Genus Stachys: A Review of Traditional Uses, Phytochemistry and Bioactivity
Abstract
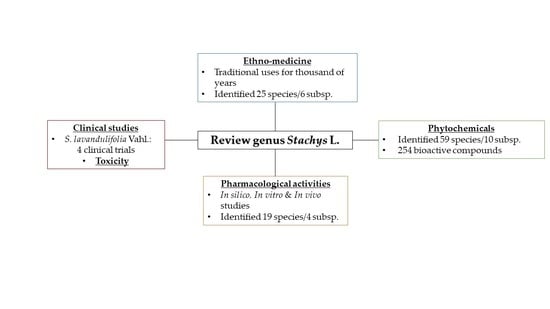
:1. Introduction
2. Materials and Methods
3. Traditional Medicinal Uses of Genus Stachys
4. Chemical Composition
4.1. Flavonoids
4.2. Phenolic Derivatives; Acetophenone Derivatives
4.3. Lignans
4.4. Phenylethanoid Glycosides; Phenylpropanoid Glucosides
4.5. Iridoids
4.6. Diterpenes
4.7. Triterpene Derivatives, Phytosterols and Phytoecdysteroids
4.8. Other Chemical Categories
5. Pharmacological Activities
5.1. Antioxidant Activity/Cytoprotective
5.2. Cytotoxicity and Antiproliferative Activity
5.3. Polycystic Ovary Syndrome (PCOS)
5.4. Anticholinesterase and Anti-Alzheimer’s Activity/Neuroprotective Activity
5.5. Anti-tyrosinase Activity
5.6. Anti-diabetic Activity
5.7. Antimicrobial Activity
5.8. Hepatoprotective
5.9. Others
6. Clinical Studies
7. Toxicity
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhattacharjee, R. Taxonomic studies in Stachys II. A new infragenic classification of Stachys L. [1980]. Notes R. Bot. Gard. Edinbourgh 2008, 38, 65–96. [Google Scholar]
- Salmaki, Y.; Heubl, G.; Weigend, M. Towards a new classification of tribe Stachydeae (Lamiaceae): Naming clades using molecular evidence. Bot. J. Linn. Soc. 2019, 190, 345–358. [Google Scholar] [CrossRef]
- Tundis, R.; Peruzzi, L.; Menichini, F. Phytochemical and biological studies of Stachys species in relation to chemotaxonomy: A review. Phytochemistry 2014, 102, 7–39. [Google Scholar] [CrossRef] [PubMed]
- Koeva-Todorovska, J. The genus Stachys L. and the genus Betonica L. Flora of PR Bulgaria; BAS Publishing House: Sofia, Bulgaria, 1979; Volume 9, pp. 388–416. [Google Scholar]
- Marin, P.; Grayer, R.; Grujic-Jovanovic, S.; Kite, G.; Veitch, N. Glycosides of tricetin methyl ethers as chemosystematic markers in Stachys subgenus Betonica. Phytochemistry 2004, 65, 1247–1253. [Google Scholar] [CrossRef]
- Strid, A.; Tan, K. Mountain Flora of Greece. II; Edinburgh University Press: Edinburgh, UK, 1991; Volume 2, pp. 97–107. [Google Scholar]
- Carnoy, A. Dictionnaire Étymologique des Noms Grecs de Plantes; Bibliothèque du Muséon: Louvain, Paris, 1959; p. 70. [Google Scholar]
- André, J. Lexique des termes de botanique en latin. In Etudes et Commentaires; Librairie, C., Ed.; Klincksieck: Paris, France, 1956; p. 79. [Google Scholar]
- Leonis, P. Lexicon Phytologicon; Librairie, C., Ed.; Athens, Greek, 1914; p. 912. (In Greek) [Google Scholar]
- Valiakos, E.; Marselos, M.; Sakellaridis, N.; Constantinidis, T.; Skaltsa, H. Ethnopharmacological approach to the herbal medicines of the “Antidotes” in Nikolaos Myrepsos’ Dynameron. J. Ethnopharmacol. 2015, 163, 68–82. [Google Scholar] [CrossRef]
- Camangi, F.; Stefani, A. Le piante nella magia e nella superstizione: Alcuni esempi di pratiche popolari in Toscana. Riv. Preist. Etnogr. Stor. Nat. 2003, 1, 1–5. [Google Scholar]
- Delazar, A.; Delnavazi, M.R.; Nahar, L.; Moghadam, S.B.; Mojarab, M.; Gupta, A.; Williams, A.S.; Rahman, M.M.; Sarker, S.D.; Mojarrab, M. Lavandulifolioside B: A new phenylethanoid glycoside from the aerial parts of Stachys lavandulifolia Vahl. Nat. Prod. Res. 2011, 25, 8–16. [Google Scholar] [CrossRef]
- Delnavazi, M.-R.; Saiyarsarai, P.; Jafari-Nodooshan, S.; Khanavi, M.; Tavakoli, S.; Hadavinia, H.; Yassa, N. Cytotoxic flavonoids from the aerial parts of Stachys lavandulifolia Vahl. Pharm. Sci. 2018, 24, 332–339. [Google Scholar] [CrossRef]
- Karioti, A.; Bolognesi, L.; Vincieri, F.F.; Bilia, A.R. Analysis of the constituents of aqueous preparations of Stachys recta by HPLC–DAD and HPLC–ESI-MS. J. Pharm. Biomed. Anal. 2010, 53, 15–23. [Google Scholar] [CrossRef]
- Gören, A.C.; Piozzi, F.; Akçiçek, E.; Kılıç, T.; Çarıkçı, S.; Mozioğlu, E.; Setzer, W.N. Essential oil composition of twenty-two Stachys species (mountain tea) and their biological activities. Phytochem. Lett. 2011, 4, 448–453. [Google Scholar] [CrossRef]
- Öztürk, M.; Duru, M.E.; Aydoğmuş-Öztürk, F.; Harmandar, M.; Mahlıçlı, M.; Kolak, U.; Ulubelen, A. GC-MS analysis and antimicrobial activity of essential oil of Stachys cretica subsp. smyrnaea. Nat. Prod. Commun. 2009, 4, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanavi, M.; Sharifzadeh, M.; Hadjiakhoondi, A.; Shafiee, A. Phytochemical investigation and anti-inflammatory activity of aerial parts of Stachys byzanthina C. Koch. J. Ethnopharmacol. 2005, 97, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Jahani, R.; Khaledyan, D.; Jahani, A.; Jamshidi, E.; Kamalinejad, M.; Khoramjouy, M.; Faizi, M. Evaluation and comparison of the antidepressant-like activity of Artemisia dracunculus and Stachys lavandulifolia ethanolic extracts: An in vivo study. Res. Pharm. Sci. 2019, 14, 544. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Mansourian, M.; Kokhdan, E.P.; Salehpour, Z.; Sadati, I.; Abbaszadeh-Goudarzi, K.; Asfaram, A.; Doustimotlagh, A.H. Antioxidant and protective effect of Stachys pilifera Benth against nephrotoxicity induced by cisplatin in rats. J. Food Biochem. 2020, 44, e13190. [Google Scholar] [CrossRef] [PubMed]
- Meremeti, A.; Karioti, A.; Skaltsa, H.; Heilmann, J.; Sticher, O. Secondary metabolites from Stachys ionica. Biochem. Syst. Ecol. 2004, 32, 139–151. [Google Scholar] [CrossRef]
- Piozzi, F.; Bruno, M. Diterpenoids from roots and aerial parts of the genus Stachys. Rec. Nat. Prod. 2011, 5, 1–11. [Google Scholar]
- Gören, A.C. Use of Stachys species (Mountain tea) as herbal tea and food. Rec. Nat. Prod. 2014, 8, 71–82. [Google Scholar]
- Kartsev, V.G.; Stepanichenko, N.N.; Auelbekov, S.A. Chemical composition and pharmacological properties of plants of the genus Stachys. Chem. Nat. Compd. 1994, 30, 645–654. [Google Scholar] [CrossRef]
- Plant List. 2013. Available online: http://www.theplantlist.org/tpl1.1/record/kew-195205 (accessed on 4 August 2020).
- The Euro + Med Plantbase. The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/query.asp (accessed on 4 August 2020).
- International Plant Name Index (IPNI). Available online: https://www.ipni.org/n/459647-1 (accessed on 4 August 2020).
- Venditti, A.; Frezza, C.; Celona, D.; Bianco, A.; Serafini, M.; Cianfaglione, K.; Fiorini, D.; Ferraro, S.; Maggi, F.; Lizzi, A.R.; et al. Polar constituents, protection against reactive oxygen species, and nutritional value of Chinese artichoke (Stachys affinis Bunge). Food Chem. 2017, 221, 473–481. [Google Scholar] [CrossRef]
- Guo, H.; Saravanakumar, K.; Wang, M.-H. Total phenolic, flavonoid contents and free radical scavenging capacity of extracts from tubers of Stachys affinis. Biocatal. Agric. Biotechnol. 2018, 15, 235–239. [Google Scholar] [CrossRef]
- Lee, J.W.; Wu, W.; Lim, S.Y. Effect of extracts from Stachys sieboldii Miq. on cellular reactive oxygen species and glutathione production and genomic DNA oxidation. Asian Pac. J. Trop. Biomed. 2018, 8, 485. [Google Scholar] [CrossRef]
- Huang, W.; Gao, X.; Zhang, Y.; Jin, C.; Wang, X. The complete chloroplast genome sequence of Stachys sieboldii Miquel. (Labiatae), a kind of vegetable crop and Chinese medicinal material plant. Mitochondrial DNA Part B 2020, 5, 1832–1833. [Google Scholar] [CrossRef]
- Asghari, G.; Akbari, M.; Asadi-Samani, M. Phytochemical analysis of some plants from Lamiaceae family frequently used in folk medicine in Aligudarz region of Lorestan province. Marmara Pharm. J. 2017, 21, 506. [Google Scholar] [CrossRef]
- Lotfipour, F.; Nazemiyeh, H.; Fathi-Azad, F.; Garaei, N.; Arami, S.; Talat, S.; Sadegpour, F.; Hasanpour, R. Evaluation of antibacterial activities of some medicinal plants from North-West Iran. Iran. J. Basic Med. Sci. 2008, 11, 80–85. [Google Scholar]
- Asnaashari, S.; Delazar, A.; Alipour, S.; Nahar, L.; Williams, A.; Pasdaran, A.; Mojarab, M.; Azad, F.; Sarker, S.D. Chemical composition, free-radical-scavenging and insecticidal activities of the aerial parts of Stachys byzantina. Arch. Biol. Sci. 2010, 62, 653–662. [Google Scholar] [CrossRef]
- Naghibi, F.; Mosaddegh, M.; Motamed, M.M.; Ghorbani, A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran. J. Pharm. Res. 2005, 2, 63–79. [Google Scholar]
- Aminfar, P.; Abtahi, M.; Parastar, H. Gas chromatographic fingerprint analysis of secondary metabolites of Stachys lanata (Stachys byzantine C. Koch) combined with antioxidant activity modelling using multivariate chemometric methods. J. Chromatogr. A 2019, 1602, 432–440. [Google Scholar] [CrossRef]
- Maleki, N.; Garjani, A.; Nazemiyeh, H.; Nilfouroushan, N.; Sadat, A.E.; Allameh, Z.; Hasannia, N. Potent anti-inflammatory activities of hydroalcoholic extract from aerial parts of Stachys inflata on rats. J. Ethnopharmacol. 2001, 75, 213–218. [Google Scholar] [CrossRef]
- Lazarević, J.; Palić, R.; Radulović, N.S.; Ristic, N.; Stojanovic, G. Chemical composition and screening of the antimicrobial and anti-oxidative activity of extracts of Stachys species. J. Serb. Chem. Soc. 2010, 75, 1347–1359. [Google Scholar] [CrossRef]
- Amin, G. Popular medicinal plants of Iran, Iranian Research Institute of medicinal plants. Tehran 1991, 80, 1–66. (In Persian) [Google Scholar]
- Rabbani, M.; Sajjadi, S.E.; Zarei, H. Anxiolytic effects of Stachys lavandulifolia Vahl on the elevated plus-maze model of anxiety in mice. J. Ethnopharmacol. 2003, 89, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Sajjadi, S.-E.; Karimi-Firozjai, M.; Ghannadian, M. Bioactivity guided isolation of apigenin from Stachys lavandulifolia Vahl. in mice with anxiolytic effects. J. Herbmed Pharmacol. 2018, 7, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Hajhashemi, V.; Ghannadi, A.; Sedighifar, S. Analgesic and anti-inflammatory properties of the hydroalcoholic, polyphenolic and boiled extracts of Stachys lavandulifolia. Res. Pharm. Sci. 2007, 2, 92–98. [Google Scholar]
- Arabsalehi, F.; Rahimmalek, M.; Ehtemam, M.H. Phytochemical and morphological variation of Stachys lavandulifolia Vahl. populations as affected by genotype × year interaction. Ind. Crop. Prod. 2018, 112, 342–352. [Google Scholar] [CrossRef]
- Veisi, H.; Kazemi, S.; Mohammadi, P.; Safarimehr, P.; Hemmati, S. Catalytic reduction of 4-nitrophenol over Ag nanoparticles immobilized on Stachys lavandulifolia extract-modified multi walled carbon nanotubes. Polyhedron 2019, 157, 232–240. [Google Scholar] [CrossRef]
- Fooladvand, Z.; Fazeli-nasab, B. Antibacterial activities of Stachys lavandulifolia Vahl. extract against eight bacteria. J. Herb. Drugs 2014, 5, 13–18. [Google Scholar]
- Kokhdan, E.P.; Sadeghi, H.; Ghafoori, H.; Sadeghi, H.; Danaei, N.; Javadian, H.; Aghamaali, M.R. Cytotoxic effect of methanolic extract, alkaloid and terpenoid fractions of Stachys pilifera against HT-29 cell line. Res. Pharm. Sci. 2018, 13, 404–412. [Google Scholar] [CrossRef]
- Maleki, F.; Valilou, M.M.S. Poulk plant (Stachys schtschegleevii) and its antibacterial specifications. Asian J. Res. Bot. 2019, 2, 1–13. [Google Scholar]
- Alizadeh, F.; Ramezani, M.; Piravar, Z. Effects of Stachys sylvatica hydroalcoholic extract on the ovary and hypophysis-gonadal axis in a rat with polycystic ovary syndrome. Middle East Fertil. Soc. J. 2020, 25, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Polat, R.; Cakilcioglu, U.; Kaltalioğlu, K.; Ulusan, M.D.; Türkmen, Z. An ethnobotanical study on medicinal plants in Espiye and its surrounding (Giresun-Turkey). J. Ethnopharmacol. 2015, 163, 1–11. [Google Scholar] [CrossRef]
- Altundag, E.; Öztürk, M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef] [Green Version]
- Mükemre, M.; Behçet, L.; Çakılcıoğlu, U.; Cakilcioglu, U. Ethnobotanical study on medicinal plants in villages of Çatak (Van-Turkey). J. Ethnopharmacol. 2015, 166, 361–374. [Google Scholar] [CrossRef]
- Lucchetti, L.; Zitti, S.; Taffetani, F. Ethnobotanical uses in the Ancona district (Marche region, Central Italy). J. Ethnobiol. Ethnomed. 2019, 15, 1–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venditti, A.; Bianco, A.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Damiano, S.; Papa, F.; Vittori, S.; Bini, L.M.; Giuliani, C.; et al. Phytochemical analysis, biological activity, and secretory structures of Stachys annua (L.) L. subsp. annua (Lamiaceae) from Central Italy. Chem. Biodivers. 2015, 12, 1172–1183. [Google Scholar] [PubMed]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M.G. Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Terrizzano, L.; Dente, F.; Mariotti, M.G. Ethnobotanical and phytomedical knowledge in the North-Western Ligurian Alps. J. Ethnopharmacol. 2014, 155, 463–484. [Google Scholar] [CrossRef]
- Mulas, M. Traditional uses of labiatae in the mediterranean area. Acta Hortic. 2006, 723, 25–32. [Google Scholar] [CrossRef]
- Pritsas, A.; Tomou, E.-M.; Tsitsigianni, E.; Papaemmanouil, C.D.; Diamantis, D.A.; Chatzopoulou, P.; Tzakos, A.G.; Skaltsa, H. Valorisation of stachysetin from cultivated Stachys iva Griseb. as anti-diabetic agent: A multi-spectroscopic and molecular docking approach. J. Biomol. Struct. Dyn. 2020, 1–15. [Google Scholar] [CrossRef]
- Fazio, C.; Passannanti, S.; Paternostro, M.; Arnold, N. Diterpenoids from Stachys mucronata. Planta Med. 1994, 60, 499. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Svanberg, I.; Köhler, P. Marsh woundwort, Stachys palustris L. (Lamiaceae): An overlooked food plant. Genet. Resour. Crop. Evol. 2011, 58, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Monigatti, M.; Bussmann, R.W.; Weckerle, C.S. Medicinal plant use in two Andean communities located at different altitudes in the Bolívar Province, Peru. J. Ethnopharmacol. 2013, 145, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-Candida activity of Brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, J.; Brendleer, T.; Jaenicke, T. PDR for Herbal Medicines; Medical Economics Company: Montvale, NJ, USA, 2000; p. 832. [Google Scholar]
