G.; Schempp, C.M. Investigation of the anti-inflammatory potential of Aloe vera gel (97.5%) in the ultraviolet erythema test. Skin Pharmacol. Physiol. 2008, 21, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Strickland, F.M.; Pelley, R.P.; Kripke, M.L. Prevention of ultraviolet radiation-induced suppression of contact and delayed hypersensitivity by Aloe barbadensis gel extract. J. Investig. Dermatol. 1994, 102, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, G.; Saini, M.R.; Goyal, P.K. Chemopreventive potential of Aloe vera against 7,12-dimethylbenz(a)anthracene induced skin papillomagenesis in mice. Integr. Cancer Ther. 2007, 6, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Goyal, P.K.; Chaudhary, G. Anti-tumor activity of Aloe vera against DMBA/croton oil-induced skin papillomagenesis in Swiss albino mice. J. Environ. Pathol. Toxicol. Oncol. 2010, 29, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, S.; Lee, M.J.; Lee, D.H.; Won, C.H.; Kim, S.M.; Chung, J.H. Dietary Aloe vera Supplementation Improves Facial Wrinkles and Elasticity and It Increases the Type I Procollagen Gene Expression in Human Skin in vivo. Ann. Dermatol. 2009, 21, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, E.; Korsholm, L.; Brandrup, F. A double-blind, placebo-controlled study of a commercial Aloe vera gel in the treatment of slight to moderate psoriasis vulgaris. J. Eur. Acad. Dermatol Venereol. 2005, 19, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Visuthikosol, V.; Chowchuen, B.; Sukwanarat, Y.; Sriurairatana, S.; Boonpucknavig, V. Effect of Aloe vera gel to healing of burn wound a clinical and histologic study. J. Med. Assoc. Thai. 1995, 78, 403–409. [Google Scholar] [PubMed]
- Schag, C.C.; Heinrich, R.L.; Ganz, P.A. Karnofsky performance status revisited: Reliability, validity, and guidelines. J. Clin. Oncol. 1984, 2, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Dinkar, C.; Vaishnav, L.K.; Rao, P.; Rai, M.P.; Fayad, R.; Baliga, M.S. The Indian Spice Turmeric Delays and Mitigates Radiation-Induced Oral Mucositis in Patients Undergoing Treatment for Head and Neck Cancer: An Investigational Study. Integr. Cancer Ther. 2014, 5, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- McQuestion, M. Evidence-based skin care management in radiation therapy. Sem. Oncol. Nurs. 2006, 22, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.L. Ionizing radiation: The good, the bad, and the ugly. J. Investig. Dermatol. 2012, 132, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; Kachikwu, E.L.; McBride, W.H. Cytokines in radiobiological responses: A review. Radiat. Res. 2012, 178, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Siomek, A. NF-κB signaling pathway and free radical impact. Acta Biochim. Pol. 2012, 59, 323–331. [Google Scholar] [PubMed]
- Harlev, E.; Nevo, E.; Lansky, E.P.; Ofir, R.; Bishayee, A. Anticancer potential of aloes: Antioxidant, antiproliferative, and immunostimulatory attributes. Planta Med. 2012, 78, 843–852. [Google Scholar] [PubMed]
- Silva, M.A.; Trevisan, G.; Hoffmeister, C.; Rossato, M.F.; Boligon, A.A.; Walker, C.I.; Klafke, J.Z.; Oliveira, S.M.; Silva, C.R.; Athayde, M.L.; et al. Anti-inflammatory and antioxidant effects of Aloe saponaria Haw in a model of UVB-induced paw sunburn in rats. J. Photochem. Photobiol. B. 2014, 5, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, M. Studies on chemical radioprotectors against X-irradiation used by soft X-ray accelerator. Yakugaku Zasshi 1995, 115, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.K.; Gehlot, P. Radioprotective effects of Aloe vera leaf extract on Swiss albino mice against whole-body gamma irradiation. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Shimpo, K.; Ida, C.; Chihara, T.; Beppu, H.; Kaneko, T.; Kuzuya, H. Aloe arborescens extract inhibits TPA-induced ear oedema, putrescine increase and tumour promotion in mouse skin. Phytother Res. 2002, 16, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Sitton, E. Early and late radiation-induced skin alterations. Part I: Mechanism of skin changes. Oncol. Nurs. Forum. 1992, 19, 801–807. [Google Scholar] [PubMed]
- Duansak, D.; Somboonwong, J.; Patumraj, S. Effects of Aloe vera on leukocyte adhesion and TNF-alpha and IL-6 levels in burn wounded rats. Clin. Hemorheol. Microcirc. 2003, 29, 239–246. [Google Scholar] [PubMed]
