Analgesic Efficacy of a Combination of Fentanyl and a Japanese Herbal Medicine “Yokukansan” in Rats with Acute Inflammatory Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Administration of Drugs
2.3. Assessment of Analgesia
2.3.1. Formalin Test
2.3.2. Immunofluorescent Staining
2.4. Cell Culture
2.5. Whole-Cell Patch-Clamp Recording
2.6. Statistical Analysis
3. Results
3.1. Formalin Test
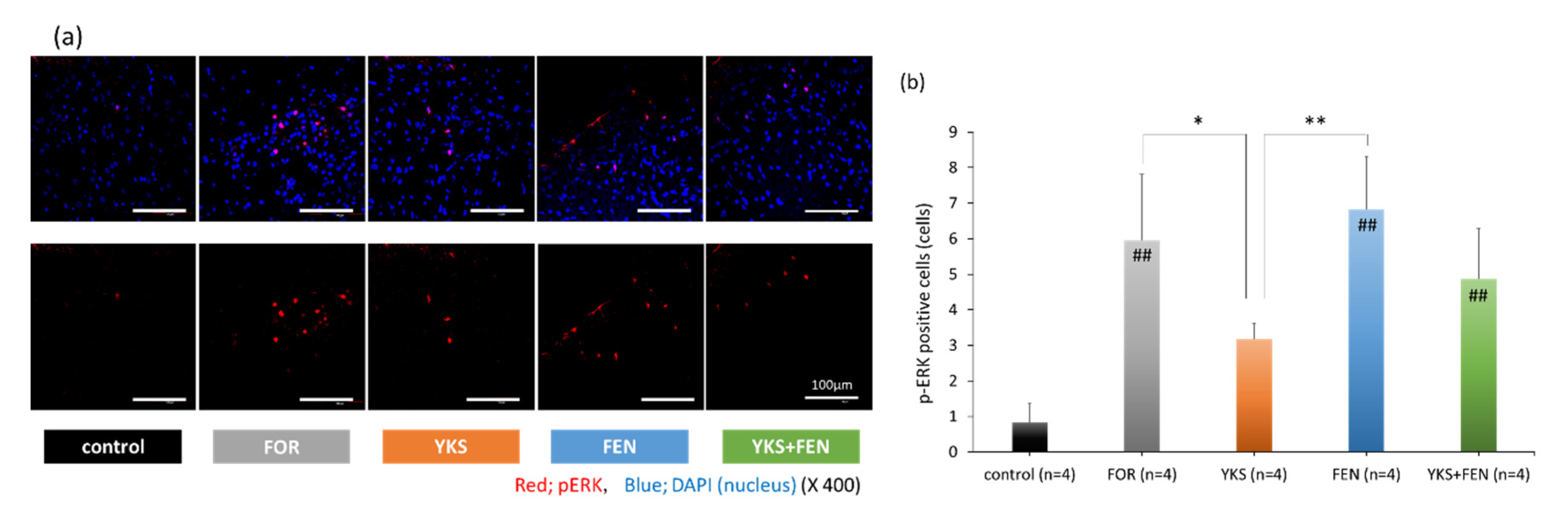
3.2. Immunofluorescent Staining of pERK(+) Cells
3.3. Whole-Cell Patch-Clamp Recording of TRPA1 Currents
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jowkar, S.; Khosravi, M.B.; Sahmeddini, M.A.; Eghbal, M.H.; Samadi, K. Preconditioning effect of remifentanil versus fentanyl in prevalence of early graft dysfunction in patients after liver transplant: A randomized clinical trial. Exp. Clin. Transplant. 2020, 18, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.; Martinez, V. Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. Br. J. Anaesth. 2014, 112, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.H.; Tran, D.H.; Lam, S.W.; Irwin, M.G. Remifentanil tolerance and hyperalgesia: Short-term gain, long-term pain? Anaesthesia 2016, 71, 1347–1362. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.Y.; Liu, K.; Wang, J.J.; Kuo, M.C.; Ho, S.T. Intraoperative high dose fentanyl induces postoperative fentanyl tolerance. Can. J. Anaesth. 1999, 46, 872–877. [Google Scholar] [CrossRef]
- Rupniewska-Ladyko, A.; Malec-Milewska, M.A. High dose of fentanyl may accelerate the onset of acute postoperative pain. Anesthesiol. Pain Med. 2019, 9, e94498. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.K.; Ma, Y.; Xie, H. TRPV1 and spinal astrocyte activation contribute to remifentanil-induced hyperalgesia in rats. Neuroreport 2019, 30, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, L.; Zhao, H.; Song, F.; Chen, G.; Zhu, H. The role of p38MAPK activation in spinal dorsal horn in remifentanil-induced postoperative hyperalgesia in rats. Neurol. Res. 2016, 38, 929–936. [Google Scholar] [CrossRef]
- Lv, C.C.; Xia, M.L.; Shu, S.J.; Chen, F.; Jiang, L.S. Attenuation of remifentanil-induced hyperalgesia by betulinic acid associates with inhibiting oxidative stress and inflammation in spinal dorsal horn. Pharmacology 2018, 102, 300–306. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Wang, J.; Chen, Y.; Yang, C.; Zhao, M.; Wang, G.; Yang, Z. Annexin 1 inhibits remifentanil-induced hyperalgesia and NMDA receptor phosphorylation via regulating spinal CXCL12/CXCR4 in rats. Neurosci. Res. 2019, 144, 48–55. [Google Scholar] [CrossRef]