- Rustaiyan, A.; Masoudi, S.; Ameri, N.; Samiee, K.; Monfared, A. Volatile constituents of Ballota aucheri Boiss., Stachys benthamiana Boiss. and Perovskia abrotanoides Karel. Growing wild in Iran. J. Essent. Oil Res. 2006, 18, 218–221. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Arshad, S.; Bader, S.; Iqbal, S.; Khan, A.; Khan, S.S.; Hussain, J.; Tareen, R.B.; Ahmed, A. New terpenoids from Stachys parviflora Benth. Magn. Reson. Chem. 2008, 46, 986–989. [Google Scholar] [CrossRef]
- Shakeri, A.; D’Urso, G.; Taghizadeh, S.F.; Piacente, S.; Norouzi, S.; Soheili, V.; Asili, J.; Salarbashi, D. LC-ESI/LTQOrbitrap/MS/MS and GC–MS profiling of Stachys parviflora L. and evaluation of its biological activities. J. Pharm. Biomed. Anal. 2019, 168, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kepekçi, R.A.; Polat, S.; Çoşkun, G.; Celik, A.; Bozkurt, A.S.; Yumrutaş, Ö.; Pehlivan, M. Preliminary characterization of phenolic acid composition and hepatoprotective effect of Stachys pumila. J. Food Biochem. 2016, 41, 12286. [Google Scholar] [CrossRef]
- Kumar, D.; Bhat, Z.A. Apigenin 7-glucoside from Stachys tibetica Vatke and its anxiolytic effect in rats. Phytomedicine 2014, 21, 1010–1014. [Google Scholar] [CrossRef]
- Ramazanov, N.S.; Bobayev, I.D.; Yusupova, U.Y.; Aliyeva, N.K.; Egamova, F.R.; Yuldasheva, N.Y.; Syrov, V.N. Phytoecdysteroids-containing extract from Stachys hissarica plant and its wound-healing activity. Nat. Prod. Res. 2016, 31, 593–597. [Google Scholar] [CrossRef]
- El–Ansari, M.A.; Abdalla, M.F.; Saleh, N.A.M.; Barron, D.; Le Quere, J.L. Flavonoid constituents of Stachys aegyptiaca. Phytochemistry 1991, 30, 1169–1173. [Google Scholar] [CrossRef]
- El-Ansari, M.A.; Nawwar, M.A.; Saleh, N.A.M. Stachysetin, a diapigenin-7-glucoside-p, p′-dihydroxy-truxinate from Stachys aegyptiaca. Phytochemistry 1995, 40, 1543–1548. [Google Scholar] [CrossRef]
- El-Desoky, S.K.; Hawas, W.U.; Sharaf, M. A new flavone glycoside from Stachys aegyptiaca. Chem. Nat. Compd. 2007, 43, 542–543. [Google Scholar] [CrossRef]
- Sharaf, M. Isoscutellarein 8-O-(6″-trans-p-coumaroyl)-β-D-glucoside from Stachys aegyptiaca. Fitoterapia 1998, 69, 355–357. [Google Scholar]
- Komissarenko, N.F.; Sheremet, I.P.; Derkach, A.I.; Pakaln, D.A. Stachyflaside from Stachys inflata and St. atherocalyx. Chem. Nat. Compd. 1976, 12, 88. [Google Scholar] [CrossRef] [Green Version]
- Komissarenko, N.F.; Derkach, A.I.; Sheremet, I.P.; Kovalev, I.P.; Gordienko, V.G.; Pakaln, D.A. Flavonoids of Stachys inflata. Chem. Nat. Compd. 1978, 14, 445–446. [Google Scholar] [CrossRef]
- Nazemiyeh, H.; Shoeb, M.; Movahhedin, N.; Kumarasamy, Y.; Talebpour, A.; Delazar, A.; Lutfun, N.; Sarker, S. Phenolic compounds and their glycosides from Stachys schtscheglevii (Lamiaceae). Biochem. Syst. Ecol. 2006, 34, 721–723. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F.; Tomás-Lorente, F. Flavonoid p-coumaroylglucosides and 8-hydroxyflavone allosylglucosides in some Labiatae. Phytochemistry 1992, 31, 3097–3102. [Google Scholar] [CrossRef]
- Lakhal, H.; Boudiar, T.; Kabouche, A.; Laggoune, S.; Kabouche, Z.; Topçu, G. Antioxidant activity and flavonoids of Stachys ocymastrum. Chem. Nat. Compd. 2011, 46, 964–965. [Google Scholar] [CrossRef]
- Skaltsa, H.; Bermejo, P.; Lazari, D.; Silván, A.M.; Skaltsounis, A.-L.; Sanz, A.; Abad, M.J. Inhibition of prostaglandin E2 and leukotriene C4 in mouse peritoneal macrophages and thromboxane B2 production in human platelets by flavonoids from Stachys chrysantha and Stachys candida. Biol. Pharm. Bull. 2000, 23, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Michailidou, A.-M. Phytochemical study of Stachys candida Bory & Chaub. Master’s Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2018. [Google Scholar]
- Serrilli, A.; Ramunno, A.; Piccioni, F.; Serafini, M.; Ballero, M. Flavonoids and iridoids from Stachys corsica. Nat. Prod. Res. 2005, 19, 561–565. [Google Scholar] [CrossRef]
- Demirtaş, I.; Gecibesler, I.H.; Yaglioglu, A.S.; Yaglioglu, A.S. Antiproliferative activities of isolated flavone glycosides and fatty acids from Stachys byzantina. Phytochem. Lett. 2013, 6, 209–214. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C. Phenolic ingredients and therapeutic potential of Stachys cretica subsp. smyrnaea for the management of oxidative stress, Alzheimer’s disease, hyperglycemia, and melasma. Ind. Crop. Prod. 2019, 127, 82–87. [Google Scholar] [CrossRef]
- Murata, T.; Endo, Y.; Miyase, T.; Yoshizaki, F. Iridoid glycoside constituents of Stachys lanata. J. Nat. Prod. 2008, 71, 1768–1770. [Google Scholar] [CrossRef]
- Derkach, A.I.; Komissarenko, N.F.; Gordienko, V.G.; Sheremet, I.P.; Kovalev, I.P.; Pakaln, D.A. Flavonoids of Stachys spectabilis. Chem. Nat. Compd. 1980, 16, 128–130. [Google Scholar] [CrossRef]
- Ertas, A.; Yener, I. A comprehensive study on chemical and biological profiles of three herbal teas in Anatolia; rosmarinic and chlorogenic acids. S. Afr. J. Bot. 2020, 130, 274–281. [Google Scholar] [CrossRef]
- Elfalleh, W.; Kirkan, B.; Sarikurkcu, C. Antioxidant potential and phenolic composition of extracts from Stachys tmolea: An endemic plant from Turkey. Ind. Crop. Prod. 2019, 127, 212–216. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Nicoletti, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Vitali, L.A.; Papa, F.; Vittori, S.; Petrelli, D.; et al. Characterization of secondary metabolites, biological activity and glandular trichomes of Stachys tymphaea hausskn. from the Monti Sibillini National Park (Central Apennines, Italy). Chem. Biodivers. 2014, 11, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Göğer, F.; Dogan, A.; Bitis, L. Two acylated isoscutellarein glucosides with anti-inflammatory and antioxidant activities isolated from endemic Stachys subnuda Montbret & Aucher ex Benth. Acta Chim. Slov. 2019, 66, 831–838. [Google Scholar] [CrossRef]
- Kostyuchenko, O.I.; Komissarenko, N.F.; Zinchenko, T.V.; Derkach, A.I. Diacetylstachyflaside from Stachys atherocalyx. Khim. Prir. Soedin. 1981, 3, 389–390. [Google Scholar]
- Kostyuchenko, O.I.; Komissarenko, N.F.; Kovalev, I.P.; Derkach, A.I.; Gordienko, V.G. Acetylspectabiflaside from Stachys atherocalyx. Chem. Nat. Compd. 1982, 18, 170–172. [Google Scholar] [CrossRef]
- Kostyuchenko, O.I.; Komissarenko, N.F.; Zinchenko, T.V.; Derkach, A.I.; Gordienko, V.G. Diacetylisostachyflaside and acetylisostachyflaside from Stachys atherocalyx. Chem. Nat. Compd. 1982, 18, 235–236. [Google Scholar] [CrossRef]
- Lenherr, A.; Lahloub, M.F.; Sticher, O. Three flavonoid glucosides containing acetylated allose from Stachys recta. Phytochemistry 1984, 23, 2343–2345. [Google Scholar] [CrossRef]
- Lenherr, A.; Meier, B.; Sticher, O. Modern HPLC as a tool for chemotaxonomical investigations: Iridoid glucosides and acetylated flavonoids in the group of Stachys recta. Planta Med. 1984, 50, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sheremet, I.P.; Komissarenko, N.F. Flavonoid glucosides of Stachys annua. Khim. Prir. Soedin 1971, 5, 583. [Google Scholar]
- Movsumov, I.S.; Yusifova, D.Y.; Suleimanov, T.A.; Mahiou-Leddet, V.; Herbette, G.; Baghdikian, B.; Ollivier, E.; Garayev, E.E.; Garayev, E.A. Biologically active compounds from chamaenerion angustifolium and Stachys annua growing in Azerbaidzhan. Chem. Nat. Compd. 2016, 52, 324–325. [Google Scholar] [CrossRef]
- Movsumov, I.S.; Garayev, E.A.; Baghdikian, B.; Mabrouki, F.; Herbette, G.; Ollivier, E.; Suleimanov, T.A.; Garayev, E.E. Flavonoids from Stachys annua growing in Azerbaijan. Chem. Nat. Compd. 2018, 54, 261–262. [Google Scholar] [CrossRef] [Green Version]
- Delazar, A.; Celik, S.; Göktürk, R.S.; Unal, O.; Nahar, L.; Sarker, S.D. Two acylated flavonoid glycosides from Stachys bombycina, and their free radical scavenging activity. Die Pharm. 2005, 60, 878–880. [Google Scholar] [CrossRef]
- Zinchenko, T.V. Flavonoids of Stachys neglecta. Farm. Zhurnal 1969, 24, 28804. [Google Scholar]
- Kotsos, M.; Aligiannis, N.; Mitaku, S.; Skaltsounis, A.-L.; Charvala, C. Chemistry of plants from Crete: Stachyspinoside, a new flavonoid glycoside and iridoids from Stachys spinosa. Nat. Prod. Lett. 2001, 15, 377–386. [Google Scholar] [CrossRef]
- Kotsos, M.P.; Aligiannis, N.; Mitakou, S. A new flavonoid diglycoside and triterpenoids from Stachys spinosa L. (Lamiaceae). Biochem. Syst. Ecol. 2007, 35, 381–385. [Google Scholar] [CrossRef]
- Afouxenidi, A.; Milošević-Ifantis, T.; Skaltsa, H. Secondary metabolites from Stachys tetragona Boiss. & Heldr. ex Boiss. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2018, 81, 83–85. [Google Scholar] [CrossRef]
- Lenherr, A.; Mabry, T.J. Acetylated allose-containing flavonoid glucosides from Stachys anisochila. Phytochemistry 1987, 26, 1185–1188. [Google Scholar] [CrossRef]
- Skaltsa, H.; Georgakopoulos, P.; Lazari, D.; Karioti, A.; Heilmann, J.; Sticher, O.; Constantinidis, T. Flavonoids as chemotaxonomic markers in the polymorfic Stachys swainsonii (Lamiaceae). Biochem. Syst. Ecol. 2007, 35, 317–320. [Google Scholar] [CrossRef]
- Laggoune, S.; Zeghib, A.; Kabouche, A.; Kabouche, Z.; Maklad, Y.A.; Leon, F.; Brouard, I.; Bermejo, J.; Calliste, C.A.; Duroux, J.L. Components and antioxidant, anti-inflammatory, anti-ulcer and antinociceptive activities of the endemic species Stachys mialhesii de Noé. Arab. J. Chem. 2016, 9, S191–S197. [Google Scholar] [CrossRef] [Green Version]
- Zinchenko, T.V. Phenolic compounds of Stachys palustris. Chem. Nat. Compd. 1973, 6, 261–262. [Google Scholar] [CrossRef]
- Litvinenko, V.I.; Aronova, B.N. Phenolic compounds of Betonica foliosa. Chem. Nat. Compd. 1968, 4, 270. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, M.-E.F.; Hamed, A.R.; El-Kashoury, E.-S.A.; Shaheen, A.M.; Tawfik, W.A.; Paré, P.W.; Abdel-Sattar, E.A. Stachaegyptin A–C: Neo clerodane diterpenes from Stachys aegyptiaca. Phytochem. Lett. 2017, 21, 151–156. [Google Scholar] [CrossRef]
- Ruiu, S.; Anzani, N.; Orruù, A.; Floris, C.; Caboni, P.; Alcaro, S.; Maccioni, E.; Distinto, S.; Cottiglia, F. Methoxyflavones from Stachys glutinosa with binding affinity to opioid receptors: In Silico, in vitro, and in vivo studies. J. Nat. Prod. 2015, 78, 69–76. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C.; Ceylan, O. Metabolite profiling and health benefits of Stachys cretica subsp. mersinaea as a medicinal food. Ind. Crop. Prod. 2019, 131, 85–89. [Google Scholar] [CrossRef]
- Venditti, A.; Serrilli, A.; Di Cecco, M.; Ciaschetti, G.; Andrisano, T.; Bianco, A. Phytochemical composition of polar fraction of Stachys germanica L. subsp. salviifolia (Ten.) Gams, a typical plant of Majella National Park. Nat. Prod. Res. 2013, 27, 190–193. [Google Scholar] [CrossRef]
- Litvinenko, V.I. Some Questions of the Chemistry and Taxonomy of the Labiatae Family, the Plant Resources of the Ukraine, Their Isolation and Rational Use; Naukova Dumka: Kiev, Ukraine, 1973; p. 128. [Google Scholar]
- Šliumpaitė, I.; Venskutonis, P.; Murkovic, M.; Ragažinskienė, O. Antioxidant properties and phenolic composition of wood betony (Betonica officinalis L., syn. Stachys officinalis L.). Ind. Crop. Prod. 2013, 50, 715–722. [Google Scholar] [CrossRef]
- Kirkan, B. Antioxidant potential, enzyme inhibition activity, and phenolic profile of extracts from Stachys cretica subsp. vacillans. Ind. Crop. Prod. 2019, 140, 111639. [Google Scholar] [CrossRef]
- Nishimura, H.; Sasaki, H.; Inagaki, N.; Chin, M.; Mitsuhashi, H.; Masao, C.; Chen, Z. Nine phenethyl alcohol glycosides from Stachys sieboldii. Phytochemistry 1991, 30, 965–969. [Google Scholar] [CrossRef]
- Ikeda, T.; Miyase, T.; Ueno, A. Phenylethanoid glycosides from Stachys riederi. Nat. Med. 1994, 48, 32–38. (In Japanese) [Google Scholar]
- Başaran, A.A.; Calis, I.; Anklin, C.; Nishibe, S.; Sticher, O. Lavandulifolioside: A new phenylpropanoid glycoside from Stachys lavandulifolia. Helv. Chim. Acta 1988, 71, 1483–1490. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Pugliese, A.; Nadjafi, F.; Menichini, F.; Loizzo, M.R. Tyrosinase, Acetyl- and Butyryl-cholinesterase inhibitory activity of Stachys lavandulifolia Vahl (Lamiaceae) and its major constituents. Rec. Nat. Prod. 2015, 9, 81–93. [Google Scholar]
- Çalis, I.; Basaran, A.; Saracoglu, C.; Sticher, O. Iridoid and phenylopropanoid glycosides from Stachys macrantha. Phytochemistry 1992, 31, 167–169. [Google Scholar] [CrossRef]
- Miyase, T.; Yamamoto, R.; Ueno, A. Phenylethanoid glycosides from Stachys officinalis. Phytochemistry 1996, 43, 475–479. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Nicoletti, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Vitali, L.A.; Petrelli, D.; Papa, F.; Vittori, S.; et al. Phytochemical analysis, biological evaluation and 2 micromorphological study of Stachys alopecuros (L.) Benth. subsp. divulsa (Ten.) Grande endemic to central Apennines, Italy. Fitoterapia 2013, 90, 94–103. [Google Scholar]
- Ahmad, V.U.; Arshad, S.; Bader, S.; Ahmed, A.; Iqbal, S.; Tareen, R.B. New phenethyl alcohol glycosides from Stachys parviflora. J. Asian Nat. Prod. Res. 2006, 8, 105–111. [Google Scholar] [CrossRef]
- Komissarenko, N.F.; Derkach, A.I.; Sheremet, I.P.; Pakaln, D.A. Iridoids of Stachys inflata and St. iberica. Khim. Prir. Soedin. 1979, 1, 99–100. [Google Scholar]
- Serrilli, A.M.; Ramunno, A.; Piccioni, F.; Serafini, M.; Ballero, M.; Bianco, A. Monoterpenoids from Stachys glutinosa L. Nat. Prod. Res. 2006, 20, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, A.M.; Camero, C.M.; D’ambola, M.; D’angelo, V.; Amira, S.; Bader, A.; Braca, A.; De Tommasi, N.; Germanò, M.P. Antiangiogenic Iridoids from Stachys ocymastrum and Premna resinosa. Planta Med. 2019, 85, 1034–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Háznagy-Radnai, E.; Czigle, S.; Janicsák, G.; Máthé, I. Iridoids of Stachys species growing in Hungary. J. Planar Chromatogr. Mod. TLC 2006, 19, 187–190. [Google Scholar] [CrossRef]
