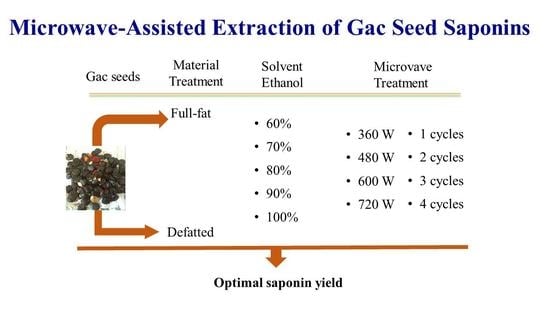
Optimisation of the Microwave-Assisted Ethanol Extraction of Saponins from Gac (Momordica cochinchinensis Spreng.) Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Solvents, Reagents and Chemicals
2.1.2. Gac Seed Kernel Powder
2.1.3. Preparation of Gac Seed Kernel Powder
2.1.4. Preparation of Defatted Gac Seed Kernel Powder
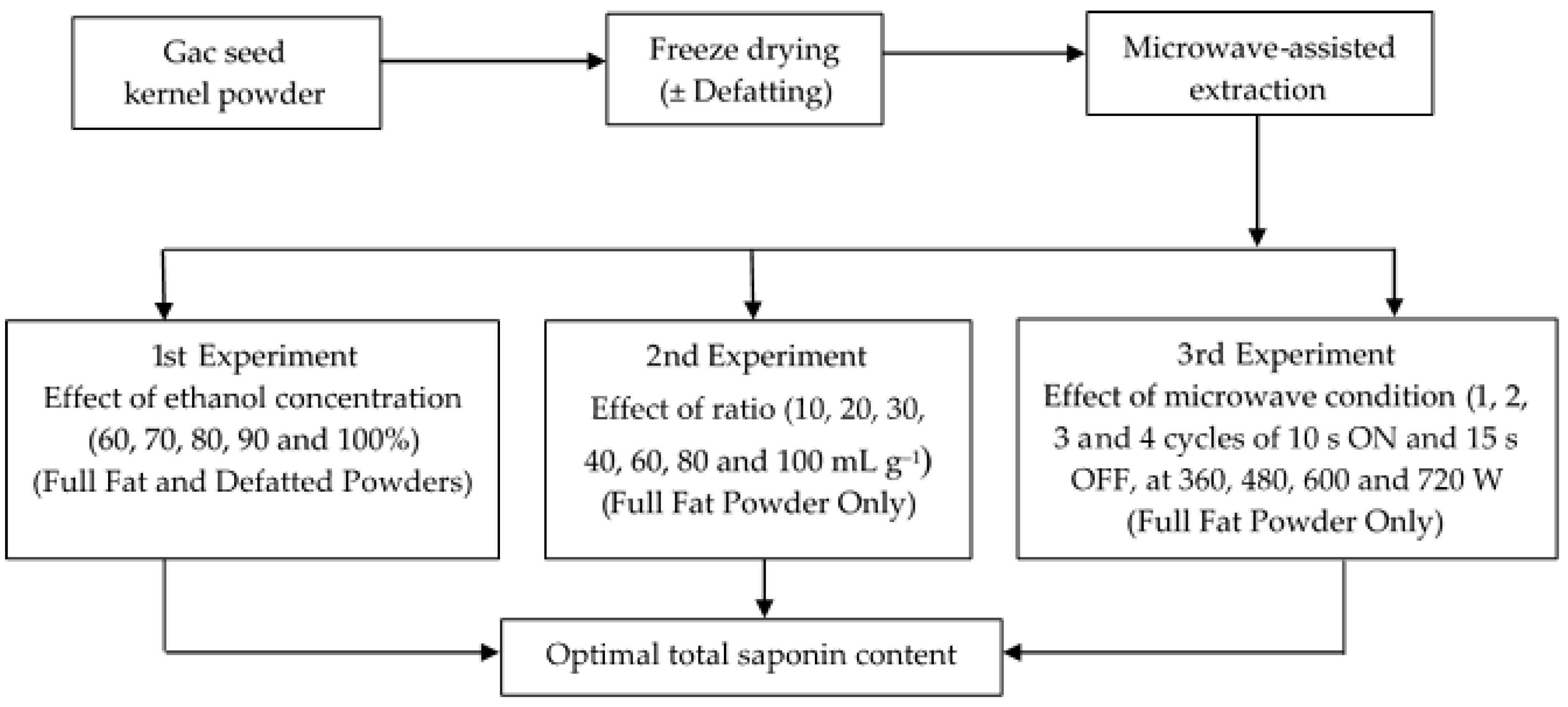
2.2. Methods
2.2.1. Microwave Assisted Extraction (MAE)
2.2.2. Extraction of Saponins from Full-Fat and Defatted Gac Seed Kernel Powders
2.2.3. Extraction of Saponins from the Full-Fat Seed Kernel Powder
2.2.4. Verifying Optimal Conditions for Gac Seed Saponin Extraction
2.3. Analytical Methods
2.3.1. Determination of Total Saponin Content (TSC)
2.3.2. Determination of Antioxidant Capacity
ABTS Assay
DPPH Assay
2.4. Statistical Analyses
3. Results
3.1. Effect of the Ethanol Concentration on the MAE of Saponins from Full-Fat and Defatted Gac Seed Kernel Powders
3.2. Effect of the Ethanol to Sample Ratio on the MAE of Saponins from the Full-Fat Gac Seed Kernel Powder
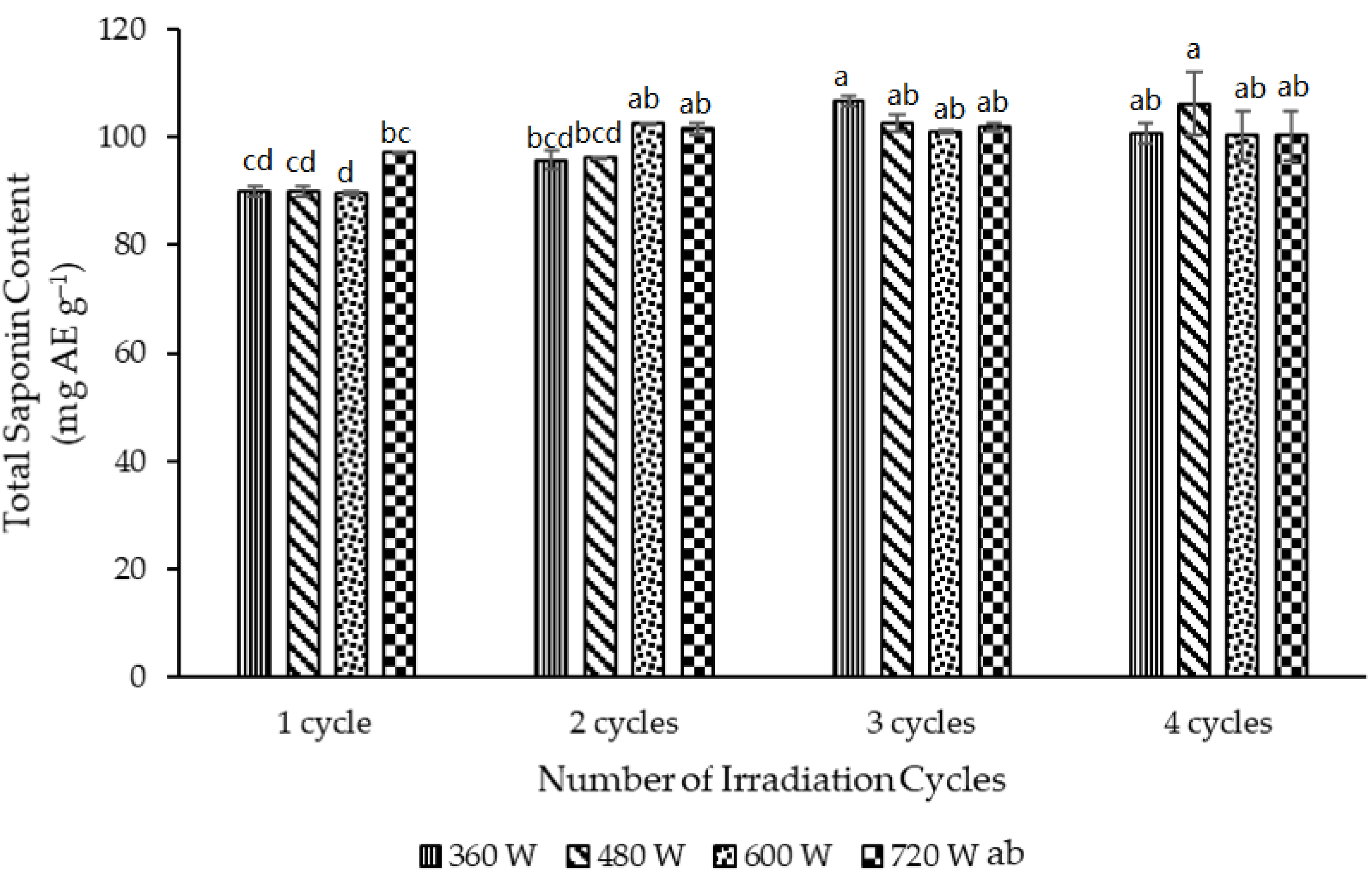
3.3. Effect of the Microwave Parameters on the MAE of Saponins from the Full-Fat Gac Seed Kernel Powder
3.4. Correlations between the TSC and the MAE Temperature
3.5. Verification of the Optimal MAE Conditions for the Extraction of Saponins from Full-Fat Gac Seed Kernel Powder
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perry, L.M.M. Medicinal Plants of East Southeast Asia: Attributed Properties and Uses; MIT Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac fruit (Momordica cochinchinensis Spreng.): A rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. Technol. 2015, 50, 567–577. [Google Scholar] [CrossRef]
- Yin, M.H.; Kang, D.G.; Choi, D.H.; Kwon, T.O.; Lee, H.S. Screening of vasorelaxant activity of some medicinal plants used in Oriental medicines. J. Ethnopharmacol. 2005, 99, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Kim, N.; Kim, B.; Kim, J.-H.; Lee, B.-Y.; Park, J.H.; Lee, M.K.; Lee, H.S.; Jang, I.