- Lu, A.; Lei, H.; Li, L.; Lai, L.; Liang, W.; Xu, S. Role of mitochondrial Ca2+ uniporter in remifentanil-induced postoperative allodynia. Eur. J. Neurosci. 2018, 47, 305–313. [Google Scholar] [CrossRef]
- Li, S.; Zeng, J.; Wan, X.; Yao, Y.; Zhao, N.; Yu, Y.; Yu, C.; Xia, Z. Enhancement of spinal dorsal horn neuron NMDA receptor phosphorylation as the mechanism of remifentanil induced hyperalgesia: Roles of PKC and CaMKII. Mol. Pain 2017, 13, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Shi, L.; Zhang, J.; Kong, M.; Liu, Y.; Zhou, Y.; Xu, L.; He, J.; Ma, Z.; Gu, X. Neuron-restrictive silencer factor in periaqueductal gray contributes to remifentanil-induced postoperative hyperalgesia via repression of the mu-opioid receptor. J. Neurol. Sci. 2015, 352, 48–52. [Google Scholar] [CrossRef]
- Ye, L.; Xiao, L.; Yang, S.Y.; Duan, J.J.; Chen, Y.; Cui, Y.; Chen, Y. Cathepsin S in the spinal microglia contributes to remifentanil-induced hyperalgesia in rats. Neuroscience 2017, 344, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Xuerong, Y.; Yuguang, H.; Xia, J.; Hailan, W. Ketamine and lornoxicam for preventing a fentanyl-induced increase in postoperative morphine requirement. Anesth. Analg. 2008, 107, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.W.; Lindsay, S.L.; Ryall, D.M.; Kokri, M.S.; Eldabe, S.S.; Lear, G.A. Does intrathecal fentanyl produce acute cross-tolerance to i.v. morphine? Br. J. Anaesth. 1997, 78, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.; Drover, D.R.; Ginosar, Y.; Cohen, S.E.; Riley, E.T. Intrathecal fentanyl added to bupivacaine and morphine for cesarean delivery may induce a subtle acute opioid tolerance. Int. J. Obstet. Anesth. 2012, 21, 29–34. [Google Scholar] [CrossRef]
- Yildirim, V.; Doganci, S.; Cinar, S.; Eskin, M.B.; Ozkan, G.; Eksert, S.; Ince, M.E.; Dogrul, A. Acute high dose-fentanyl exposure produces hyperalgesia and tactile allodynia after coronary artery bypass surgery. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3425–3434. [Google Scholar]
- Richebé, P.; Rivat, C.; Laulin, J.P.; Maurette, P.; Simonnet, G. Ketamine improves the management of exaggerated postoperative pain observed in perioperative fentanyl-treated rats. Anesthesiology 2005, 102, 421–428. [Google Scholar] [CrossRef]