- Derkach, A.I.; Komissarenko, N.F.; Pakaln, D.A. Iridoids from some Stachys L. species. Rast Nye Resur. 1987, 23, 92–95. [Google Scholar]
- Litvinenko, V.I.; Aronova, B.N. Iridoids of Betonica foliosa. Chem. Nat. Compd. 1968, 4, 269–270. [Google Scholar] [CrossRef]
- Jeker, M.; Sticher, O.; Calis, I.; Rüedi, P. Allobetonicoside and 6-O-Acetylmioporoside: Two new iridoid glycosides from Betonica officinalis L. Helv. Chim. Acta 1989, 72, 1787–1791. [Google Scholar] [CrossRef]
- Zinchenko, T.V. Stachys and Betonica iridoids. Farm. Zhurnal 1972, 27, 86–87. [Google Scholar]
- Muñoz, O.; Peña, R.C.; Montenegro, G. Iridoids from Stachys grandidentata. Z. Nat. C 2001, 56, 902–903. [Google Scholar] [CrossRef] [Green Version]
- Melek, F.R.; Radwan, A.S.; El-Ansari, M.A.; El-Gindi, O.D.; Hilal, S.H.; Genenah, A.A. Diterpenes from Stachys aegyptiaca. Fitoterapia 1992, 63, 276. [Google Scholar]
- Mohamed, A.E.-H.H.; Mohamed, N.S. A new trans-neo clerodane diterpene from Stachys aegyptiaca. Nat. Prod. Res. 2013, 28, 30–34. [Google Scholar] [CrossRef]
- Mohamed, T.A.; ElShamy, A.I.; Hamed, A.R.; Shams, K.A.; Hegazy, M.-E.F. Cytotoxic neo-clerodane diterpenes from Stachys aegyptiaca. Phytochem. Lett. 2018, 28, 32–36. [Google Scholar] [CrossRef]
- Hussien, T.A.; Mahmoud, A.A.; Mohamed, N.S.E.-D.; Shahat, A.A.; El-Seedi, H.R.; Hegazy, M.-E.F. New rare ent-clerodane diterpene peroxides from Egyptian Mountain Tea (Qourtom) and its chemosystem as herbal remedies and phytonutrients agents. Molecules 2020, 25, 2172. [Google Scholar] [CrossRef] [PubMed]
- Derkach, A.I. Biologically active substances of some species of the genus Stachys L. of the flora of the Ukraine. Rastit. Nye Resur. 1998, 34, 57–61. [Google Scholar]
- Piozzi, F.; Savona, G.; Hanson, J.R. Kaurenoid diterpenes from Stachys lanata. Phytochemistry 1980, 19, 1237–1238. [Google Scholar] [CrossRef]
- Orgiyan, T.M.; Popa, D.P. Diterpenoids from Stachys annua. Khim. Prir. Soedin. 1969, 5, 5–6. [Google Scholar] [CrossRef]
- Popa, D.P.; Orgiyan, T.M. The stereochemistry of stachysolone. Khim. Prir. Soedin. 1972, 8, 717–719. [Google Scholar] [CrossRef]
- Popa, D.P.; Orgiyan, T.M. Minor diterpenoids of Stachys annua. Chem. Nat. Compd. 1974, 10, 410. [Google Scholar] [CrossRef]
- Piozzi, F.; Paternostro, M.; Servettaz, O.; Arnold, N. Occurrence of (+)-6-desoxyandalusol in Stachys ionica and Stachys distans. Biochem. Syst. Ecol. 2002, 30, 887–889. [Google Scholar] [CrossRef]
- Adinolfi, M.; Barone, G.; Lanzetta, R.; Laonigro, G.; Mangoni, L.; Parrilli, M. Diterpenes from Stachys recta. J. Nat. Prod. 1984, 47, 541–543. [Google Scholar] [CrossRef]
- Fazio, C.; Paternostro, M.P.; Passannanti, S.; Piozzi, F. Further neo-clerodane diterpenoids from Stachys rosea. Phytochemistry 1994, 37, 501–503. [Google Scholar] [CrossRef]
- Popa, D.P.; Pasechnik, G.S. Structure of stachysic acid—A new diterpenoid of the kaurane series. Chem. Nat. Compd. 1974, 10, 454–457. [Google Scholar] [CrossRef]
- Bankova, V.; Koeva-Todorovska, J.; Stambolijska, T.; Ignatova-Groceva, M.-D.; Todorova, D.; Popov, S. Polyphenols in Stachys and Betonica species (Lamiaceae). Z. Nat. C 1999, 54, 876–880. [Google Scholar] [CrossRef]
- Paternostro, M.P.; Maggio, A.M.; Piozzi, F.; Servettaz, O. Lavdane diterpenes from Stachys plumose. J. Nat. Prod. 2000, 63, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Miyase, T.; Yamamoto, R.; Ueno, A. Betonicosides A–D and betonicolide, diterpenoids from the roots of Stachys officinalis. Chem. Pharm. Bull. 1996, 44, 1610–1613. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.A.; Zinchenko, T.V. Triterpenoids and steroids from Stachys palustris. Farm. Zhurnal 1975, 30, 91–92. [Google Scholar] [PubMed]
- Yamamoto, R.; Miyase, T.; Ueno, A. Stachyssaponins I-VIII, new oleanane-type triterpene saponins from Stachys riederi CHAMISSO. Chem. Pharm. Bull. 1994, 42, 1291–1296. [Google Scholar] [CrossRef] [Green Version]
- Takeda, Y.; Zhang, H.-J.; Masuda, T.; Honda, G.; Otsuka, H.; Sezik, E.; Yesilada, E.; Sun, H. Megastigmane glucosides from Stachys byzantina. Phytochemistry 1997, 44, 1335–1337. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, M.; Penn, M.; El Mowafi, A.; Storebakken, T.; Chunfang, C.; Øverland, M.; Krogdahl, Å. Effect of stachyose, raffinose and soya-saponins supplementation on nutrient digestibility, digestive enzymes, gut morphology and growth performance in Atlantic salmon (Salmo salar, L.). Aquaculture 2011, 314, 145–152. [Google Scholar] [CrossRef]
- Yin, J.; Yang, G.; Wang, S.; Chen, Y. Purification and determination of stachyose in Chinese artichoke (Stachys sieboldii Miq.) by high-performance liquid chromatography with evaporative light scattering detection. Talanta 2006, 70, 208–212. [Google Scholar] [CrossRef]
- Harada, S.; Tsujita, T.; Ono, A.; Miyagi, K.; Mori, T.; Tokuyama, S. Stachys sieboldii (Labiatae, Chorogi) protects against learning and memory dysfunction associated with ischemic brain injury. J. Nutr. Sci. Vitaminol. 2015, 61, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarikurkcu, C.; Kocak, M.S.; Uren, M.C.; Calapoglu, M.; Tepe, A.S. Potential sources for the management global health problems and oxidative stress: Stachys byzantina and S. iberica subsp. iberica var. densipilosa. Eur. J. Integr. Med. 2016, 8, 631–637. [Google Scholar] [CrossRef]
- Abi-Rizk, A.; El Rayess, Y.; Iriti, M.; Tabet, E.; Mezher, R.; El Beyrouthy, M. Chemical composition, antitumor and antioxidant effects of four lebanese plants extracts on human pulmonary adenocarcinoma. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, M.; Erol, E.; Beladjila, K.A.; Çetintaş, Y.; Duru, M.E.; Öztürk, M.; Kabouche, A.; Kabouche, Z. Antioxidant, anticholinesterase and antibacterial activities of Stachys guyoniana and Mentha aquatica. Pharm. Biol. 2016, 55, 324–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigorakis, S.; Makris, D.P. Characterisation of polyphenol-containing extracts from Stachys mucronata and evaluation of their antiradical activity. Medicines 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Slapšytė, G.; Dedonytė, V.; Adomėnienė, A.; Lazutka, J.R.; Kazlauskaitė, J.; Ragažinskienė, O.; Venskutonis, P.R. Genotoxic properties of Betonica officinalis, Gratiola officinalis, Vincetoxicum luteum and Vincetoxicum hirundinaria extracts. Food Chem. Toxicol. 2019, 134, 110815. [Google Scholar] [CrossRef]
- Kokhdan, E.P.; Ahmadi, K.; Sadeghi, H.; Sadeghi, H.; Dadgary, F.; Danaei, N.; Aghamaali, M.R. Hepatoprotective effect of Stachys pilifera ethanol extract in carbon tetrachloride-induce hepatotoxicity in rats. Pharm. Biol. 2017, 55, 1389–1393. [Google Scholar] [CrossRef] [Green Version]
- Mansourian, M.; Mirzaei, A.; Azarmehr, N.; Vakilpour, H.; Kokhdan, E.P.; Doustimotlagh, A.H. Hepatoprotective and antioxidant activity of hydroalcoholic extract of Stachys pilifera. Benth on acetaminophen-induced liver toxicity in male rats. Heliyon 2019, 5, e03029. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.Y.; Yadav, A.K.; Jang, B.C.; Kim, Y.C. Antioxidant and cytoprotective effects of Stachys riederi var. japonica ethanol extract on UVA-irradiated human dermal fibroblasts. Int. J. Mol. Med. 2019, 43, 1497–1504. [Google Scholar]
- Lee, J.K.; Lee, J.; Kim, Y.-K.; Lee, Y.; Ha, J.-H. Stachys sieboldii Miq. root attenuates weight gain and dyslipidemia in rats on a high-fat and high-cholesterol diet. Nutrients 2020, 12, 2063. [Google Scholar] [CrossRef]
- Ravichandran, V.A.; Kim, M.; Han, S.K.; Cha, Y. Stachys sieboldii extract supplementation attenuates memory deficits by modulating BDNF-CREB and its downstream molecules, in animal models of memory impairment. Nutrients 2018, 10, 917. [Google Scholar] [CrossRef] [Green Version]
- Rahzani, K.; Malekirad, A.A.; Zeraatpishe, A.; Hosseini, N.; Seify, S.M.R.; Abdollahi, M. Anti-oxidative stress activity of Stachys lavandulifolia aqueous extract in human. Cell J. 2013, 14, 314–317. [Google Scholar] [PubMed]
- Jalilian, N.; Modarresi, M.; Rezaei, M.; Ghaderi, L.; Bozorgmanesh, M. Phytotherapeutic management of polycystic ovary syndrome: Role of aerial parts of wood betony (Stachys lavandulifolia). Phytother. Res. 2013, 27, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Monji, F.; Hashemian, F.; Surmaghi, M.-H.S.; Mohammadyari, F.; Ghiyaei, S.; Soltanmohammadi, A. Therapeutic effects of standardized formulation of Stachys lavandulifolia Vahl on primary dysmenorrhea: A randomized, double-blind, crossover, placebo-controlled pilot study. J. Altern. Complement. Med. 2018, 24, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, A.R.; Jadidi, A.; Hezave, A.; Safarabadi, M.; Pour, S.A.; Ghassami, K.; Mohammadbeigi, A. An analgesic effect of Stachys lavandulifolia in patients with migraine: A double-blind randomised clinical trial study. Adv. Hum. Biol. 2019, 9, 76. [Google Scholar] [CrossRef]
- Jafarzadeh, L.; Rafieian-Kopaei, M.; Samani, R.A.; Asgari, A. The effect of hydroalcoholic extract of Stachys lavandulifolia Vahl on pregnant mice. EXCLI J. 2012, 11, 357–362. [Google Scholar]
- Taghikhani, M.; Nasri, H.; Asgari, A.; Afrough, H.; Namjoo, A.; Ansari-Samani, R.; Shahinfard, N.; Rafieian-Kopaei, M. The renal toxicity of hydroalcoholic extract of Stachys lavandulifolia Vahl in Wistar rats. Life Sci. J. 2012, 9, 3025–3031. [Google Scholar]
- Modarresi, M.; Hosseinzadeh, L.; Nematy, N.; Siavash-Haghighi, Z.; Ghanbari, K. Acute and subchronic toxicological evaluation of Stachys lavandulifolia aqueous extract in Wistar rats. Res. Pharm. Sci. 2015, 9, 165. [Google Scholar]
| Species | Geographical Origin of the Reported Traditional Use | Traditional Medicinal Use | Preparation and/or Administration/ Parts of the Plant | Ref. |
|---|---|---|---|---|
| S. acerosa Boiss. | Iran | Common cold | Decoction | [31] |
| S. affinis Bunge (=S. sieboldii Miq.) | China | Infections, colds, heart diseases, tuberculosis, pneumonia | Edible food (tubers) | [27,28] |
| China | Common cold, heart diseases, for pain relief, as antioxidant, to treat ischemic brain injury, dementia, various gastrointestinal related diseases | - | [29] | |
| S. annua (L.) L | Italy | Headache | Infusion of leaves; also, external use to wash face | [51] |
| S. annua (L.) L subsp. annua | Italy | Anti-catarrhal, febrifuge, tonic, vulnerary, against evil eye | Aerial parts | [52] |
| S. arvensis (L.) L. | - | Against evil eye | - | [55] |
| S. balansae Boiss. & Kotschy | - | Hypotonic diseases, cardiac neuroses | Liquid and alcoholic extracts | [23] |
| S. byzantina K. Koch. | - | Anti-inflammatory, antitumor, anticancer, antispasmodic, sedative and diuretic agent, and in the treatment of digestive disorders, wounds, infections, asthma, rheumatic and inflammatory disorders, dysentery, epilepsy, common cold and neuropathy | - | [33] |
| Iran | Infected wounds, cutting | Decoction, Demulcent (Leaves) | [34,35] | |
| Brazil | Antiinflammatory | Infusion of leaves | [60] | |
| S. cretica subsp. anatolica Rech. f. | Turkey | Colds, stomach ailments | Infusion, decoction, internal | [49] |
| S. cretica L. subsp. mersinaea (Boiss.) Rech. f. | Turkey | Colds, stomach ailments | Infusion, decoction, internal | [49] |
| S. fruticulosa M. Bieb. | Iran | Anti- inflammatory | Aerial parts | [32] |
| S. geobombycis C.Y.Wu | China, Japan and Europe | Tonic | - | [22] |
| S. germanica L. | Iran | Gastrodynia, for painful menstruation | Infusion of flowers | [34] |
| - | Skin disorders (Veterinary use) | - | [55] | |
| S. glutinosa L. | - | As antispasmoic and against chicken louse | - | [55] |
| S. iberica subsp. georgica Rech. f. | Turkey | Colds, antipyretic | Decoction, internal | [49] |
| S. iberica subsp. stenostachya (Boiss.) Rech. f. | Turkey | Colds, antipyretic, stomach ache | Decoction, internal | [49] |
| S. inflata Benth. | Iran | Infections, asthmatic, rheumatic, inflammatory disorders | Extracts of aerial parts (non flowering stems) | [36,37] |
| Iran | Common cold, Analgesic, high blood pressure | Decoction of aerial parts | [31] | |
| S. iva Griseb. | Greece | Common cold and gastrointestinal disorders | Decoction, infusion | [56] |
| S. kurdica Boiss & Hohen var. kurdica | Turkey | Cold, stomach-ache | Decoction of branches/flowers Drink one glass of the plant on an empty stomach in the morning | [50] |
| S. lavandulifolia Vahl. | Iran | Treat pain and inflammation | Boiled extracts of the aerial parts | [12] |
| Iran | Sedative, gastrotonic and spasmolytic properties, treatment of some gastrointestinal disorders, colds and flu | Herbal tea of flowering aerial parts | [13] | |
| Iran | Headache, renal calculus common cold, sedative flavoring agent, abdominal pain | Decoction of aerial parts, Food additive (aerial parts) | [31] | |
| Turkey | Antipyretic, cough | Decoction, internal | [49] | |
| Iran | Painful and inflammatory disorders | Boiled extracts of aerial parts | [41] | |
| Iran | Anxiolytic influence | Herbal tea | [38,39,40,41,42,43,44] | |
| S. mucronata Sieb. | Greece | Antirheumatic and antineuralgic remedy | Decoction for massage | [57] |
| For wounds and ulcers | Washed with the decoction and covered with a poultice of fresh leaves for cicatrization | |||
| Antidiarrhoic agent | Infusion of fresh leaves | |||
| Pugative | Infusion of roots | |||
| S. obliqua Waldst. & Kit. | Turkey | Cold, stomach ailments, fever and cough | Herb, infusion, decoction | [22] |