-J.; Kim, J.S. Gastroprotective action of Cochinchina momordica seed extract is mediated by activation of CGRP and inhibition of cPLA2/5-LOX pathway. Dig. Dis. Sci. 2009, 54, 2549–2560. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Chin, Y.-W.; Chung, Y.H.; Park, Y.H.; Yoo, H.; Min, D.S.; Lee, B.; Kim, J. Anti-gastritis and wound healing effects of Momordicae semen extract and its active component. Immunopharmacol. Immunotoxicol. 2013, 35, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Kim, N.; Kim, B.; Kim, J.-H.; Lee, B.-Y.; Park, J.H.; Lee, M.K.; Lee, H.S.; Kim, J.S.; Jung, H.C. Enhancement of gastric ulcer healing and angiogenesis by Cochinchina momordica seed extract in rats. J. Korean Med. Sci. 2010, 25, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Tien, P.G.; Kayama, F.; Konishi, F.; Tamemoto, H.; Kasono, K.; Hung, N.T.K.; Kuroki, M.; Ishikawa, S.-E.; Van, C.N.; Kawakami, M. Inhibition of tumor growth and angiogenesis by water extract of Gac fruit (Momordica cochinchinensis Spreng.). Int. J. Oncol. 2005, 26, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Y.-M.; Zhan, Y.-Z.; Liu, C.-X. Momordica cochinchinensis seed extracts suppress migration and invasion of human breast cancer ZR-75-30 cells via down-regulating MMP-2 and MMP-9. Asian Pac. J. Cancer Prev. 2014, 15, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Kim, J.H.; Lee, S.; Jung, K.; Kim, K.H.; Cho, J.Y. Src/Syk-Targeted Anti-inflammatory actions of Triterpenoidal saponins from Gac (Momordica cochinchinensis) seeds. Am. J. Chin. Med. 2017, 45, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Chin, Y.-W.; Yoon, K.D.; Chae, H.-S.; Kim, C.Y.; Yoo, H.; Kim, J. Anti-inflammatory properties of a triterpenoidal glycoside from Momordica cochinchinensis in LPS-stimulated macrophages. Immunopharmacol. Immunotoxicol. 2013, 35, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Roh, H.-S.; Lee, S.; Jung, K.; Baek, K.-H.; Kim, K.H. Antiproliferative effect of Momordica cochinchinensis seeds on human lung cancer cells and isolation of the major constituents. Rev. Bras. Farmacogn. 2017, 27, 329–333. [Google Scholar] [CrossRef]