- Li, Q.B.; Chang, L.; Ye, F.; Luo, Q.H.; Tao, Y.X.; Shu, H.H. Role of spinal cyclooxygenase-2 and prostaglandin E2 in fentanyl-induced hyperalgesia in rats. Br. J. Anaesth. 2018, 120, 827–835. [Google Scholar] [CrossRef]
- Chang, L.; Ye, F.; Luo, Q.; Tao, Y.; Shu, H. Increased hyperalgesia and proinflammatory cytokines in the spinal cord and dorsal root ganglion after surgery and/or fentanyl administration in rats. Anesth. Analg. 2018, 126, 289–297. [Google Scholar] [CrossRef]
- Li, Z.; Yin, P.; Chen, J.; Jin, S.; Liu, J.; Luo, F. CaMKIIα may modulate fentanyl-induced hyperalgesia via a CeLC-PAG-RVM-spinal cord descending facilitative pain pathway in rats. PLoS ONE 2017, 12, e0177412. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wei, W. Role of gabapentin in preventing fentanyl- and morphine-withdrawal-induced hyperalgesia in rats. J. Anesth. 2012, 26, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Caires, S.; Steenkamp, V. Use of Yokukansan (TJ-54) in the treatment of neurological disorders: A review. Phytother. Res. 2010, 24, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, Y.; Mizoguchi, K. Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacol. Ther. 2016, 166, 84–95. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tajima, K.; Kawagoe, I.; Kanai, M.; Mitsuhata, H. Efficacy of traditional herbal medicine, Yokukansan on patients with neuropathic pain. Masui. Jpn. J. Anesthesiol. 2009, 58, 1248–1255, (In Japanese, English Abstract). [Google Scholar]
- Yamaguchi, K. Traditional Japanese herbal medicines for treatment of odontopathy. Front. Pharmacol. 2015, 6, 176. [Google Scholar] [CrossRef]
- Sugasawa, Y. Effect of Yokukansan, Japanese Herbal Medicine, on Phantom-limb pain. Middle East J. Anaesthesiol. 2016, 23, 499–500. [Google Scholar]
- Akiyama, H.; Hasegawa, Y. Effectiveness of the traditional Japanese Kampo medicine Yokukansan for chronic migraine: A case report. Medicine (Baltimore) 2019, 98, e17000. [Google Scholar] [CrossRef]
- Suzuki, Y.; Mitsuhata, H.; Yuzurihara, M.; Kase, Y. Antiallodynic effect of herbal medicine yokukansan on peripheral neuropathy in rats with chronic constriction injury. Evid. Based Complement. Altern. Med. 2012, 2012, 953459. [Google Scholar] [CrossRef]