| S. officinalis (L.) Trevisan (=S. betonica Benth.; Betonica officinalis L.) | Serbia, Egypt, Montenegro | Skin disorders, antibacterial purposes, against headache, nervous tension, anxiety, menopausal problems, as a tobacco snuff | Tea of dried leaves | [22] |
| Italy | Dye wool yellow | Plant | [51] | |
| Italy | Wounds, in the sores of pack animals | Oily extract of flowers | [54] | |
| S. palustris L. | - | Disinfectant, anti-spasmodic and for treatment of wounds | - | [17,61] |
| Poland | Wounds, additive in food | - | [58] | |
| - | Antiseptic, to relieve gout, to stop haemorrhage | - | [62] | |
| S. parviflora Benth. (=Phlomidoschema parviflorum (Benth.) Vved.) | - | Cramps, arthralgia, epilepsy, falling sickness, dracunculiasis | - | [63,64] |
| S. pilifera Benth. | Iran | Toothache, edible, tonic, analgesic, edema, expectorant, tussive | Decoction of aerial parts | [31] |
| Iran | Asthma, rheumatoid arthritis and infections | - | [45] | |
| S. pumila Banks & Sol. | Anatolia | Antibacterial and healing effects | Tea of the whole part | [21] |
| Anatolia | Sedative, antispasmodic, diuretic and emmenagogic properties | Tea of the leaves | [21] | |
| - | Bronchitis, asthma, stomach pain and gall and liver disorders | - | [65] | |
| S. recta L. | Europe | Anxiolytic properties | Herbal tea, Oral administration | [11] |
| Italy | Headache | Infusion of leaves to wash face | [51] | |
| Italy | Bad influence/spirit | Decoction | [53] | |
| Italy | Depurative | Decoction of the aerial parts | [54] | |
| S. recta L. subsp. recta | Italy | Tootache and other pain | Aerial parts applied in body parts | [53] |
| against anxiety, pain and toothache | Decoction of flowering tops for bath or to wash face, hands and wrists for 3 days | |||
| S. schtschegleevii Sosn. ex Grossh. | Iran | Antiinflamatory | Aerial parts | [32,34] |
| Iran | Infectious diseases of the respiratoy tract (for colds and sinusitis), for asthma, rheumatism and other inflammatory disorders | - | [46] | |
| S. sieboldii Miq. (=S. affinis Bunge) | China | Cold and against infections, promoting blood circulation | Dried whole plant | [30] |
| S. sylvatica L. | - | Disinfectant, anti-spasmodic and for treatment of wounds | - | [17] |
| Iran | Diuretic, digestive, emmenagogue, antispasmodic, anti-inflammatory, sedative, tonic properties and for the treatment of women with PCOS | - | [47] | |
| Turkey | Cardiac disorders | Infusion of aerial parts | [48] | |
| S. tibetica Vatke | India | For fever, cough, phobias and various mental disorder | Whole plant is boiled and made into a decoction. Drink one teacup decoction twice a day to treat fever for 5–7 days | [66] |
| S. turcomanica Trautv. | Iran | Foot inflammation, toothache, bronchitis and common cold | Infusion, Demulcent, Vapor (Whole plant) | [34] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Ambleia | |||
| S. aegyptiaca Pers. | Aerial parts | Apigenin (1), Apigenin 7-O-β-D-glucoside (cosmoside) (2), Apigenin 7-O-[6′″-O-acetyl]-allosyl-(1→2)-β-D-glucoside (3), Apigenin 6,8-di-C-glucoside (Vicenin-2) (10), Isoscutellarein 7-O-allosyl-(1→2)-β-D-glucoside (13), Isoscutellarein-7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucoside (15), Luteolin (34), Luteolin-7-O-[6′″-O-acetyl]-allosyl]-(1→2)-β-D-glucoside (39), 6,8 Di-C-β-D-glucopyranosyl luteolin (Lucenin-2) (40), Chrysoeriol (42) Chrysoeriol 7-O-β-D-glucoside (43), Hypolaetin 7-O-[6′″-O-acetyl]-allosyl-(1→2)-[3″-O-acetyl]- β-D-glucoside (54), Apigenin 7-O-diglucoside (not determined), Luteolin 7-O-diglucoside (not determined) | [68] |
| Aerial parts | Apigenin-7-(3″-E-p-coumaroyl)-β-D-glucoside (4), Apigenin 7-(6″-p-coumaroyl)-β-D-glucoside (6) | [69] | |
| Aerial parts | Isoscutellarein (11), 3′,4′-Dimethyl-luteolin-7-O-β-D-glucoside (41) | [70] | |
| Isoscutellarein 8-O-(6″-trans-p-coumaroyl)-β-D-glucoside (18) | [71] | ||
| S. inflata Beth. | Scutellarein 7-O-β-D-mannopyranosyl-(1→2)-β-D-glucoside (stachyflaside) (31) | [72] | |
| Isoscutellarein (11), 4′-Μethyl-isoscutellarein (12), Scutellarein (29) | [73] | ||
| S. schtschegleevii Sosn. ex Grossh. | Stems | Apigenin 7-O-β-D-glucoside (2), Apigenin 7-(6″-E-p-coumaroyl)-β-D-glucopyranoside (6), 3′-Hydroxy-isoscutellarein-7-O-[6′″-O-acetyl]-β-D-glucopyranoside (14), Chrysoeriol 7-(6″-E-p-coumaroyl)-β-D-glucopyranoside (47) | [74] |
| Section Campanistrum | |||
| S. arvensis (L.) L. | Aerial parts # | 8-Hydroxyflavone-allosylglucosides (not determined) | [75] |
| S. ocymastrum (L.) Briq. (= S. hirta L.) | Aerial parts # | 8-Hydroxyflavone-allosylglucosides (not determined) | [75] |
| Aerial parts | Apigenin (1), Apigenin 7-(6″-E-p-coumaroyl)-β-D-glucopyranoside (6), Isoscutellarein 7-O-allosyl-(1→2)- glucopyranoside (13), Luteolin (34) | [76] | |
| Section Candida | |||
| S. candida Bory & Chaubard | Aerial parts | Chrysoeriol (42), Chrysoeriol 7-(3″-E-p-coumaroyl)-β-D-glucopyranoside (46) | [77] |
| Aerial parts | Apigenin 7-O-β-D- glucopyranoside (2), Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), Isoscutellarein 7-O-[6′″-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]- glucopyranoside (17), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21), Chrysoeriol 7-O-β-D- glucopyranoside (43), Chrysoeriol 7-(3″-E-p-coumaroyl)-β-D-glucopyranoside (46), 4′-Methyl-hypolaetin-7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [78] | |
| S. chrysantha Boiss. and Heldr. | Aerial parts | Isoscutellarein 7-O-[6′″-O-acetyl]-allosyl(1→2)-[6″-O-acetyl]-glucoside (17), Luteolin 7-O-β-D-glucoside (37), Chrysoeriol (42), Chrysoeriol 7-O-β-D- glucopyranoside (43), Chrysoeriol 7-(3″-E-p-coumaroyl)-β-D-glucopyranoside (46) | [77] |
| S. iva Griseb. | Flowering aerial parts | Apigenin (1), Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-[6″-O-acetyl]-β-D-glucopyranoside (17), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21), 4′-Methyl-hypolaetin-7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [56] |
| Section Corsica | |||
| S. corsica Pers. | Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21) | [79] | |
| Section Eriostomum | |||
| S. alpina L. | Aerial parts # | 8-Hydroxyflavone-allosylglucosides (not determined) | [75] |
| Leaves # | Hypolaetin 7-O-acetyl-allosyl-(1→2)-glucoside (not determined), Isoscutellarein-7-O-acetyl-allosyl-glucoside (not determined), Hypolaetin-4′-methyl- 7-O- acetyl-allosyl-glucoside (not determined) | [5] | |
| S. byzantina K. Koch. | Aerial parts | Apigenin (1), Apigenin 7-O-β-glucoside (2), Apigenin 7-(6″-E-p-coumaroyl)-β-D-glucopyranoside (6) | [33] |
| Aerial parts | Apigenin 7-(6″-E-p-coumaroyl)-β-D-glucopyranoside (6), Isoscutellarein 7-O-β-D-allopyranosyl-(1→2)-[6″-O-acetyl]-β-D-glucopyranoside (16), 4′-Methyl-isoscutellarein-7-O-β-D-allopyranosyl-(1→2)-[6″-O-acetyl]-β-D-glucopyranoside (20) | [80] | |
| S. cretica subsp. smyrnaea Rech. f. | Aerial parts # | Apigenin (1) | [81] |
| S. germanica L. | Aerial parts # | Hypolaetin 7-allosyl-(1→2)-glucoside monoacetyl, Isoscutellarein 7-allosyl-(1→2)-glucoside monoacetyl, Hypolaetin 7-allosyl-(1→2)-glucoside diacetyl, Isoscutellarein-7-allosyl-(1→2)-glucoside diacetyl(not determined) | [75] |
| Leaves # | Apigenin 7-O-glucoside (2), Chrysoeriol 7-O-acetyl-allosyl-glucoside (not determined), 4′-Methyl-hypolaetin 7-O-acetyl-allosyl-(1→2)-glucoside (not determined), Apigenin 7-O-p-coumaroyl-glucoside (not determined) | [5] | |
| S. heraclea All. | Aerial parts # | 8-Hydroxyflavone-allosylglucosides (not determined) | [75] |
| S. lanata Crantz. (=S. germanica L. subsp. germanica) | Aerial parts | Apigenin 7-O-β-D-glucopyranoside (2), Apigenin 7-(3″-Z-p-coumaroyl)-β-D-glucopyranoside (5), Apigenin 7-(6″-Z-p-coumaroyl)-β-D-glucopyranoside (7), Apigenin 7-O-(3′′,6′′-di-O-E-p-coumaroyl)-β-D-glucopyranoside (Anisofolin A) (8), Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), Isoscutellarein 4′-methyl ether 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21), 4′-Methyl-hypolaetin-7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [82] |
| S. spectabilis Choisy ex DC. | Epigeal parts | Isostachyflaside (25), Spectabiflaside (28), Scutellarein 7-O-β-D-mannopyranosyl-(1→2)-β-D-glucopyranoside (stachyflaside) (31) | [83] |
| S. thirkei K. Koch. | Whole plant # | Apigenin (1) | [84] |
| S. tmolea Boiss. | Aerial parts # | Apigenin (1), Apigenin-7-O-glucoside (2) | [85] |
| S. tymphaea Hausskn. (=S. germanica subsp. tymphaea (Hausskn.) R. Bhattacharjee) | Flowering aerial parts | Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21), 4′-Methyl-hypolaetin-7-O-[6′″-O-acetyl]- allopyranosyl -(1→2)-[6″-O-acetyl]-glucopyranoside (58) | [86] |
| Section Fragilicaulis | |||
| S. subnuda Montbret & Aucher ex Benth | Aerial parts | Ιsoscutellarein 7-O-allosyl-(1→2)-glucoside # (13), Isoscutellarein 7-O-[6″′-O-acetyl]-allosyl-(1→2)-glucoside (15), 4′-Methyl-isoscutellarein-7-O-β-D-allopyranosyl-(1→2)-β-D-glucoside # (19), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucoside (21), 4′-Methyl-isoscutellarein-7-O-[6′″-O-acetyl]-allosyl(1→2)-[6″-O-acetyl]-glucoside # (24) | [87] |
| Section Olisia | |||
| S. atherocalyx C. Koch | Stachyflaside (31) | [72] | |
| Diacetylstachyflaside (not determined), Diacetylspectabiflaside (not determined), Spectabiflaside (28) | [88] | ||
| 5,8,4′-Trihydroxy-3′-methoxy-7-O-(β-D-glucopyranosyl-2″-O-β-D-mannopyranosyl)-flavone (Spectabiflaside) (28), Acetyl-sectabiflaside (not determined), | [89] | ||
| Acetyl-isostachyflaside (26), Di-acetyl-isostachyflaside (27), Spectabiflaside (28) | [90] | ||
| Leaves # | Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein-7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21), 4′-Methyl-hypolaetin-7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [91] | |
| S. angustifolia M. Bieb. | Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21) | [92] | |
| S. annua (L.) L. | Epigeal parts | 4′-Methyl-isoscutellarein (12), 7-O-β-D-glucopyranosyl- 5,6-dihydroxy-4′-methoxyflavone (Stachannin A) (32), 4′-Methoxy-scutellarein-7-[O-β-D-mannopyranosyl-(1→2)-β-D-glucopyranoside] (Stachannoside B) (33) | [93] |
| Leaves # | Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein-7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21), 4′-Methyl-hypolaetin-7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [92] | |
| Aerial parts | 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21) | [94] | |
| Aerial parts | 4′-O-Methyl-isoscutellarein-7-O-[4′″-O-acetyl]allopyranosyl-(1→2)- glucopyranoside (Annuoside) (23) | [95] | |
| Subterranean organs | 4′-O-Methyl-isoscutellarein (12), 4′-O-Methyl-isoscutellarein 7-O-(6′″-O-acetyl)allopyranosyl-(1→2)-glucopyranoside (21) | [95] | |
| S.annua (L.) L. subsp. annua | Flowering aerial parts | 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21), Hypolaetin 7-O-[6′″-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]-glucopyranoside (53), 4′-Methyl-hypolaetin-7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [52] |
| S. beckeana Dörfler & Hayek | Leaves # | Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (21), 4′-Methyl-hypolaetin-7-O-[6′″-O acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [92] |
| S. bombycina Boiss. | Aerial parts | Apigenin 7-(6″-E-p-coumaroyl)-β-D-glucopyranoside (6), Stachyspinoside (44) | [96] |
| S. parolinii Vis. | Leaves # | Isoscutellarein 7-O-[6′″-O-acetyl]-β- D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-hypolaetin-7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [92] |
| S. leucoglossa Griseb. | Leaves # | Isoscutellarein 7-O-[6′″-O-acetyl]-allosyl(1→2)-[6″-O-acetyl]-glucoside (17), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21), 4′-Methyl-hypolaetin-7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [92] |
| S. neglecta Klok. ex Kossko (=S. annua (L.) L.) | Apigenin (1), Apigenin 7-O-β-D-glucoside (2), Luteolin (34), Luteolin 7-O-β-D-glucoside (37) | [97] | |
| S. recta L. | Leaves | Isoscutellarein 7-O-[6′″-O-acetyl]-allosyl(1→2)-[6″-O-acetyl]-glucoside (17), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]- allosyl](1→2)-β-D-glucoside (21), 4′-Methyl-hypolaetin 7-O-[6′″-O-acetyl]-β-D-allosyl(1→2)-β-D-glucoside (56) | [91,92] |
| Aerial parts | Apigenin 7-(3″-E-p-coumaroyl)-β-D-glucopyranoside (4), Apigenin 7-(6″-E-p-coumaroyl)-β-D-glucopyranoside (6), Isoscutellarein 7-O-[allosyl(1→2)]- glucopyranoside (13), Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allosyl-(1→2)-β-D-glucopyranoside (15), Isoscutellarein 7-O-[6′″-O-acetyl]-allosyl(1→2)-[6″-O-acetyl]- glucopyranoside (17), 4′-Methylisoscutellarein 7- O-[allosyl-(1→2)]- glucopyranoside (19), 4′-Methyl-isoscutellarein 7-O-β-D-[6″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (20), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]- allosyl-(1→2)-β-D-glucopyranoside (21), 4′-Methyl-isoscutellarein 7-O-[6′″-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]-glucoside (24), Hypolaetin 7-O-allosyl-(1→2)-glucopyranoside # (50), 4′-Methyl-hypolaetin 7-O-allosyl(1→2)-glucoside # (55), 4′-Methyl-hypolaetin-7-O-[6″-O-acetyl]-allosyl-(1→2)- glucopyranoside (57), 4′-Methyl-hypolaetin 7-O-[6′″-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]- glucopyranoside (58) | [14] | |
| S. labiosa Bertol. (=S. recta subsp. labiosa (Bertol.) Briq.) | Leaves | Isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein-7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21), 4′-Methyl-hypolaetin-7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [92] |
| S. subcrenata Vis.(=S. recta L. subsp. subcrenata (Vis.) Briq.) | Leaves | Isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein-7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21), 4′-Methyl-hypolaetin-7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | [92] |