- Masayo, I.; Hikaru, O.; Tatsuo, Y.; Masako, T.; Yoshie, R.; Shuji, H.; Kunihide, M.; Ryuichi, H. Studies on the constituents of Momordica cochinchinensis Spreng. I. Isolation and characterization of the seed saponins, Momordica saponins I and II. Chem. Pharm. Bull. 1985, 33, 464–478. [Google Scholar]
- Akihisa, T.; Tokuda, H.; Ichiishi, E.; Mukainaka, T.; Toriumi, M.; Ukiya, M.; Yasukawa, K.; Nishino, H. Anti-tumor promoting effects of multiflorane-type triterpenoids and cytotoxic activity of karounidiol against human cancer cell lines. Cancer Lett. 2001, 173, 9–14. [Google Scholar] [CrossRef]
- Kan, L.; Hu, Q.; Chao, Z.; Song, X.; Cao, X. Chemical constituents of unsaponifiable matter from seed oil of Momordica cochinchinensis. China J. Chin. Mater. Med. 2006, 31, 1441–1444. [Google Scholar]
- Desai, P.J.; Parikh, P. Extraction of natural products using microwaves as a heat source. Sep. Purif. Rev. 2010, 39, 1–32. [Google Scholar] [CrossRef]
- Tatke, P.; Jaiswal, Y. An overview of microwave assisted extraction and its applications in herbal drug research. Res. J. Med. Plant 2011, 5, 21–31. [Google Scholar] [CrossRef]
- Le, A.V.; Roach, P.D.; Nguyen, M.H.; Parks, S.E. The microwave- and ultrasonic-assisted aqueous extraction of bioactive compounds from Gac (Momordica cochinchinensis S.) seeds. In Proceedings of the 4th World Congress on Medicinal Plants and Natural Products Research, Osaka, Japan, 8–9 August 2018. [Google Scholar]
- Nguyen, V.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Microwave-assisted extraction as an advanced technique for optimization of saponin yield and antioxidant potential from Phyllanthus amarus. Sep. Sci. Technol. 2017, 52, 2721–2731. [Google Scholar] [CrossRef]
- Li, J.; Zu, Y.-G.; Fu, Y.-J.; Yang, Y.-C.; Li, S.-M.; Li, Z.-N.; Wink, M. Optimization of microwave-assisted extraction of Triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2010, 11, 637–643. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.-Y.; Gong, X.-F. Microwave-assisted extraction used for the isolation of total Triterpenoid saponins from Ganoderma atrum. J. Food Eng. 2007, 81, 162–170. [Google Scholar] [CrossRef]