- Ebisawa, S.; Andoh, T.; Shimada, Y.; Kuraishi, Y. Yokukansan improves mechanical allodynia through the regulation of interleukin-6 expression in the spinal cord in mice with neuropathic pain. Evid. Based Complement. Altern. Med. 2015, 2015, 870687. [Google Scholar] [CrossRef]
- Suga, H.; Sunagawa, M.; Ikemoto, H.; Nakanishi, T.; Fujiwara, A.; Okada, M. The analgesic and anti-stress effects of a Kampo medicine (Yokukansan) in rats with chronic constriction injury—A comparative study with kamishoyosan. J. Integr. Med. Ther. 2015, 2, 5. [Google Scholar] [CrossRef]
- Honda, Y.; Sunagawa, M.; Yoneyama, S.; Ikemoto, H.; Nakanishi, T.; Iwanami, H.; Hisamitsu, T. Analgesic and anti-stress effects of Yokukansan in rats with adjuvant arthritis. Kampo Med. 2013, 64, 78–85. [Google Scholar] [CrossRef]
- Takemoto, M.; Sunagawa, M.; Okada, M.; Ikemoto, H.; Suga, H.; Katayama, A.; Otake, H.; Hisamitsu, T. Yokukansan, a Kampo medicine, prevents the development of morphine tolerance through the inhibition of spinal glial cell activation in rats. Integr. Med. Res. 2016, 5, 41–47. [Google Scholar] [CrossRef]
- Katayama, A.; Kanada, Y.; Tsukada, M.; Akanuma, Y.; Takemura, H.; Ono, T.; Suga, H.; Mera, H.; Hisamitsu, T.; Sunagawa, M. Yokukansan (Kampo medicinal formula) prevents the development of morphine tolerance by inhibiting the secretion of orexin A. Integr. Med. Res. 2018, 7, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Z.; Ikarashi, Y.; Kase, Y. Isoliquiritigenin is a novel NMDA receptor antagonist in kampo medicine yokukansan. Cell Mol. Neurobiol. 2011, 31, 1203–1212. [Google Scholar] [CrossRef]
- Kawakami, Z.; Omiya, Y.; Mizoguchi, K. Comparison of the effects of Yokukansan and Yokukansankachimpihange on glutamate uptake by cultured astrocytes and glutamate-induced excitotoxicity in cultured PC12 cells. Evid. Based Complement. Altern. Med. 2019, 2019, 9139536. [Google Scholar] [CrossRef]
- Takeda, A.; Itoh, H.; Tamano, H.; Yuzurihara, M.; Oku, N. Suppressive effect of Yokukansan on excessive release of glutamate and aspartate in the hippocampus of zinc-deficient rats. Nutr. Neurosci. 2008, 11, 41–46. [Google Scholar] [CrossRef]
- Vissers, K.C.; Geenen, F.; Biermans, R.; Meert, T.F. Pharmacological correlation between the formalin test and the neuropathic pain behavior in different species with chronic constriction injury. Pharmacol. Biochem. Behav. 2006, 84, 479–486. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef]