| S. baldaccii (Maly) Hand.—Mazz. (=S. recta L. subsp. baldaccii (K. Maly) Hayek) | Leaves # | Isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl]-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein-7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21) | [92] |
| S. spinosa L. | Aerial parts | Chrysoeriol 7-O-[6′″-O-acetyl-allosyl]-(1→2)-glucoside (Stachyspinoside) (44) | [98] |
| Aerial parts | Chrysoeriol 7-O-[6′′-O-acetyl-allosyl]-(1→2)-glucoside (Isostachyspinoside) (45) | [99] | |
| S. tetragona Boiss. & Hayek | Leaves # | Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allopyranosyl-(1→2)-β-D-glucopyranoside (21) | [92] |
| Aerial parts | Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), Isoscutellarein 7-O-[6′″-O-acetyl]- β-D-allosyl-(1→2)-[6″-O-acetyl]-β-D glucopyranoside (17) | [100] | |
| Section Swainsoniana | |||
| S. anisochila Vis. & Pancic | Leaves | Isoscutellarein 7-O-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (13), Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), Isoscutellarein 7-O-[6′″-O-acetyl]- β-D-allosyl-(1→2)-[6″-O-acetyl]-β-D-glucopyranoside (17), 4′-Methyl-isoscutellarein-7-O-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (19), Hypolaetin 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (51), Hypolaetin 7-O-[6′″-O-acetyl]- β-D-allopyranosyl-(1→2)-[6″-O-acetyl]-β-D glucopyranoside (53), 4′-Methyl-hypolaetin-7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56), 4′-Methyl-hypolaetin 7-O-[6′″-O-acetyl]-β-D-allosyl-(1→2)-[6″-O-acetyl]-β-D glucopyranoside (58) | [101] |
| Leaves | Apigenin 7-O-(p-coumaroyl)-β-D-glucopyranoside (not determined) | [5] | |
| S. decumbens Pers. (=S. mollissima Willd.) | Aerial parts # | 8-Hydroxyflavone-allosylglucosides (not determined) | [75] |
| S. menthifolia Vis. (=S. grandiflora Host.) | Leaves # | Isoscutellarein 7-O-β-D-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein-7-O-β-D-[6′″-O-acetyl]-β-D allopyranosyl-(1→2)-β-D-glucopyranoside (21) | [92] |
| S. swainsonii Benth. subsp. swainsonii | Aerial parts | Apigenin (1), Apigenin 7-O-β-D-glucopyranoside (2), Apigenin 7-O-β-D-glucoside (2), Luteolin 7-O-β-D-glucopyranoside (37), Chrysoeriol (42), Chrysoeriol 7-O-β-D-glucopyranoside (43), Stachyspinoside (44) | [102] |
| S. swainsonii subsp. argolica (Boiss.) Phitos and Damboldt | Aerial parts | Apigenin (1), Luteolin 7-O-β-D-glucopyranoside (37), Chrysoeriol (42), Chrysoeriol-7-O-β-D-glucopyranoside (43), Chrysoeriol 7-(3″-E-p-coumaroyl)-β-D-glucopyranoside (46) | [102] |
| S. swainsonii subsp. melangavica D. Persson | Aerial parts | Apigenin (1), Apigenin 7-O-β-D- glucopyranoside (2), Luteolin 7-O-β-D-glucopyranoside (37), Chrysoeriol-7-O-β-D-glucopyranoside (43), Stachyspinoside (44) | [102] |
| S. swainsonii subsp. scyronica (Boiss.) Phitos and Damboldt | Aerial parts | Apigenin (1), Apigenin 7-O-β-D- glucopyranoside (2), Luteolin 7-O-β-D-glucopyranoside (37), Chrysoeriol-7-O-β-D-glucopyranoside (43), Stachyspinoside (44) | [102] |
| S. ionica Halácsy | Aerial parts | Apigenin (1), Apigenin 7-(6″-E-p-coumaroyl)-β-D-glucopyranoside (6), Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15), 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (21) | [20] |
| Section Stachys | |||
| S. sieboldii Miq. (=S. affinis Bunge) | Aerial parts | Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allosyl]-(1→2)-β-D-glucoside (15), 4′-Methyl-isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucoside (21) | [20] |
| S. mialhesii Noé | Aerial parts | Apigenin 7-(6″-E-p-coumaroyl)-β-D-glucopyranoside (6), Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (15) | [103] |
| S. palustris L. | 5-(glycuroglucosyl)-7-methoxybaicalein (Palustrin) (63), 5-(glucuronosyl)-7-methoxybaicalein (Palustrinoside) (64) | [104] | |
| Leaves # | Vicenin-2 (10), Apigenin 7-O-p-coumaroyl-β-D-glucopyranoside (not determined) | [5] | |
| S. sylvatica L. | Aerial parts # | 8-Hydroxyflavone-allosyl-glucosides (not determined) | [75] |
| Leaves # | Chrysoeriol 7-O-acetylallosylglucoside (not determined), Apigenin 7-O-p-coumaroyl-β-D-glucopyranoside (not determined) | [5] | |
| S. plumosa Griseb. | Leaves # | Apigenin-7-O-β-D-glucoside (2), Luteolin 7-O-β-D-glucoside (37), Chrysoeriol 7-O-acetyl-allosyl-glucoside (not determined), Isoscutellarein 7-O-acetyl-allosyl-glucoside (not determined), Apigenin 7-O-p-coumaroyl-β-D-glucopyranoside (not determined) | [5] |
| Section Zietenia | |||
| S. lavandulifolia Vahl. | Aerial parts | Apigenin (1), Hydroxygenkwanin (Luteolin 7-Methyl ether) (35), Chrysoeriol (42) | [13] |
| S. tibetica Vatke | Roots | Apigenin 7-O-β-D-glucoside (2) | [66] |
| Subgenus Betonica | |||
| Section Betonica | |||
| S. alopecuros (L.) Benth. | Aerial parts | p-coumaroyl-glucosides (not determined) # | [75] |
| Leaves # | Isoscutellarein 7-O-glucoside (11a), Luteolin 7-O-glucuronide (36), Luteolin 7-O-glucoside (37), Chrysoeriol 7-O-glucoside (43), Hypolaetin 7-O-glucoside (49), Hypolaetin 7-O-glucuronide (49a), Selgin 7-O-glucoside (59), Tricin 7-O-glucuronide (60), Tricin 7-O-glucoside (61), Apigenin 7-O-p-coumaroyl glucopyranoside (not determined) | [5] | |
| S. foliosa Regel. (=S. betoniciflora Rupr.; Betonica foliosa Rupr.) | Four flavonoids (not determined) | [105] | |
| S. monieri (Gouan) P.W. Ball. (=S. officinalis (L.) Trevis subsp. officinalis) | Aerial parts | p-coumaroyl-glucosides (not determined) # | [75] |
| S. officinalis (L.) Trevis (=Betonica officinalis L.) | Apigenin (1), 5, 6, 4′-trihydroxyflavone-7-O-β-D-glucoside (30) | [20] | |
| Leaves # | Apigenin 8-C-glucoside (Vitexin) (9), Luteolin 7-O-glucuronide (36), Luteolin 6-C-glucoside (isoorientin) (38), Tricin 7-O-glucuronide (60), Tricin 7-O-glucoside (61), Tricetin 3′,4′,5′-trimethyl-7-O-glucoside (62), Apigenin 7-O-p-coumaroyl glucopyranoside (not determined) | [5] | |
| Aerial parts | p-coumaroyl-glucosides (not determined) # | [75] | |
| Section Macrostachya | |||
| S. scardica Griseb. (=Betonica scardica Griseb.) | Leaves # | Apigenin 8-C-glucoside (9), Luteolin 7-O-glucoside (37), Luteolin 6-C-glucoside (38), Hypolaetin 7-O-glucoside (49), Selgin 7-O-glucoside (59), Tricin 7-O-glucuronide (60), Tricin 7-O-glucoside (61), Tricetin 3′,4′,5′-trimethyl-7-O-glucoside (isolation) (62), Apigenin 7-O-p-coumaroyl glucopyranoside (not determined) | [5] |
| Species | Plant Parts | Compound | Ref | |
|---|---|---|---|---|
| Subgenus Stachys | ||||
| Section Ambleia | ||||
| S. aegyptiaca Pers. | Aerial parts | Xanthomicrol (69), Sideritiflavone (70), 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone (75), 5,4′-Dihydroxy - 6,7,8,3′-tetramethoxyflavone (76), 5,3′,4′-Trihydroxy-3,6,7,8-tetramethoxyflavone (82), Calycopterin (83), Chrysosplenetin (84), 5-Hydroxy-3,6,7,8,4′- pentamethoxyflavone (88), 5,4′-Dihydroxy -3,6,7,8,3′- pentamethoxyflavone (89) | [68] | |
| Aerial parts | 5,7,3′-Trihydroxy-6,4′-dimethoxyflavone (67), 5,7,3′-Trihydroxy-6,8,4′-trimethoxyflavone (68) | [70] | ||
| Aerial parts | Xanthomicrol (69), Eupatilin-7-methyl ether (73), Calycopterin (83), 5-Hydroxy-3,6,7,4′-tetramethoxy flavone (85), 5,8-Dihydroxy-3,6,7,4′-tetramethoxy flavone (86), 5-Hydroxy-auranetin (88), 4′-Hydroxy-3,5,7,3′- tetramethoxy flavone (90) | [106] | ||
| S. schtschegleevii Sosn. ex Grossh. | Stems | Cirsimaritin (66), Xanthomicrol (69) | [74] | |
| Section Aucheriana | ||||
| S. glutinosa L. | Xanthomicrol (69), Sideritiflavone (70), 8-Methoxycirsilineol (71), Eupatilin (72a) | [107] | ||
| Section Candida | ||||
| S. candida Bory & Chaubard | Aerial parts | Xanthomicrol (69), Calycopterin (83) | [77,78] | |
| S. chrysantha Boiss. and Heldr. | Aerial parts | Xanthomicrol (69), Calycopterin (83) | [77] | |
| Section Swainsoniana | ||||
| S. swainsonii Benth. subsp. swainsonii | Aerial parts | Eupatorin (72), Penduletin (81), 5-Hydroxyauranetin (88) | [102] | |
| S. swainsonii subsp. argolica (Boiss.) Phitos and Damboldt | Aerial parts | Xanthomicrol (69), Eupatorin (72), Salvigenin (74) | [102] | |
| S. swainsonii subsp. melangavica D. Persson | Aerial parts | Eupatorin (72), 5-Hydroxyauranetin (88) | [102] | |
| S. swainsonii subsp. scyronica (Boiss.) Phitos and Damboldt | Aerial parts | Eupatorin (72), Penduletin (81), 5-Hydroxyauranetin (88) | [102] | |
| S. ionica Halácsy | Aerial parts | Xanthomicrol (69), Salvigenin (74), Chrysosplenetin (84), 5-Hydroxy-3,6,7,4′-tetramethoxyflavone (85), Casticin (87) | [20] | |
| S. lavandulifolia Vahl. | Aerial parts | Velutin (Luteolin 7,3′-dimethyl ether) (65), Viscosine (5,7,4′-trihydroxy-3,6-dimethoxyflavone (78), Kumatakenin (Kaempferol 3,7-dimethyl ether) (79), Pachypodol (Quercetin 3,7,3′-trimethyl ether) (80), Penduletin (81), Chrysosplenetin (84), | [13] | |
| Subgenus Betonica | ||||
| Section Betonica | ||||
| S. officinalis (L.) Trevis = (Betonica officinalis L.) | 5,4′-Dyhydroxy-7,3′,5′-trimethoxyflavone (77) | [20] | ||
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Eriostomum | |||
| S. cretica subsp. smyrnaea Rech. f. | Aerial parts # | Kaempferol (91) | [81] |
| Section Olisia | |||
| S. tetragona Boiss. & Hayek | Aerial parts | Kaempferol (91) | [100] |
| Section Swainsoniana | |||
| S. swainsonii Benth. subsp. swainsonii | Aerial parts | Isorhamnetin (92) | [99] |
| S. swainsonii subsp. argolica (Boiss.) Phitos and Damboldt | Aerial parts | Isorhamnetin (92) | [99] |
| Section Stachys | |||
| S. palustris L. | Leaves # | Quercetin-3-O-rutinoside (93), Isorhamnetin-3-O-rutinoside (94) | [5] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Ambleia | |||
| S. aegyptiaca Pers. | Aerial parts | Naringenin (96) | [69] |
| Section Eriostomum | |||
| S. cretica subsp. smyrnaea Rech. f. | Aerial parts # | Hesperidin (97) | [81] |
| Section Swainsoniana | |||
| S. swainsonii Benth. subsp. swainsonii | Aerial parts | Eriodictyol (95) | [102] |
| S. swainsonii subsp. argolica (Boiss.) Phitos and Damboldt | Aerial parts | Eriodictyol (95) | [102] |
| S. swainsonii subsp. melangavica D. Persson | Aerial parts | Eriodictyol (95) | [102] |
| S. swainsonii subsp. scyronica (Boiss.) Phitos and Damboldt | Aerial parts | Eriodictyol (95) | [102] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Ambleia | |||
| S. aegyptiaca Pers. | Aerial Parts | Diapigenin-7-O-(6″-trans,6″-cis-p, p′-dihydroxy-µ-truxinyl)glucoside (stachysetin) (98) | [69] |
| Section Eriostomum | |||
| S. lanata Crantz. (=S. germanica L. subsp. germanica) | Aerial parts | Stachysetin (98) | [82] |
| Section Candida | |||
| S. iva Griseb. | Flowering aerial parts | Stachysetin (98) | [56] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Candida | |||
| S. candida Bory & Chaubard | Aerial parts | Chlorogenic acid (103) | [78] |
| S. iva Griseb | Flowering aerial parts | Chlorogenic acid (103) | [56] |
| Section Eriostomum | |||
| S. cretica subsp. smyrnaea Rech. f. | Aerial parts # | Chlorogenic acid (103) | [81] |
| S. cretica subsp. vacillans Rech. f. | Aerial parts # | Vanillic acid (100), Syringic acid (101), Chlorogenic acid (103) | [105] |
| S. cretica subsp. mersinaea (Boiss.) Rech. f. | Aerial parts # | Chlorogenic acid (103) | [108] |
| S. lanata Crantz. (=S. germanica L. subsp. germanica) | Roots | Chlorogenic acid (103) | [82] |
| S. tmolea Boiss | Aerial parts # | 4-Hydroxybenzoic acid (99), Chlorogenic acid (103) | [85] |
| S. thirkei K. Koch | Aerial parts # | Chlorogenic acid (103) | [84] |
| S. germanica L. subsp. salviifolia (Ten.) Gams. | Aerial parts | Arbutin (107) | [109] |
| Section Olisia | |||
| S. atherocalyx C. Koch. | Νeochlorogenic acid (105), p-Coumaric acid (106), Caffeic acid (108) | [110] | |
| S. recta L. | Aerial parts # | 1-Caffeoylquinic acid (102), Chlorogenic acid (103), 4-Caffeoylquinic acid (104) | [14] |
| Section Stachys | |||
| S. palustris L. | 1-Caffeoylquinic acid (102), Chlorogenic acid (103), 4-Caffeoylquinic acid (104), Caffeic acid (108) | [104] | |
| Cryptochlorogenic acid (104), Neochlorogenic acid (105) | [23] | ||
| Subgenus Betonica | |||
| Section Betonica | |||
| S. officinalis L. (=Betonica officinalis L.) | Leaves # | Chlorogenic acid (103) | [111] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Eriostomum | |||
| S. lanata Crantz. (=S. germanica L. subsp. germanica) | Roots | Androsin (109), Neolloydosin (110), Glucoacetosyringone (111) | [82] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Stachys | |||
| S. mialhesii Noé | Aerial Parts | (+) -Sesamin (112), (+) -Paulownin (113) | [103] |
| Section Olisia | |||
| S. tetragona Boiss. & Heldr. | Aerial parts | (7S-8R)-Urolignoside (114) | [100] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Ambleia | |||
| S. schtschegleevii Sosn. ex Grossh. | Stems | Acteoside (118), Betunyoside F (128) | [74] |
| Section Candida | |||
| S. candida Bory & Chaubard | Aerial parts | Acteoside (118) | [78] |
| S. iva Griseb. | Flowering aerial parts | Acteoside (118), Leucosceptoside A (131), Lavandulifolioside (129) | [56] |
| Section Eriostomum | |||
| S. byzantina Κ. Koch | Aerial parts | Verbascoside (118), 2′-O-Arabinosyl verbascoside (122), Aeschynanthoside C (133) | [33] |
| S. cretica L. subsp. vacillans Rech. f. | Aerial parts # | Verbascoside (118) | [112] |
| S. germanica L. subsp. salviifolia (Zen.) Gams | Aerial parts | Verbascoside (118) | [109] |
| S. lanata Crantz (=S. germanica L. subsp. germanica) | Aerial parts | Leonoside B (134), Martynoside (135) | [82] |
| Roots | Rhodioloside (115), Verbasoside (116), Verbascoside (118), Isoacteoside (119), Darendoside B (120), Campneoside II (121), 2-Phenylethyl-D-xylopyranosyl-(1→6)-D-glucopyranoside (117), Campneoside I (136) | [82] | |
| S. tymphaea Hausskn. (=S. germanica subsp. tymphaea (Hausskn.) R. Bhattacharjee) | Flowering aerial parts | Verbascoside (118), Stachysoside A (129) | [86] |
| Section Olisia | |||