- Kerem, Z.; German-Shashoua, H.; Yarden, O. Microwave-assisted extraction of bioactive saponins from chickpea (Cicer arietinum L.). J. Sci. Food Agric. 2005, 85, 406–412. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Belanger, J.M.; Pare, J.J.; Yaylayan, V.A. Application of the microwave-assisted process (MAP™☆) to the fast extraction of ginseng saponins. Food Res. Int. 2003, 36, 491–498. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Q3C—Tables and List. In USHaH Services; Food and Drug Administration: Silver Spring, MD, USA, 2012. [Google Scholar]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Wimalasiri, D.; Piva, T.; Urban, S.; Huynh, T. Morphological and genetic diversity of Momordica cochinchinenesis (Cucurbitaceae) in Vietnam and Thailand. Genet. Resour. Crop Evol. 2016, 63, 19–33. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med. 1976, 29, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical Cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Tan Vuong, Q.V.; Stathopoulos, C.E.; Parks, S.E.; Roach, P.D. Optimized aqueous extraction of saponins from bitter melon for production of a saponin-enriched bitter melon powder. J. Food Sci. 2014, 79, E1372–E1381. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Investigation of phytochemicals and antioxidant capacity of selected Eucalyptus species using conventional extraction. Chem. Pap. 2016, 70, 567–575. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Influence of solvents and novel extraction methods on bioactive compounds and antioxidant capacity of Phyllanthus amarus. Chem. Pap. 2016, 70, 556–566. [Google Scholar]
- Ishida, B.K.; Turner, C.; Chapman, M.H.; McKeon, T.A. Fatty acid and carotenoid composition of Gac (Momordica cochinchinensis Spreng.) fruit. J. Agric. Food Chem. 2004, 52, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Le, A.V.; Roach, P.D.; Nguyen, M.H.; Parks, S.E. Optimisation of process parameters for supercritical carbon dioxide extraction of oil from Gac seed kernel powder. Adv. J. Food Sci. Technol. 