- Sałat, K.; Filipek, B. Antinociceptive activity of transient receptor potential channel TRPV1, TRPA1, and TRPM8 antagonists in neurogenic and neuropathic pain models in mice. J. Zhejiang Univ. Sci. B 2015, 16, 167–178. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Gereau, R.W., IV; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Nagayasu, K.; Nishitani, N.; Shirakawa, H.; Sekiguchi, K.; Ikarashi, Y.; Kase, Y.; Kaneko, S. Yokukansan inhibits morphine tolerance and physical dependence in mice: The role of α₂A-adrenoceptor. Neuroscience 2012, 227, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Derouiche, S.; Maruyama, K.; Tominaga, M. Emerging perspectives on pain management by modulation of TRP channels and ANO1. Int. J. Mol. Sci. 2019, 20, 3411. [Google Scholar] [CrossRef]
- Forster, A.B.; Reeh, P.W.; Messlinger, K.; Fischer, M.J. High concentrations of morphine sensitize and activate mouse dorsal root ganglia via TRPV1 and TRPA1 receptors. Mol. Pain 2009, 5, 17. [Google Scholar] [CrossRef]
- Kanada, Y.; Katayama, A.; Ikemoto, H.; Takahashi, K.; Tsukada, M.; Nakamura, A.; Ishino, S.; Hisamitsu, T.; Sunagawa, M. Inhibitory effect of the Kampo medicinal formula Yokukansan on acute stress-induced defecation in rats. Neuropsychiatr. Dis. Treat. 2018, 14, 937–944. [Google Scholar] [CrossRef]
- Gamal-Eltrabily, M.; Espinosa de Los Monteros-Zúñiga, A.; Manzano-García, A.; Martínez-Lorenzana, G.; Condés-Lara, M.; González-Hernández, A. The Rostral Agranular Insular Cortex, a New Site of Oxytocin to Induce Antinociception. J. Neurosci. 2020, 40, 5669–5680. [Google Scholar] [CrossRef]
- Zhuang, Z.Y.; Gerner, P.; Woolf, C.J.; Ji, R.R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005, 114, 149–159. [Google Scholar] [CrossRef]
- Karim, F.; Wang, C.C.; Gereau, R.W., IV. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J. Neurosci. 2001, 21, 3771–3779. [Google Scholar] [CrossRef]
- Tsuda, M.; Ishii, S.; Masuda, T.; Hasegawa, S.; Nakamura, K.; Nagata, K.; Yamashita, T.; Furue, H.; Tozaki-Saitoh, H.; Yoshimura, M.; et al. Reduced pain behaviors and extracellular signal-related protein kinase activation in primary sensory neurons by peripheral tissue injury in mice lacking platelet-activating factor receptor. J. Neurochem. 2007, 102, 1658–1668. [Google Scholar] [CrossRef]