| S. recta L. | Aerial parts | Acteoside (118), Isoacteoside (119), β-OH-Acteoside (121), Betunyoside E (127), Campneoside I (136), Forsythoside B (137), β-OH-Forsythoside B methyl ether (138) | [14] |
| S. tetragona Boiss. & Heldr. | Aerial parts | Acteoside (118), Betonioside F (128), Leucosceptoside A (131), Stachysoside D (134), Forsythoside B (137), Lamiophloside A (141) | [100] |
| Section Stachys | |||
| S. affinis Bunge (=S. sieboldii Miq.) | Tubers | Acteoside (118), Leucosceptoside A (131), Martynoside (135) | [27] |
| Stachysosides A (129), B (139), C (140) | [113] | ||
| S. riederi Cham. | Whole plants | Acteoside (118), Campneoside II (121), Lavandulifolioside (129), Leonoside A (139) | [114] |
| Section Zietenia | |||
| S. lavandulifolia Vahl | Aerial parts | Acteoside (118), Lavandulifolioside (129) | [115] |
| Aerial parts | Verbascoside (118), Lavandulofolioside A (129), Lavandufolioside B (130), Leucosceptoside A (131) | [12] | |
| Aerial parts | Acteoside (118) | [116] | |
| Subgenus Betonica | |||
| Section Betonica | |||
| S. macrantha (C. Koch.) Stearn (=Betonica grandiflora Willd.) | Aerial parts | Verbascoside (118), Leucosceptoside A (131), Martynoside (135), Lavandulifolioside (129) | [117] |
| S. officinalis (L.) Trevis. (=Betonica officinalis L.) | Aerial parts | Acteoside (118), Acteoside isomer (isoacteoside) (119), Campneoside II (121), Betonyosides A-F (123–128), Leucosceptoside B (132), Forsythoside B (137) | [118] |
| S. alopecuros (L.) Benth subsp. divulsa (Ten.) Grande | Flowering aerial parts | Verbascoside (118) | [119] |
| Former Stachys species | |||
| S. parviflora Benth. (=Phlomidoschema parviflorum (Benth.) Vved.) | Whole plant | Parvifloroside A (142), Parvifloroside B (143) | [120] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Eriostomum | |||
| S. lanata Crantz. (=S. germanica L. subsp. germanica) | Roots | Coniferin (144), Syringin (145) | [82] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Ambleia | |||
| S. inflata Benth. | Ajugol (146), Ajugoside (147), | [121] | |
| Section Aucheriana | |||
| S. glutinosa L. | Aerial parts | Harpagide (148), Acetylharpagide (150), Monomelittoside (165), Melittoside (166), Allobetonicoside (161), 5-Allosyloxy-aucubin (167) | [122] |
| Section Campanistrum | |||
| S. ocymastrum (L.) Briq. (=S. hirta L.) | Leaves | 6β-Acetoxyipolamiide (172), 6β-Hydroxyipolamiide (173), Ipolamiide (174), Ipolamiidoside (175), Lamiide (176) | [123] |
| Section Candida | |||
| S. iva Griseb. | Flowering Aerial parts | Harpagide (148), 8-Acetylharpagide (150), 8-Epi-loganic acid (157), Gardoside (160), 8-Epi-loganin (159), Monomelittoside (165), Melittoside (166) | [56] |
| Section Corsica | |||
| S. corsica Pers. | Harpagide (148), Acetylharpagide (150) | [79] | |
| Section Eriostomum | |||
| S. alpina L. | Stems, Leaves # | Ajugoside (147), Harpagide (148), Acetylharpagide (150), Harpagoside (154), Aucubin (164), Catalpol (163) | [124] |
| S. balansae Boiss. & Kotschy | Ajugol (146), Ajugoside (147) | [125] | |
| S. germanica L. | Harpagide (148) | [125] | |
| Leaf, Inflorescence # | Ajugoside (147), Harpagide (148), Acetylharpagide (150), Harpagoside (154), Aucubin (164), Catalpol (163) | [124] | |
| S. spectabilis Choisy ex DC. | Ajugol (146), Ajugoside (147), Harpagide (148) | [125] | |
| S. byzantina Κ. Koch. | Aerial parts # | Ajugoside (147), Harpagide (148), Acetylharpagide (150), Harpagoside (154), Catalpol (163), Aucubin (164) | [124] |
| S. germanica L. subsp. salviifolia (Zen.) Gams | Flowering Aerial parts | Harpagide (148) | [86] |
| Aerial parts | Ajugol (146), Harpagide (148), 7-Hydroxyharpagide (149), 5-Allosyloxy-aucubin (167) | [109] | |
| S. lanata Crantz. (=S. germanica L. subsp. germanica) | Roots | Stachysosides E (168), G (170), H (171) | [82] |
| Aerial parts | Stachysosides E (168), F (169) | [82] | |
| S. tymphaea Hausskn. (=S. germanica subsp. tymphaea (Hausskn.) R. Bhattacharjee) | Aerial parts | Harpagide (148) | [86] |
| Section Olisia | |||
| S. angustifolia M. Bieb. | Ajugoside (147), Acetylharpagide (150), Harpagide (148), Melittoside (166) | [92] | |
| S. annua (L.) L. | Ajugoside (147), Acetylharpagide (150), Melittoside (166) | [92] | |
| S. atherocalyx C. Koch. | Ajugol (146), Harpagide (148), Acetylharpagide (150), Melittoside (166) | [92,125] | |
| S. beckeana Dörfl. & Hayek | Harpagide (148), Ajugol (146), Acetylharpagide (150), Melittoside (166) | [92] | |
| S. iberica M. Bieb. | Ajugol (146), Ajugoside (147), Harpagide (148), Acetylharpagide (150) | [121] | |
| S. recta L. | Ajugol (146), Harpagide (148), Acetylharpagide (150), Melittoside (166) | [92] | |
| Leaves | 8-Acetylharpagide (150), Melittoside# (166) | [14] | |
| Aerial parts # | Ajugoside (147), Harpagide (148), Acetylharpagide (150), Harpagoside (154), Catalpol (163), Aucubin (164) | [124] | |
| S. baldaccii (Maly) Hand-Mazz (=S. recta L. subsp. baldaccii (K. Maly) Hayek) | Ajugol (146), Ajugoside (147), Harpagide (148), Acetylharpagide (150), Melittoside (166) | [92] | |
| S. subcrenata Vis. (=S. recta subsp. subcrenata) | Ajugol (146), Harpagide (148), Acetylharpagide (150), Melittoside (166) | [92] | |
| S. labiosa Bertol. | Ajugol (146), Harpagide (148), Acetylharpagide (150), Melittoside (166) | [92] | |
| S. leucoglossa Griseb. | Ajugol (146), Harpagide (148), Acetylharpagide (150), Melittoside (166) | [92] | |
| S. spinosa L. | Aerial parts | Ajugol (146), Harpagide (148), 7-O-Acetyl-8-epi-loganic acid (158) | [98] |
| S. tetragona Boiss. & Heldr. | Ajugol (146), Ajugoside (147), Harpagide (148), Acetylharpagide (150), Melittoside (166) | [92] | |
| Aerial parts | 8-Acetyl-harpagide (150), 5-O-Allopyranosyl-monomelittoside (167) | [100] | |
| Section Stachys | |||
| S. affinis Bunge (= S. sieboldii Miq.) | Tubers | Harpagide (148), Acetylharpagide (150), Melittoside (166), 5-Allosyloxy-aucubin (167) | [27] |
| S. palustris L. | Aerial parts # | Ajugoside (147), Harpagide (148), Acetylharpagide (150), Harpagoside (154), Catalpol (163), Aucubin (164) | [124] |
| S. sylvatica L. | Aerial parts # | Ajugoside (147), Harpagide (148), Acetylharpagide (150), Harpagoside (154), Catalpol (163), Aucubin (164) | [124] |
| Section Swainsoniana | |||
| S. anisochila Vis. & Pancic | Acetylharpagide (150), Melittoside (166) | [92] | |
| S. ionica Halácsy | 8-epi-loganic acid (157), Gardoside (160) | [20] | |
| S. menthifolia Vis. (= S. grandiflora Host.) | Ajugol (146), Harpagide (148), Acetylharpagide (150), Melittoside (166) | [92] | |
| Aerial parts # | Ajugoside (147) Harpagide (148), Acetylharpagide (150), Harpagoside (154), Catalpol (163), Aucubin (164) | [124] | |
| Section Zietenia | |||
| S. lavandulifolia Vahl. | Ajugol (146), Ajugoside (147) | [125] | |
| Aerial parts | Melittoside (166), Monomelittoside (165), 5-O-Allopyranosyl-monomelittoside (167) | [12] | |
| SubgenusBetonica | |||
| Section Betonica | |||
| S. alopecuros (L.) Benth subsp. divulsa (Ten.) Grande | Flowering aerial parts | Harpagide (148), Acetylharpagide (150), 4′-O-β-D-galactopyranosyl-teuhircoside (162) | [119] |
| S. foliosa Rupr. (=S. betoniciflora Rupr.; Betonica foliosa Rupr.) | Harpagide (148), Acetylharpagide (150) | [126] | |
| S. betonicaeflora Rupr. | Harpagide (148), Acetylharpagide (150) | [126] | |
| S. macrantha (C. Koch.) Stearn (=Betonica grandiflora Steph. ex Willd.) | Aerial parts | Ajugol (146), Ajugoside (147), Harpagide (148), 8-O-Acetyl-harpagide (150), Reptoside (153), Macranthoside [=8-O- (3, 4-dimethoxy-cinnamoyl-harpagide)] (156), Allobetonicoside (161) | [117] |
| S. officinalis (L.) Trevis. (=Betonica officinalis L.) | Aerial parts | Acetylharpagide (150), Reptoside (153), 6-O-Acetylmioporoside (155), Allobetonicoside (161) | [127] |
| Harpagide (148), Acetylharpagide (150) | [128] | ||
| Aerial parts # | Ajugoside (147), Harpagide (148), Acetylharpagide (150), Harpagoside (154), Catalpol (163), Aucubin (164) | [124] | |
| Unknown Section | |||
| S. grandidentata Lindl. ** | Aerial parts | Ajugol (146), Harpagide (148), Acetylharpagide (150), 5-Desoxy-harpagide (151), 5-Desoxy-8-acetyl-harpagide (152), Monomelittoside (165), Melittoside (166) | [129] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| section Ambleia | |||
| S. aegyptiaca Pers. | Stachysolone (177), 11a,18-Dihydroxy-ent-kaur-16-ene (210) | [130] | |
| Aerial parts | Stachysperoxide (189), Stachysolone (177), 7,13-Diacetyl-stachysolone (180) | [131] | |
| Aerial parts | Stachaegyptin A-C (190–192), Roseostachenone (184), Stachysolone (177), 7,13-Diacetyl-stachysolone (180) | [106] | |
| Aerial parts | Stachaegyptins D, E (193, 194) | [132] | |
| Aerial parts | Stachaegyptins A (190), F-H (195–197), Stachysperoxide (189) | [133] | |
| S. inflata Benth. | Annuanone (181), Stachylone (182), Stachone (183) | [134] | |
| Section Aucheriana | |||
| S. glutinosa L. | Aerial parts | Roseostachenone (184), 3α,4α-Epoxyroseostachenol (188) | [107] |
| Section Eriostomum | |||
| S. balansae Boiss. & Kotschy | Annuanone (181), Stachylone (182) | [134] | |
| S. lanata Crantz. (=S. germanica L. subsp. germanica) | Ent-3α-acetoxy-kaur-16-en-19-oic acid (207), Ent-3α,19-dihydroxy-kaur-16-ene (208), Ent-3α-hydroxy-kaur-16-en-19-oic acid (209) | [135] | |
| Section Mucronata | |||
| S. mucronata Sieb. | Aerial parts | Ribenone [=3β-hydroxy-13-epi-ent-manoyl oxide] (198), Ribenol [=3-keto-13-epi-ent-manoyl oxide] (199) | [57] |
| Section Olisia | |||
| S. annua (L.) L. | Stachysolone (177) | [136,137] | |
| Annuanone (181), Stachylone (182), Stachone (183) | [138] | ||
| S. atherocalyx C. Koch. | Annuanone (181), Stachylone (182), Stachone (183) | [134] | |
| S. distans Benth | Aerial parts | (+)-6-Deoxyandalusol (201) | [139] |
| S. iberica M. Bieb. | Annuanone (181), Stachylone (182), Stachone (183) | [134] | |
| S. recta L. | Aerial parts | 7,13-Diacetate stachysolone (180), 7-Acetate stachysolone (178), 13-Acetate stachysolone (179) | [140] |
| Section Roseostachys | |||
| S. rosea Boiss. | Aerial parts | Roseostachenone (184), Roseostachone (185), 13-epi-sclareol (200), Roseostachenol (186), Roseotetrol (187) | [141] |
| Section Stachys | |||
| S. mialhesii Noé | Aerial parts | Horminone (211) | [103] |
| S. palustris L. | Annuanone (181) | [134] | |
| S. sylvatica L. | Stachysic acid (204) | [142] | |
| Annuanone (181), Stachylone (182), Stachone (183) | [134] | ||
| Stachysic acid (204), 6β-Hydroxy-ent-kaur-16-ene (205), 6β,18-Dihydroxy-ent-kaur-16-ene (206) | [142] | ||
| Betolide (214) | [143] | ||
| Section Swainsoniana | |||
| S. ionica Halácsy | Aerial parts | (+)-6-Deoxyandalusol (201) | [139] |
| S. plumosa Griseb. | Aerial parts | (+)-6-Deoxyandalusol (201), 13-Epi-jabugodiol (202), (+)-Plumosol (203) | [144] |
| Section Zietenia | |||
| S. lavandulifolia Vahl. | Aerial parts | Stachysolone (177) | [116] |
| Subgenus Betonica | |||
| Section Betonica | |||
| S. officinalis (L.) Trevis. (=Betonica officinalis L.) | Betolide (214) | [145] | |
| Betonicolide (215), Betonicosides A-D (216–219) | [145] | ||
| Roots | Betolide (214) | [143] | |
| S. scardica (Griseb.) Hayek (=Betonica scardica Griseb.) | Roots | Betolide (214) | [143] |
| Former Stachys species | |||
| S. parviflora Benth. (=Phlomidoschema parviflorum (Benth.) Vved.) | Whole plant | Stachyrosane 1 (212) Stachyrosane 2 (213) | [133] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Eriostomum | |||
| S. byzantina K. Koch | Aerial parts | Stigmasterol (220), | [17] |
| β-Sitosterol (221), Lawsaritol (223), Stigmastan-3,5-dien-7-one (224) | [35] | ||
| S. hissarica Regel | - | 20-Hydroxyecdysone (239), Polipodin B (240), Integristeron A (241), 2-Desoxy-20-hydroxyecdysone (242), 2-Desoxyecdyson (243) | [67] |
| Section Olisia | |||
| S. annua (L.) L. | Aerial parts | β-Sitosterol (221), Ursolic acid (226) | [95] |
| S. spinosa L. | Aerial parts | Stigmasterol (220), β-Sitosterol (221), Oleanolic acid (227), 12α-Hydroxy-oleanolic lactone (228) | [99] |
| S. tetragona Boiss. & Heldr. | Aerial parts | Stigmasterol (220), β-Sitosterol (221), Oleanolic acid (227), | [100] |
| Section Stachys | |||
| S. palustris L. | β-Sitosterol (221), α-amyrin (225) | [146] | |
| S. riederi Cham. | Whole plant | Stachyssaponins I-VIII (231–238) | [147] |
| Subgenus Betonica | |||
| Section Betonica | |||
| S. alopecuros (L.) Benth subsp. divulsa (Ten.) Grande | Flowering aerial parts | 3-O-β-Sitosterol-glucoside (222) | [119] |
| Former Stachys species | |||
| S. parviflora Benth. (=Phlomidoschema parviflorum (Benth.) Vved.) | Aerial parts | Stachyssaponin A (229), Stachyssaponin B (230) | [63] |
| Species | Plant Parts | Compound | Ref |
|---|---|---|---|
| Subgenus Stachys | |||
| Section Eriostomum | |||
| S. byzantina K. Koch. | Aerial parts | Byzantionoside A (244), Byzantionoside B (245), Icariside B2 (246), (6R, 9R)- and (6R, 9S)-3-oxo-α-ionol glucosides (247), Blumeol C glucoside (248) | [148] |
| S. lanata Crantz (=S. germanica L. subsp. germanica) | Aerial parts | Vomifoliol (249), Dehydrovomifoliol (250) | [82] |
| Roots | Citroside A (251) | [82] | |

| Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| R=OH | |||||||
| Apigenin (1) | H | H | OH | H | H | OH | H |
| Apigenin 7-O-β-D-glucoside (cosmoside) (2) | H | H | O-glc | H | H | OH | H |
| Apigenin 7-O-[6′″-O-acetyl]-β-D-allosyl-(1→2)-β-D-glucoside (3) | H | H | O-[6′″-acetyl-allosyl]-(1→2)-glc | H | H | OH | H |
| Apigenin 7-(3″-E-p-coumaroyl)-β-D-glucoside (4) | H | H | O-(3″-E-p-coumaroyl)-glc | H | H | OH | H |
| Apigenin 7-(3″-Z-p-coumaroyl)-β-D-glucoside (5) | H | H | O-(3″-Z-p-coumaroyl)-glc | H | H | OH | H |
| Apigenin 7-(6″-E-p-coumaroyl)-β-D-glucoside (6) | H | H | O-(6″-E-p-coumaroyl)-glc | H | H | OH | H |