2017, 13, 170–177. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Aguilar-Garcia, C.; Gavino, G.; Baragano-Mosqueda, M.; Hevia, P.; Gavino, V.C. Correlation of tocopherol, tocotrienol, γ-oryzanol and total polyphenol content in rice bran with different antioxidant capacity assays. Food Chem. 2007, 102, 1228–1232. [Google Scholar] [CrossRef]



| R2 (p Value) | ||||
|---|---|---|---|---|
| TSC | Number of Cycle | Power | Cycle and Power | |
| Temperature | 0.439 (p < 0.001) | 0.925 (p < 0.001) | 0.002 (p > 0.5) | 0.926 (p < 0.001) |
| Number of cycle | 0.362 (p < 0.001) | - | - | - |
| Power | 0.000 (p > 0.5) | - | - | - |
| Cycle and Power | 0.188 (p > 0.1) | - | - | - |
| Extract | TSC (mg AE g−1) | ABTS (µmol TE g−1) | DPPH (µmol TE g−1) | Energy Consumption (kWh) |
|---|---|---|---|---|
| Optimal treatment 1 † | 105.69 ± 2.40 a | 1.47 ± 0.12 a | Undetected | 0.003 |
| Optimal treatment 2 ‡ | 109.23 ± 2.69 a | 1.80 ± 0.31 a | Undetected | 0.005 |
| Control (no microwave) § | 109.64 ± 4.79 a | 1.63 ± 0.10 a | Undetected | 0.325 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, A.V.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Optimisation of the Microwave-Assisted Ethanol Extraction of Saponins from Gac (Momordica cochinchinensis Spreng.) Seeds. Medicines 2018, 5, 70. https://doi.org/10.3390/medicines5030070
Le AV, Parks SE, Nguyen MH, Roach PD. Optimisation of the Microwave-Assisted Ethanol Extraction of Saponins from Gac (Momordica cochinchinensis Spreng.) Seeds. Medicines. 2018; 5(3):70. https://doi.org/10.3390/medicines5030070
Chicago/Turabian StyleLe, Anh V., Sophie E. Parks, Minh H. Nguyen, and Paul D. Roach. 2018. "Optimisation of the Microwave-Assisted Ethanol Extraction of Saponins from Gac (Momordica cochinchinensis Spreng.) Seeds" Medicines 5, no. 3: 70. https://doi.org/10.3390/medicines5030070
APA StyleLe, A. V., Parks, S. E., Nguyen, M. H., & Roach, P. D. (2018). Optimisation of the Microwave-Assisted Ethanol Extraction of Saponins from Gac (Momordica cochinchinensis Spreng.) Seeds. Medicines, 5(3), 70. https://doi.org/10.3390/medicines5030070