- Budai, M.M.; Varga, A.; Milesz, S.; Tőzsér, J.; Benkő, S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol. Immunol. 2013, 56, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, G.; Jia, J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing. J. Ethnopharmacol. 2008, 120, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Chithra, P.; Sajithlal, G.B.; Chandrakasan, G. Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. J. Ethnopharmacol. 1998, 59, 179–186. [Google Scholar] [CrossRef]
- Chithra, P.; Sajithlal, G.B.; Chandrakasan, G. Influence of Aloe vera on collagen characteristics in healing dermal wounds in rats. Mol. Cell Biochem. 1998, 181, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Chithra, P.; Sajithlal, G.B.; Chandrakasan, G. Influence of Aloe vera on collagen turnover in healing of dermal wounds in rats. Indian J. Exp. Biol. 1998, 36, 896–901. [Google Scholar] [PubMed]
- Hosseinimehr, S.J.; Khorasani, G.; Azadbakht, M.; Zamani, P.; Ghasemi, M.; Ahmadi, A. Effect of aloe cream versus silver sulfadiazine for healing burn wounds in rats. Acta Dermatovenerol Croat. 2010, 18, 2–7. [Google Scholar] [PubMed]
- Lv, R.L.; Wu, B.Y.; Chen, X.D.; Jiang, Q. The effects of aloe extract on nitric oxide and endothelin levels in deep-partial thickness burn wound tissue in rat. Zhonghua Shao Shang Za Zhi 2006, 22, 362–365. [Google Scholar] [PubMed]
- Roberts, D.B.; Travis, E.L. Acemannan-containing wound dressing gel reduces radiation-induced skin reactions in C3H mice. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1047–1052. [Google Scholar] [CrossRef]
- Atiba, A.; Nishimura, M.; Kakinuma, S.; Hiraoka, T.; Goryo, M.; Shimada, Y.; Ueno, H.; Uzuka, Y. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-β and fibroblast growth factor production. Am. J. Surg. 2011, 201, 809–818. [Google Scholar] [CrossRef] [PubMed]



| Details | JBO (Johnson’s Baby Oil) | AVC (Aloe Vera-Based Cream) |
|---|---|---|
| Age (Yrs) | 55.2 ± 9.66 | 55.9 ± 8.99 |
| Gender | ||
| Male | 24 | 26 |
| Female | 6 | 4 |
| Tumor Site | ||
| Alveolus | 2 | 1 |
| Buccal mucosa | 2 | 1 |
| glottis | 1 | 2 |
| larynx | 2 | 3 |
| Maxilla | 3 | 3 |
| Pyriform sinus | 1 | 2 |
| Pharynx (Oro and Hypo) | 3 | 1 |
| Posterior cricoid | 1 | 1 |
| Retromolar trigone | 1 | 1 |
| Supraglottis | 5 | 5 |
| Tongue | 6 | 7 |
| Vocal cord | 3 | 3 |
| TNM stage | ||
| Primary | ||
| T1 | 1 | 3 |
| T2 | 15 | 14 |
| T3 | 12 | 10 |
| T4 | 1 | 3 |
| TX | 1 | - |
| Regional nodes | ||
| N0 | 6 | 7 |
| N1 | 12 | 16 |
| N2 | 4 | 3 |
| N2a | - | 2 |
| N2b | 4 | 1 |
| N2c | 3 | - |
| N3 | 2 | - |
| NX | - | - |
| Metastasis | ||
| M0 | 28 | 29 |
| MX | 2 | 1 |
| Radiation details | ||
| Dose of radiation (Gy) | 69.0 ± 1.9 | 67.0 ± 3.7 |
| Total Fraction (in 7 weeks) | 34.3 ± 0.7 | 33.7 ± 1.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.; Hegde, S.K.; Baliga-Rao, M.P.; Palatty, P.L.; George, T.; Baliga, M.S. An Aloe Vera-Based Cosmeceutical Cream Delays and Mitigates Ionizing Radiation-Induced Dermatitis in Head and Neck Cancer Patients Undergoing Curative Radiotherapy: A Clinical Study. Medicines 2017, 4, 44. https://doi.org/10.3390/medicines4030044
Rao S, Hegde SK, Baliga-Rao MP, Palatty PL, George T, Baliga MS. An Aloe Vera-Based Cosmeceutical Cream Delays and Mitigates Ionizing Radiation-Induced Dermatitis in Head and Neck Cancer Patients Undergoing Curative Radiotherapy: A Clinical Study. Medicines. 2017; 4(3):44. https://doi.org/10.3390/medicines4030044
Chicago/Turabian StyleRao, Suresh, Sanath Kumar Hegde, Manjeshwar Poonam Baliga-Rao, Princy Louis Palatty, Thomas George, and Manjeshwar Shrinath Baliga. 2017. "An Aloe Vera-Based Cosmeceutical Cream Delays and Mitigates Ionizing Radiation-Induced Dermatitis in Head and Neck Cancer Patients Undergoing Curative Radiotherapy: A Clinical Study" Medicines 4, no. 3: 44. https://doi.org/10.3390/medicines4030044
APA StyleRao, S., Hegde, S. K., Baliga-Rao, M. P., Palatty, P. L., George, T., & Baliga, M. S. (2017). An Aloe Vera-Based Cosmeceutical Cream Delays and Mitigates Ionizing Radiation-Induced Dermatitis in Head and Neck Cancer Patients Undergoing Curative Radiotherapy: A Clinical Study. Medicines, 4(3), 44. https://doi.org/10.3390/medicines4030044