| Apigenin 7-(6″-Z-p-coumaroyl)-β-D-glucoside (7) | H | H | O-(6″-Z-p-coumaroyl)-glc | H | H | OH | H |
| Apigenin 7-(3″,6″-p-dicoumaroyl)- β-D-glucoside (Anisofolin A) (8) | H | H | O-(3″,6″-p-dicoumaroyl)-glc | H | H | OH | H |
| Apigenin 8-C-glucoside (9) | H | H | OH | C-glc | H | OH | H |
| Apigenin 6,8-di-C-glucoside (Vicenin-2) (10) | H | C-glc | OH | C-glc | H | OH | H |
| Isoscutellarein (11) | H | H | OH | OH | H | OH | H |
| Isoscutellarein 7-O-glucoside (11a) | H | H | O-glc | OH | H | OH | H |
| 4′-Methyl-isoscutellarein (12) | H | H | OH | OH | H | OCH3 | H |
| Isoscutellarein 7-O-allosyl-(1→2)-glucoside (13) | H | H | O-allosyl-(1→2)- glc | OH | H | OH | H |
| 3′-Hydroxy-isoscutellarein-7-O-[6′″-O-acetyl]-β-D-glucoside (14) | H | H | O-[6′″-O-acetyl]- glc | OH | OH | OH | H |
| Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allosyl-(1→2)-β-D-glucoside (15) | H | H | O-[6′″-O-acetyl]-allosyl-(1→2)-glc | OH | H | OH | H |
| Isoscutellarein 7-O-β-D-allosyl-(1→2)-[6″-O-acetyl]-β-D-glucoside (16) | H | H | O-[6″-O-acetyl]-allosyl-(1→2)-glc | OH | H | OH | H |
| Isoscutellarein 7-O-[6′″-O-acetyl]-β-D-allosyl-(1→2)-[6″-O-acetyl]- β-D-glucoside (17) | H | H | O-[6′″-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]-glc | OH | H | OH | H |
| Isoscutellarein 8-O-(6″-trans-p-coumaroyl)-β-D-glucoside (18) | H | H | OH | O-(6”-trans-p-coumaroyl)-glc | H | OH | H |
| 4′-Methyl-isoscutellarein 7-O-β-D-allosyl-(1→2)-β-D-glucoside (19) | H | H | O-allosyl-(1→2)-glc | OH | H | OCH3 | H |
| 4′-Methyl- isoscutellarein 7-O-β-D-allosyl-(1→2)-[6″-O-acetyl]-β-D-glucoside (20) | H | H | O-allosyl-(1→2)-[6″-O-acetyl]-glc | OH | H | OCH3 | H |
| 4′-Methyl-isoscutellarein 7-O-β-D-[6′″-O-acetyl]-allosyl-(1→2)-β-D-glucoside (21) | H | H | O-[6′″-O-acetyl]-allosyl-(1→2)-glc | OH | H | OCH3 | H |
| 4′-Methyl-isoscutellarein 7-O- [2″-O-acetyl]-β-D-allosyl-(1→2)-β-D-glucoside (22) | H | H | O-[2″-O-acetyl]-allosyl-(1→2)-glc | OH | H | OCH3 | H |
| 4′-Methyl-isoscutellarein 7-O-β-D-[4′′′-O-acetyl]-allosyl]-(1→2)-β-D-glucoside (annuoside) (23) | H | H | O-[4′′′-O-acetyl]-allosyl-(1→2)-glc | OH | H | OCH3 | H |
| 4′-Methyl-isoscutellarein 7-O-[6′′′-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]-glucoside (24) | H | H | O-[6′′′-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]-glc | OH | H | OCH3 | H |
| Isostachyflaside (25) | H | H | OH | OH | H | O-mannosyl- (1→2)-glc | H |
| Acetyl-isostachyflaside (26) | H | H | OH | OH | H | O-[acetyl]-mannosyl- (1→2)-glc | H |
| Di-acetyl- isostachyflaside (27) | H | H | OH | OH | H | O-[diacetyl-mannosyl]- (1→2)-glc | H |
| Spectabiflaside (28) | H | H | O-mannosyl- (1→2)-glc | OH | OCH3 | OH | H |
| Scutellarein (29) | H | OH | OH | H | H | OH | H |
| Scutellarein 7-O-β-D-glucoside[5,6, 4′-trihydroxyflavone-7-O-β-D-glucoside] (30) | H | OH | O-glc | H | H | OH | H |
| Scutellarein 7-O-β-D-mannnosyl- (1→2)-β-D-glucoside (stachyflaside) (31) | H | OH | O-mannosyl- (1→2)-glc | H | H | OH | H |
| 7-O-β-D-glucopyranosyl-5,6-dihydroxy-4′-methoxyflavone (Stachannin A) (32) | H | OH | O-glc | H | H | OCH3 | H |
| 4′-Methoxy-scutellarein 7-[O-β-D-mannosyl-(1→2)-β-D-glucoside (Stachannoside B) (33) | H | OH | O-mannosyl- (1→2)-glc | H | H | OCH3 | H |
| Luteolin (34) | H | H | OH | H | OH | OH | H |
| Luteolin 7-methyl ether (35) | H | H | OCH3 | H | OH | OH | H |
| Luteolin 7-O-β-D-glucuronide (36) | H | H | O-glcA | H | OH | OH | H |
| Luteolin 7-O-β-D-glucoside (37) | H | H | O-glc | H | OH | OH | H |
| Luteolin 6-C-glucoside (isoorientin) (38) | H | -C-glc | OH | H | OH | OH | H |
| Luteolin 7-O-[6′′′-O-acetyl]-allosyl-(1→2)-glucoside (39) | H | H | O-[6′′′-O-acetyl]-allosyl-(1→2)-glc | H | OH | OH | H |
| 6,8 Di-C-β-D-glucopyranosyl luteolin (Lucenin-2) (40) | H | C-glc | OH | C-glc | OH | OH | H |
| 3′,4′-Dimethyl-luteolin-7-O-β-D-glucoside (41) | H | H | O-glc | H | OCH3 | OCH3 | H |
| Chrysoeriol (42) | H | H | OH | H | OCH3 | OH | H |
| Chrysoeriol 7-O-β-D-glucoside (43) | H | H | O-glc | H | OCH3 | OH | H |
| Chrysoeriol 7-O-[6′′′-O-acetyl]-β-D-allosyl-(1→2)-glucoside (Stachyspinoside) (44) | H | H | O-[6′′′-O-acetyl]- allosyl-(1→2)-glc | H | OCH3 | OH | H |
| Chrysoeriol 7-O-[6″-O-acetyl]-β-D-allosyl-(1→2)-glucoside (Isostachyspinoside) (45) | H | H | O-[6″-O-acetyl]- allosyl-(1→2)-glc | H | OCH3 | OH | H |
| Chrysoeriol 7-(3″-E-p-coumaroyl)-β-D-glucoside (46) | H | H | O-(3″-E-p-coumaroyl)-glc | H | OCH3 | OH | H |
| Chrysoeriol 7-(6″-E-p-coumaroyl)-β-D-glucoside (47) | H | H | O-(6″-E-p-coumaroyl)-glc | H | OCH3 | OH | H |
| Hypolaetin (48) | H | H | OH | OH | OH | OH | H |
| Hypolaetin-7-O-glucoside (49) | H | H | O-glc | OH | OH | OH | H |
| Hypolaetin-7-O-glucuronide (49a) | H | H | O-glcA | OH | OH | OH | H |
| Hypolaetin 7-O-allosyl-(1→2)-glucoside (50) | H | H | O-allosyl-(1→2)-glc | OH | OH | OH | H |
| Hypolaetin 7-O-[6′′′-O-acetyl]-β-D-allosyl-(1→2)-β-D-glucoside (51) | H | H | O-[6′′′-O-acetyl]- allosyl-(1→2)- glc | OH | OH | OH | H |
| Hypolaetin 7-O-[6″-O-acetyl]-allosyl-(1→2)glucoside (52) | H | H | O-[6″-O-acetyl]- allossyl-(1→2)- glc | OH | OH | OH | H |
| Hypolaetin 7-O-[6′′′-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]-glucoside (53) | H | H | O-[6′′′-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]- glc | OH | OH | OH | H |
| Hypolaetin 7-O-[6′′′-O-acetyl]-allosyl-(1→2)-[3″-O-acetyl]-glucoside (54) | H | H | O-[6′′′-O-acetyl]-allosyl-(1→2)-[3″-O-acetyl]- glc | OH | OH | OH | H |
| 4′-Methyl-hypolaetin-7-O-allosyl-(1→2)-glucoside (55) | H | H | O-allosyl-(1→2)-glc | OH | OH | OCH3 | H |
| 4′-Methyl-hypolaetin-7-O-[6′′′-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (56) | H | H | O-[6′′′-O-acetyl]-allosyl-(1→2)- glc | OH | OH | OCH3 | H |
| 4′-Methyl-hypolaetin-7-O-[6″-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (57) | H | H | O-[6″-O-acetyl]-allosyl-(1→2)- glc | OH | OH | OCH3 | H |
| 4′-Methyl-hypolaetin-7-O-[6′′′-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]-glucoside (58) | H | H | O-[6′′′-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]- glc | OH | OH | OCH3 | H |
| Selgin 7-O-glucoside (59) | H | H | O-glc | H | OCH3 | OH | OH |
| Tricin 7-O-glucuronide (60) | H | H | O-glcA | H | OCH3 | OH | OCH3 |
| Tricin 7-O-glucoside (61) | H | H | O-glc | H | OCH3 | OH | OCH3 |
| Tricetin 3′,4′,5′-trimethyl-7-O-glucoside (62) | H | H | O-glc | H | OCH3 | OCH3 | OCH3 |
| R=O-glcA-glc (2→1) | |||||||
| Palustrin (63) | H | OH | OCH3 | H | H | H | H |
| R=O-glcA | |||||||
| Palustrinoside (64) | H | OH | OCH3 | H | H | H | H |

| Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| R=OH | |||||||
| Velutin (luteolin 7,3′-dimethyl ether) (65) | H | H | OCH3 | H | OCH3 | OH | H |
| Cirsimaritin (66) | H | OCH3 | OCH3 | H | H | OH | H |
| 5,7,3′-Trihydroxy-6,4′-dimethoxyflavone (67) | H | OCH3 | OH | H | OH | OCH3 | H |
| 5,7,3′-Trihydroxy-6,8,4′-trimethoxyflavone (68) | H | OCH3 | OH | OCH3 | OH | OCH3 | H |
| Xanthomicrol (69) | H | OCH3 | OCH3 | OCH3 | H | OH | H |
| Sideritiflavone (70) | H | OCH3 | OCH3 | OCH3 | OH | OH | H |
| 8-Methoxycirsilineol (71) | H | OCH3 | OCH3 | OCH3 | OCH3 | OH | H |
| Eupatorin (72) | H | OCH3 | OCH3 | H | OH | OCH3 | H |
| Eupatilin (72a) | H | OCH3 | OH | H | OCH3 | OCH3 | H |
| Eupatilin-7-methyl ether (73) | H | OCH3 | OCH3 | H | OCH3 | OCH3 | H |
| Salvigenin (74) | H | OCH3 | OCH3 | H | H | OCH3 | H |
| 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone (75) | H | OCH3 | OCH3 | OCH3 | OCH3 | OCH3 | H |
| 5, 4′-Dihydroxy - 6,7,8,3′-tetramethoxyflavone (76) | H | OCH3 | OCH3 | OCH3 | OCH3 | OH | H |
| 5, 4′-Dihydroxy-7,3′,5′-trimethoxyflavone (77) | H | H | OCH3 | H | OCH3 | OH | OCH3 |
| Viscosine (5,7,4′-trihydroxy-3,6-dimethoxyflavone) (78) | OCH3 | OCH3 | OH | H | H | OH | H |
| Kumatakenin (kaempferol 3,7-dimethyl ether) (79) | OCH3 | H | OCH3 | H | H | OH | H |
| Pachypodol (quercetin 3,7,3′-trimethyl ether) (80) | OCH3 | H | OCH3 | H | OCH3 | OH | H |
| Penduletin (81) | OCH3 | OCH3 | OCH3 | H | H | OH | H |
| 5,3′,4′-Trihydroxy-3,6,7,8-tetramethoxyflavone (82) | OCH3 | OCH3 | OCH3 | OCH3 | OH | OH | H |
| Calycopterin (83) | OCH3 | OCH3 | OCH3 | OCH3 | H | OH | H |
| Chrysosplenetin (84) | OCH3 | OCH3 | OCH3 | H | OCH3 | OH | H |
| 5-Hydroxy-3,6,7,4′-tetramethoxyflavone (85) | OCH3 | OCH3 | OCH3 | H | H | OCH3 | H |
| 5,8-Dihydroxy-3,6,7,4′-tetramethoxyflavone (86) | OCH3 | OCH3 | OCH3 | OH | H | OCH3 | H |
| Casticin (87) | OCH3 | OCH3 | OCH3 | H | OH | OCH3 | H |
| 5-Hydroxy-3,6,7,8,4′- pentamethoxyflavone (5-hydroxyauranetin) (88) | OCH3 | OCH3 | OCH3 | OCH3 | H | OCH3 | H |
| 5,4′-Dihydroxy -3,6,7,8,3′- pentamethoxyflavone (89) | OCH3 | OCH3 | OCH3 | OCH3 | OCH3 | OH | H |
| R=OCH3 | |||||||
| 4′-Hydroxy- 3,5,7,3′-tetramethoxyflavone (90) | OCH3 | H | OCH3 | H | OCH3 | OH | H |

| Name | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| Kaempferol (91) | OH | OH | H | OH |
| Isorhamnetin (92) | OH | OH | OCH3 | OH |
| Quercetin 3-O-rutinoside (93) | O-rut | OH | OH | OH |
| Isorhamnetin 3-O-rutinoside (94) | O-rut | OH | OCH3 | OH |

| Name | R | R1 | R2 |
|---|---|---|---|
| Eriodictyol (95) | OH | OH | OH |
| Naringenin (96) | H | OH | OH |
| Hesperidin (97) | OH | OCH3 | O-rut |
| Stachysetin (98) |
|---|
 |
 | 4-Hydroxybenzoic acid R=H, R1=H, R2=H (99) | |
| Vanillic acid R=H, R1=H, R2=OCH3 (100) | ||
| Syringic acid R=H, R1= OCH3, R2=OCH3 (101) | ||
 | 1-Caffeoylquinic acid R1=caffeoyl-, R2=R3=R4=H (102) | |
| 3-Caffeoylquinic acid (Chlorogenic acid) R1=H, R2=caffeoyl-, R3=R4=H (103) | ||
| 4-Caffeoylquinic acid (cryptochlorogenic acid) R1=R2=H, R3=caffeoyl-, R4=H (104) | ||
| 5-Caffeoylquinic acid (neohlorogenic acid) R1=R2=R3=H, R4=caffeoyl- (105) | ||
 p-Coumaric acid (106) |  Arbutin (107) |  Caffeic acid (108) |
 |
|---|
| Androsin R=R1=H (109) |
| Neolloydosin R=H, R1=Xyl (110) |
| Glucoacetosyringone R=OCH3, R1=H (111) |
 |  |
|---|---|
| Sesamin R=H (112) Paulownin R=OH (113) | (7S-8R)-Urolignoside (114) |

 Caffeic acid Caffeic acid |  Ferulic acid Ferulic acid | ||||||
| Name | R1 | R2 | R3 | R4 | R5 | R6 | R |
| Rhodioloside (Salidroside) (115) | H | H | H | H | H | OH | H |
| Verbasoside (decaffeoyl-acteoside) (116) | H | H | Rha | H | OH | OH | H |
| 2-Phenylethyl-D-xylopyranosyl-(1→6)-D-glucopyranoside (117) | Xyl | H | H | H | H | H | H |
| Acteoside (Verbascoside) (118) | H | Caf | Rha | H | OH | OH | H |
| Isoacteoside (119) | Caf | H | Rha | H | OH | OH | H |
| Darendoside B (deacyl-martynoside) (120) | H | H | Rha | H | OH | OCH3 | H |
| β-OH-Acteoside (Campneoside II) (121) | H | Caf | Rha | OH | OH | OH | H |
| 2′-O-Arabinosyl verbascoside (122) | H | Caf | Rha | H | OH | OH | Ara |
| Betonyoside A (123) | H | Fer | Rha | OH | OH | OH | H |
| Betonyoside B/C (isomers) (124/125) | Fer | H | Rha | OH | OH | OH | H |
| Betonyoside D (126) | Api | Cis-fer | Rha | H | OH | OCH3 | H |
| Betonyoside E (127) | Api | Fer | Rha | OH | OH | OH | H |
| Betonyoside F (128) | H | Caf | Rha-Api | H | OH | OH | H |
| Lavandulifolioside A (Stachysoside A) (129) | H | Caf | Rha-Ara | H | OH | OH | H |
| Lavandulifolioside B (130) | H | 4′-methyl-Fer | Rha-Ara | H | OCH3 | OH | H |
| Leucosceptoside A (131) | H | Fer | Rha | H | OH | OH | H |
| Leucosceptoside B (132) | Api | Fer | Rha | H | OH | OCH3 | H |
| Aeschynanthoside C (133) | H | Fer | Xyl | H | OH | OCH3 | H |
| Leonoside B (Stachysoside D) (134) | H | Fer | Rha-Ara | H | OH | OCH3 | H |
| Martynoside (135) | H | Fer | Rha | H | OH | OCH3 | H |
| Campneoside I (136) | H | Caf | Rha | OCH3 | OH | OH | H |
| Forsythoside B (137) | Api | Caf | Rha | H | OH | OH | H |
| β-OH-Forsythoside B methyl ether (138) | Api | Caf | Rha | OCH3 | OH | OH | H |
| Leonoside A (Stachysoside B) (139) | H | Fer | Rha-Ara | H | OH | OH | H |
| * Stachysoside C (140) | H | Fer | Rha-Ara | H | OH | OH | H |
| Lamiophloside A (141) | Api | Fer | Rha | H | OCH3 | OH | H |
| Parvifloroside A (142) | H | Caf | H | H | OH | OH | Rha |
| Parvifloroside B (143) | Caf | H | H | H | OH | OH | Rha |
 | |
|---|---|
| Coniferin R=H (144) | Syringin R=OCH3 (145) |

| Name | R | R1 | R2 | R3 | R4 | |||
|---|---|---|---|---|---|---|---|---|
| Ajugol (146) | H | OH | H | H | H | |||
| Ajugoside (147) | H | OH | Ac | H | H | |||
| Harpagide (148) | H | OH | H | OH | H | |||
| 7-Hydroxyharpagide (149) | H | OH | H | OH | OH | |||
| 8-Acetylharpagide (Acetylharpagide) (150) | H | OH | Ac | OH | H | |||
| 5-Desoxyharpagide (151) | OH | OH | H | H | H | |||
| 5-Desoxy-8-acetylharpagide (152) | OH | OH | Ac | H | H | |||
| Reptoside (153) | H | H | Ac | OH | H | |||
| Harpagoside (154) | H | OH | Cinnamoyl- | OH | H | |||
| 6-O-Acetylmioporoside (155) | AcO | H | H | H | H | |||
| Macranthoside (156) | H | OH | 3,4-dimethoxy cinnamoyl- | OH | H | |||
 | 8-Epi-loganic acid R=R′=H (157) | |||||||
| 7-O-Acetyl-8-epi-loganic acid R=Ac, R′=H (158) | ||||||||
| 8-Epi-loganin R=H, R′=CH3 (159) | ||||||||
 | Gardoside (160) | |||||||
 | Allobetonicoside R=Allose, R1=Glc (161) | |||||||
| 4′-O-β-D-galactopyranosyl-teuhircoside R=H, R1=Glc-Gal (162) | ||||||||
 | Catalpol (163) | |||||||
 | Aucubin R=H (164) | |||||||
| Monomelittoside R=OH (165) | ||||||||
| Melittoside R=O-Glc (166) | ||||||||
| 5-O-Allopyranosyl-monomelittoside; 5-Allosyloxy-aucubin R=O-Alo (167) | ||||||||
 | Name | R1 | R2 | R3 | ||||
| Stachysoside E (168) | H | p-(E)-coumaroyl- | H | |||||
| Stachysoside F (169) | H | p-(Z)-coumaroyl- | H | |||||
| Stachysoside G (170) | H | H | p-(E)-coumaroyl- | |||||
| Stachysoside H (171) | p-(E)-coumaroyl- | H | H | |||||
 | Name | R1 | R2 | R3 | ||||
| 6β-Acetoxyipolamiide (172) | OAc | H | OH | |||||
| 6β-Hydroxyipolamiide (173) | OH | H | OH | |||||
| Ipolamiide (174) | H | H | OH | |||||
| Ipolamiidoside (175) | H | H | OAc | |||||
 | |||||
|---|---|---|---|---|---|
| Name | R1 | R2 | |||
| Stachysolone (177) | H | H | |||
| 7-Monoacetyl-stachysolone (178) | Ac | H | |||
| 13-Monoacetyl-stachysolone (179) | H | Ac | |||
| 7,13-Diacetyl-stachysolone (180) | Ac | Ac | |||
 |  |  | |||
| Annuanone (181) | Stachylone (182) | Stachone (183) | |||
 |  |  | |||
| Roseostachenone (184) | Roseostachone (185) | Roseostachenol (186) | |||
 |  | ||||
| Roseotetrol (187) | 3α,4α-Epoxyroseostachenol (188) | ||||
 | |||||
| Stachysperoxide (189) | |||||
 |  | ||||
| Stachaegyptin A (190) | Stachaegyptin B (191) | ||||
 |  | ||||
| Stachaegyptin C (192) | Stachaegyptin D (193) | ||||
 |  | ||||
| Stachaegyptin E (194) | Stachaegyptin F (195) | ||||
 |  | ||||
| Stachaegyptin G (196) | Stachaegyptin H (197) | ||||
 |  | ||||
| Ribenone R=O (198) Ribenol R=αOH,βH(199) | 13-Epi-sclareol (200) | ||||
 |  | ||||
| (+)-6-Deoxyandalusol (201) | 13-Epi-jabugodiol (202) | ||||
 | |||||
| (+)-Plumosol (203) | |||||
 | |||||
| Name | R | R′ | R1 | R2 | R3 |
| Stachysic acid (204) | COOH | CH3 | H | OAc | H |
| 6β-hydroxy-ent-kaur-16-ene (205) | CH3 | CH3 | H | OH | H |
| 6β,18-dihydroxy-ent-kaur-16-ene (206) | CH2OH | CH3 | H | OH | H |
| Ent-3α-acetoxy-kaur-16-en-19-oic acid (207) | CH3 | COOH | OAc | H | H |
| 3α,19-Dihydroxy-ent-kaur-16-ene (208) | CH3 | CH2OH | OH | H | H |
| Ent-3α-hydroxy-kaur-16-en-19-oic acid (209) | CH3 | COOH | OH | H | H |
| 11a,18-Dihydroxy-ent-kaur-16-ene (210) | CH2OH | CH3 | H | H | OH |
 Horminone (211) | |||||
 Stachyrosane 1 (212) |  Stachyrosane 2 (213) | ||||
 Betolide (214) |  Betonicolide (215) | ||||
 | Name | R1 | R2 | R3 | |
| Betonicoside A (216) | O-Glc | CH2OH | O-Glc | ||
| Betonicoside B (217) | O-Glc | CH2OH | OH | ||
| Betonicoside C (218) | OH | CH2OH | O-Glc | ||
| Betonicoside D (219) | OH | CH2O-Glc | OH | ||
 | Stigmasterol (220) | R= | |
| R1=H | |||
| β-Sitosterol (221) | R=  | ||
| R1= H | |||
| 3-O-β-Sitosterol-glucoside (222) | R=  | ||
| R1=Glc | |||
 Lawsaritol (223) |  Stigmastan-3,5-dien-7-one (224) | ||
 α-Amyrin R=CH3 (225) Ursolic acid R=COOH (226) |  Oleanolic acid (227) | ||
 12α-hydroxy-oleanolic lactone (228) | |||
 | |||
| Stachyssaponin A (229) | R=Glc-Rha, R1=H, R2=Glc-Ara, R3=H, R4=OH | ||
| Stachyssaponin B (230) | R=Glc, R1=Ara, R2=H, R3=Glc, R4=H | ||
 | Stachyssaponin I R=OGlc-Ara, R1=Ara (231) Stachyssaponin II R=OGlc-Ara, R1=Ara-Rha (232) Stachyssaponin III R=OGlc-Xyl, R1=Ara-Rha (233) Stachyssaponin IV R=OGlc-Ara, R1=Ara-Rha-Xyl (234) Stachyssaponin V R=OGlc-Ara, R1=Ara-Rha-Xyl-3Ac (235) Stachyssaponin VI R=OGlc-Ara, R1= Ara-Rha-Xyl-4Ac (236) Stachyssaponin VII R=OGlc-Ara, R1=Ara-Rha-(3Glc)-Xyl (237) Stachyssaponin VIII R=OGlc-Xyl, R1=Ara-Rha-Xyl (238) | ||
 | 20-Hydroxyecdysone (239) R1=R2=R3=R5=H, R4=OH, R6=CH3 | ||
| Polipodin B (240) R1=R2=R5=H, R3=R4=OH, R6=CH3 | |||
| Integristeron A (241) R2=R3=R5=H, R1=R4=OH, R6=CH3 | |||
 | 2-Desoxy-20-hydroxyecdysone (242) R1=OH, R2=H | ||
| 2-Desoxyecdyson (243) R1=R2=H | |||
 |  |
| Byzantionoside A (244) | Byzantionoside B (245) |
 |  |
| Icariside B2 (246) | (6R, 9R)- and (6R, 9S)-3-oxo-α-ionol glucosides (247) |
 |  |
| Blumeol C glucoside (248) | Vomifoliol (249) |
 |  |
| Dehydrovomifoliol (250) | Citroside A (251) |
| Species | Extract or Compound | Activity a | Ref |
|---|---|---|---|
| S. aegyptiaca Pers. | Stachysolon diacetate (180) | Cytotoxicity HepG2 cell line IC50: 59.5 μM | [132] |
| S. affinis Bunge (=S. sieboldii Miq.) | Ethyl acetate fraction | Antioxidant DPPH IC50: 0.85 ± 0.04 μg/mL Superoxide radical scavenging activity: 38.63–61.41% | [28] |
| Ethanol | Cytotoxicity K562 cell line; SH-SY5Y cell line; Caco-2 cell line: n.a. Anti-ROS K562 cell line; SH-SY5Y cell line; Caco-2 cell line EC50: 0.0023 mg/mL; 0.05 mg/mL; 0.026 mg/mL | [27] | |
| S. byzantina K. Koch. | Methanol | Antioxidant Phosphomolybdenum (mmol TEs/g extract): 1.49 ± 0.12 ABTS (mg TEs/g extract): 143.85 ± 0.47 Nitric oxide (mmol TEs/g extract): 1.84 ± 0.02 CUPRAC (mg TEs/g extract): 134.73 ± 10.37 | [153] |
| Water | DPPH (mg TEs/g extract): 125.26 ± 1.47 Superoxide anion (mg TEs/g extract): 50.68 ± 2.05 FRAP (mg TEs/g extract): 98.73 ± 2.14 Chelating effect (mg EDTAEs/g extract): 16.69 ± 0.96 | ||
| Ethyl acetate | Anti-Alzheimer’s AChE inhibition (mg GALAEs/g extract): 2.08 ± 0.01 BChE inhibition (mg GALAEs/g extract): 4.09 ± 0.04 Anti-tyrosinase Tyrosinase inhibition (mg KAEs/g extract): 33.27 ± 0.54 Anti-diabetic α-Amylase inhibition (mmol ACEs/g extract): 0.31 ± 0.01 α-Glucosidase inhibition (mmol ACEs/g extract): 1.95 ± 0.20 | ||
| S. cretica L. subsp. smyrnaea Rech. f. | Methanol | Antioxidant Ferrous ion chelating (mg EDTAEs/g dp): 4.82 ± 0.04 Phosphomolybdenum (mg TEs/g dp): 71.94 ± 4.56 DPPH (mg TEs/g dp): 9.10 ± 0.04 ABTS (mg TEs/g dp): 17.36 ± 0.07 CUPRAC (mg TEs/g dp): 14.67 ± 0.02 FRAP (mg TEs/g dp): 12.98 ± 0.11 | [81] |
| Methanol | Anti-Alzheimer’s AChE inhibition (µg GALAEs/g dp): 343.78 ± 10.79 | ||
| Ethyl acetate | BChE inhibition (µg GALAEs/g dp): 167.68 ± 2.68 | ||
| Ethyl acetate | Anti-tyrosinase Tyrosinase inhibition (mg KAEs/g dp): 2.45 ± 0.05 | ||
| Methanol | Anti-diabetic α-Amylase inhibition (mg ACEs/g dp): 61.47 ± 0.05 α-Glucosidase inhibition (mg ACEs/g dp): 47.84 ± 0.78 | ||
| S. cretica L. subsp. mersinaea (Boiss.) Rech. f. | Water | Antioxidant Phosphomolybdenum (mmol TEs/g extract): 2.17 ± 0.21 DPPH (mg TEs/g extract): 176.21 ± 2.52 | [108] |
| Methanol | ABTS (mg TEs/g extract): 292.67 ± 1.53 CUPRAC (mg TEs/g extract): 256.79 ± 2.02 FRAP (mg TEs/g extract): 236.44 ± 2.96 Ferrous ion chelating (mg EDTAEs/g extract): 18.57 ± 0.04 | ||
| Methanol | Anti-Alzheimer’s AChE inhibition (mg GALAEs/g extract): 2.03 ± 0.15 | ||
| Ethyl acetate | BChE inhibition (mg GALAEs/g extract): 0.39 ± 0.01 | ||
| Ethyl acetate | Anti-tyrosinase Tyrosinase inhibition (mg KAEs/g extract): 16.58 ± 0.31 | ||
| Ethyl acetate | Anti-diabetic α-Amylase inhibition (mg ACEs/g extract): 396.50 ± 4.63 | ||
| Methanol | α-Glucosidase inhibition (mg ACEs/g extract): 734.47 ± 4.32 | ||
| S. cretica L. subsp. vacillans Rech. f. | Methanol | Antioxidant (mg TE/g extract) DPPH: 191.47 ± 5.77 ABTS: 213.93 ± 21.83 CUPRAC: 579.23 ± 13.99 FRAP: 254.40 ± 8.58 | [112] |
| Water | Ferrous ion chelating (mg EDTAE/g extract): 68.72 ± 0.80 | ||
| Methanol | Anti-tyrosinase Tyrosinase inhibition (mg KAE/g extract): 314.04 ± 2.05 | ||
| Methanol | Anti-diabetic α-Amylase inhibition (mg ACE/g extract): 433.99 ± 5.10 | ||
| S. ehrenbergii Βoiss. | Methanol | Antioxidant ABTS IC50: 52 ± 7.5 mg/mL Cytotoxicity A549 cell line IC50: 420 ± 104 μg/mL | [154] |
| S. glutinosa L. | Dichloromethane; Xanthomicrol (69) | Opioid Receptors binding affinity (in silico) Ki for MOR = 10.3 μg/mL, Ki for DOR = 9.0 μg/mL; Ki for MOR = 0.83 μM, Ki for DOR = 3.6 μM Antinociceptive (in vivo) | [107] |
| S. guyoniana Noë ex Batt. | Chloroform n-Butanol Chloroform n-Butanol | Antioxidant β-carotene IC50: 2.30 ± 1.27 μg/mL DPPH IC50: 2.91 ± 0.14 μg/mL ABTS IC50: 7.29 ± 0.23 μg/mL CUPRAC A0.50: 0.15 ± 0.05 μg/mL Metal chelating assay (%) of inhibition at 100 μg/mL: 48.00 ± 1.71 | [155] |
| n-Butanol | Anticholinesterase AChE inhibition IC50: 5.78 ± 0.01 μg/mL BChE inhibition IC50: 39.10 ± 1.41 μg/mL | ||
| n-Butanol; Chloroform | Antibacterial MIC value: S. aureus 32 ± 0.90 μg/mL, E. aerogenes 32 ± 0.70 μg/mL; E. coli 64 ± 0.60 μg/mL | ||
| S. hissarica Regel | - | Wound Healing (in vivo) | [67] |
| S. iberica var. densipilosa R. Bhattacharjee | Ethyl acetate; | Antioxidant ABTS (mg TEs/g extract): 138.16 ± 0.49, Nitric oxide (mmol TEs/g extract): 1.81 ± 0.01, Superoxide anion (mg TEs/g extract): 41.31 ± 1.64, CUPRAC (mg TEs/g extract): 111.47 ± 4.67; | [153] |
| Water | DPPH (mg TEs/g extract): 82.52 ± 1.62 FRAP (mg TEs/g extract): 89.15 ± 0.82 Chelating effect (mg EDTAEs/g extract): 9.24 ± 0.87 | ||
| Ethyl acetate | Anti-Alzheimer’s AChE inhibition (mg GALAEs/g extract): 2.16 ± 0.01 BChE inhibition (mg GALAEs/g extract): 4.20 ± 0.01 Anti-tyrosinase Tyrosinase inhibition (mg KAEs/g extract): 16.59 ± 0.33 Anti-diabetic α-Amylase inhibition (mmol ACEs/g extract): 0.34 ± 0.02 α-Glucosidase inhibition (mmol ACEs/g extract): 6.17 ± 0.51 | ||
| S. iva Griseb. | Stachysetin (98) | Anti-diabetic (in silico) Dipeptyl peptidase IV, peroxisome proliferator-active receptor gamma, aldose reductase, glycogen kinase, pancreatic alpha amylase precursor | [56] |
| S. mialhesii Noé | n-Butanol; Isoscutellarein-7-O-[6”′-O-acetyl]-β-D-allopyranosyl-(1→2)-β-D-glucoside (15) | Antioxidant DPPH IC50: 0.047 ± 0.0048 mg/mL; 0.066 ± 0.002 mg/mL | [103] |
| n-Butanol | Acute toxicity (in vivo) Not toxic (10 g/kg of extract) Antinociceptive(in vivo) Inhibition of the writhing response induced by acetic acid (dose: 10,000; 5000 mg/kg) 77.11%, 58.22% Antiinflammatory (in vivo) Carrageenan-induced paw edema (dose: 5000 mg/kg) 52.03% Ulcerogenic (in vivo) n.a. | ||
| S. mucronata Sieb. | n-Butanol fraction | Anti-radical | [156] |
| S. lavandulifolia Vahl. | Methanol Soxhlet extract; Arbutin (107), Ethanol; Arbutin (107), Methanol Soxhlet extract; Arbutin (107), | Antioxidant DPPH IC50: 25.0 ± 1.1 μg/mL; 62.5 ± 0.9 μg/mL, ABTS IC50: 19.9 μg/mL; 45.7 μg/mL, FRAP (μM Fe(II)/g): 44.5 ± 1.0; 12.2 ± 0.6, | [116] |
| Methanol; Ethanol | β-carotene IC50: 29.3 µg/mL (30 min), 60.3 µg/mL (60 min); 33.0 µg/mL (30 min), 34.6 µg/mL (60 min) | ||
| Ethanol | Anti-tyrosinase Tyrosinase inhibition IC50: 33.4 ± 0.8 μg/mL | ||
| Hexane Dichloromethane | Anti-Alzheimer’s AChE inhibition IC50:13.7 ± 1.2 μg/mL BChE inhibition IC50: 143.9 μg/mL | ||
| Chloroform | Cytotoxicity Brine Shrimp lethality test: 121.8 ± 5.6 μg/mL | [13] | |
| Apigenin (1); Chrysosplenetin (84) | MRC-5 cell line IC50: 35.67 μg/mL; MDA-MB-231 cell line IC50: 88.23 μg/mL, HT-29 cell line IC50: 116.50 μg/mL | ||
| S. officinalis (L.) Trevis (=Betonica officinalis L.) | Acetone Methanol | Genotoxicity | [157] |
| S. ocymastrum (L.) Briq. (=S. hirta L.) | 6β-Acetoxyipolamiide (172); 6β-Hydroxyipolamiide (173); Ipolamiide (174); Ipolamiidoside (175) | Antiangiogenic(in vivo) | [123] |
| S. parviflora Benth. (=Phlomidoschema parviflorum (Benth.) Vved.) | Methanol | Antioxidant DDPH IC50: 76.87 ± 0.57 µg/mL BCB IC50: 188.47 ± 0.76 µg/mL Cytotoxicity A2780 cell line IC50: n.a HCT cell line IC50: n.a B16F10 cell line IC50: n.a Antibacterial MIC: Bacillus cereus 0.12 mg/mL | [64] |
| S. pilifera Benth. | Terpenoid fraction | Cytotoxicity HT29 cell line IC50: 46.44 μg/mL | [45] |
| 70% Methanol Alkaloid fraction | Antiproliferative Caspase-8 increased 99% Caspase-9 increased 85.38% | ||
| 70% Ethanol | Hepatoprotective (in vivo) | [158] | |
| Hydroalcoholic | Antioxidant(in vivo) Hepatoprotective(in vivo) | [159] | |
| Hydroalcoholic | Antioxidant (in vivo) Renoprotective (in vivo) | [19] | |
| Water | Neuroprotective (in vivo) | [152] | |
| S. riederi var. japonica (Miq.) H. Hara | 80% Ethanol | Antioxidant/Cytoprotective UVA-irradiated human dermal fibroblasts (HDFs) Cytotoxicity HDFs: l.a./n.a | [160] |
| S. sieboldii Miq. (=S. affinis Bunge) | n-Hexane fraction n-Hexane; 85% MeOH; n-BuOH; water fractions | Antioxidant ROS inhibition: 63% Increased GSH levels Inhbited oxidative DNA damage >90% | [29] |
| (Root powder) | Anti-obesity (in vivo) Anti-dyslipidemic (in vivo) | [161] | |
| 20% Ethanol | Memory protective (in vivo) | [162] | |
| S. sylvatica L. | Hydroalcoholic | Polycystic ovary syndrome(in vivo) (500 mg/kg) (mIU/mL), FSH 5.95 ± 0.02 mIU/mL, LH 6.48 ± 0.09 mIU/mL, Estrogen 0.9 ± 0.07 mIU/mL, LH/FSH 6.48/5.59 mIU/mL | [47] |
| S. thirkei K. Koch. | Methanol | Antioxidant β-carotene IC50: 47.79 ± 0.59 μg/mL DPPH IC50: 49.31 ± 0.38 μg/mL ABTS IC50: 13.34 ± 0.02 μg/mL CUPRAC absorbance%: 1.88 ± 0.02 | [84] |
| Acetone | Anticholinesterase AChE inhibition IC50: 52.46± 1.26% BChE inhibition IC50: 75.04 ± 1.91% | ||
| Methanol | Cytotoxicity A549 and L929 Fibroblast cells (100 mg/mL): n.a. | ||
| Acetone; Methanol | Antimicrobial Inhibition zone diameter: S. aureus (11 mm), S. pyogenes (10 mm), E. coli (10 mm), P. aeruginosa (n.a.), C. albicans: n.a.; S. aureus (10 mm), S. pyogenes (10 mm), E. coli (10 mm), P. aeruginosa (n.a.), C. albicans: n.a. MIC values: 250 ± 0.6 μg/mL, 300 ± 0.4 μg/mL, 250 ± 0.3 μg/mL, n.a., n.a.; 300 ± 0.1 μg/mL, 250 ± 0.2 μg/mL, 250 ± 0.4 μg/mL, n.a., n.a. | ||
| S. tmolea Boiss. | Water | Antioxidant DPPH (mg TEs/g dp): 50.88 ± 1.55 ABTS (mg TEs/g dp): 44.39 ± 3.24 CUPRAC (mg TEs/g dp): 87.57 ± 0.83 FRAP (mg TEs/g dp): 51.80 ± 2.17 Phosphomolybdenum (mg TEs/g dp): 40.58 ± 3.45 Ferrous ion chelating (mg EDTAEs/g dp): 1.10 ± 0.03 | [85] |
| Type of Data | No of Studies * | Years of Publication |
|---|---|---|
| Ethnobotanical | 48 | since 1914 |
| Phytochemical | 91 | since 1968 |
| Pharmacological | 22 (in vitro) | since 2015 |
| 8 (in vivo) | ||
| 2 (in silico) | ||
| Clinical studies | 4 | since 2013 |
| Reviews | 4 | since 1994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomou, E.-M.; Barda, C.; Skaltsa, H. Genus Stachys: A Review of Traditional Uses, Phytochemistry and Bioactivity. Medicines 2020, 7, 63. https://doi.org/10.3390/medicines7100063
Tomou E-M, Barda C, Skaltsa H. Genus Stachys: A Review of Traditional Uses, Phytochemistry and Bioactivity. Medicines. 2020; 7(10):63. https://doi.org/10.3390/medicines7100063
Chicago/Turabian StyleTomou, Ekaterina-Michaela, Christina Barda, and Helen Skaltsa. 2020. "Genus Stachys: A Review of Traditional Uses, Phytochemistry and Bioactivity" Medicines 7, no. 10: 63. https://doi.org/10.3390/medicines7100063
APA StyleTomou, E.-M., Barda, C., & Skaltsa, H. (2020). Genus Stachys: A Review of Traditional Uses, Phytochemistry and Bioactivity. Medicines, 7(10), 63. https://doi.org/10.3390/medicines7100063