- Ma, Y.; Bao, Y.; Wang, S.; Li, T.; Chang, X.; Yang, G.; Meng, X. Anti-inflammation effects and potential mechanism of saikosaponins by regulating nicotinate and nicotinamide metabolism and arachidonic acid metabolism. Inflammation 2016, 39, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luo, C.; Wang, P.; He, Q.; Zhou, J.; Peng, H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-κB pathways in LPS-stimulated RAW 264.7 cells. Exp. Ther. Med. 2013, 5, 1345–1350. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Dai, Y.Y.; Pan, Y.F.; Wu, X.M.; Yang, Y.; Bian, K.; Zhang, D.D. Total flavonoids from radix glycyrrhiza exert anti-inflammatory and antitumorigenic effects by inactivating iNOS signaling pathways. Evid. Based Complement. Altern. Med. 2018, 2018, 6714282. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Park, G.H.; Song, K.S. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology 2013, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Roeckel, L.A.; Le Coz, G.M.; Gavériaux-Ruff, C.; Simonin, F. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience 2016, 338, 160–182. [Google Scholar] [CrossRef] [PubMed]
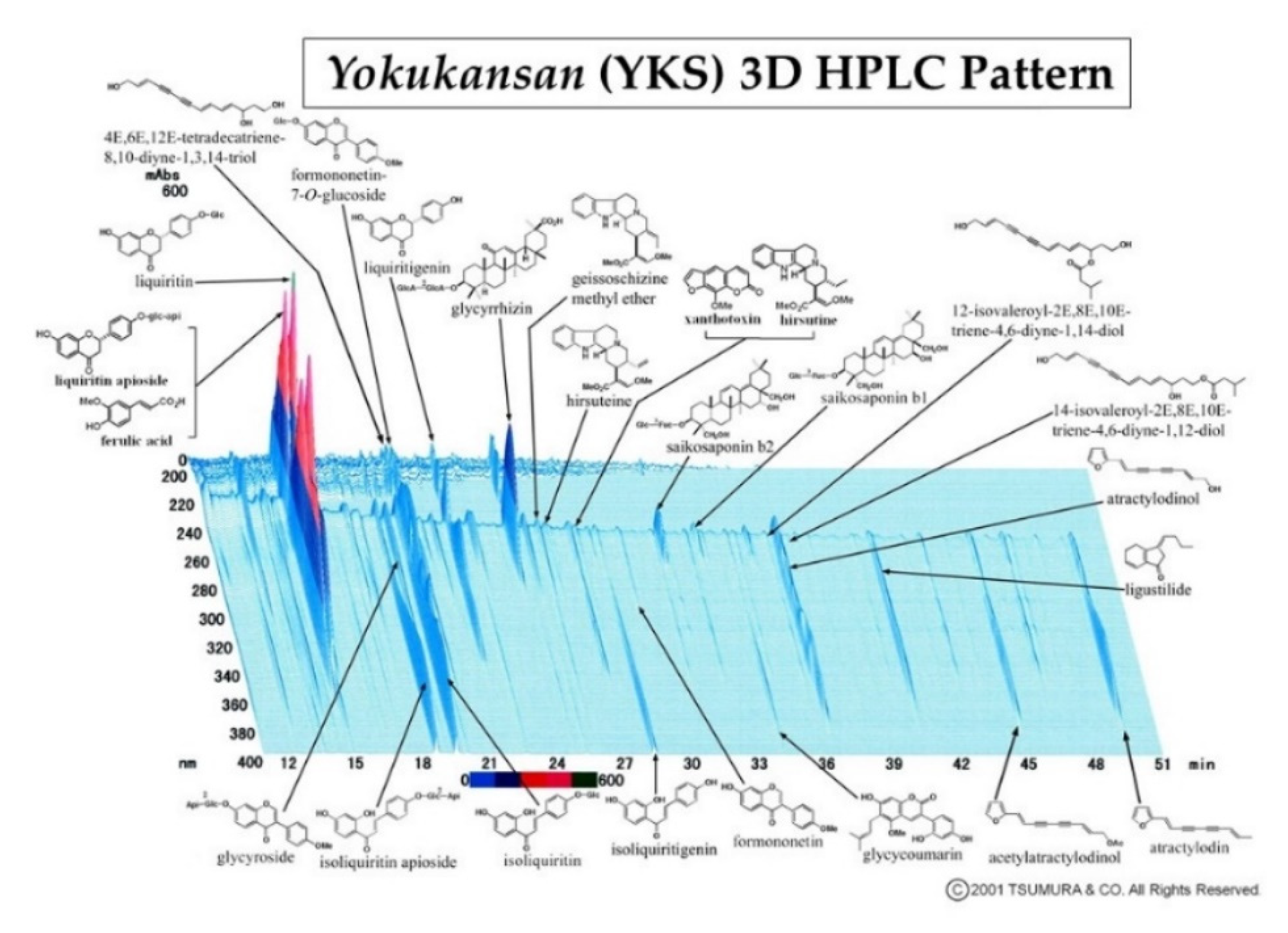


| Uncariae cum Uncis ramulus | 3.0 g |
| Cnidii rhizoma | 3.0 g |
| Bupleuri radix | 2.0 g |
| Atratylodis Lanceae rhizoma | 4.0 g |
| Poria | 4.0 g |
| Angelicae radix | 3.0 g |
| Glycyrrhizae radix | 1.5 g |
| Groups | Days 1–7 | Day 8 | ||
|---|---|---|---|---|
| 10 min before Test | Formalin Test | |||
| control | Powdered chow | Saline (i.p.) | Saline (50 µL; s.c.) | Observation of pain-related behavior (60 min) |
| FOR | Powdered chow | Saline (i.p.) | Formalin (5%, 50 µL; s.c.) | |
| YKS | Powdered chow mixed with YKS (3%) | Saline (i.p.) | Formalin (5%, 50 µL; s.c.) | |
| FEN | Powdered chow | Fentanyl (0.08 µg/kg; i.p.) | Formalin (5%, 50 µL; s.c.) | |
| YKS+FEN | Powdered chow mixed with YKS (3%) | Fentanyl (0.08 µg/kg; i.p.) | Formalin (5%, 50 µL; s.c.) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akanuma, Y.; Kato, M.; Takayama, Y.; Ikemoto, H.; Adachi, N.; Ohashi, Y.; Yogi, W.; Okumo, T.; Tsukada, M.; Sunagawa, M. Analgesic Efficacy of a Combination of Fentanyl and a Japanese Herbal Medicine “Yokukansan” in Rats with Acute Inflammatory Pain. Medicines 2020, 7, 75. https://doi.org/10.3390/medicines7120075
Akanuma Y, Kato M, Takayama Y, Ikemoto H, Adachi N, Ohashi Y, Yogi W, Okumo T, Tsukada M, Sunagawa M. Analgesic Efficacy of a Combination of Fentanyl and a Japanese Herbal Medicine “Yokukansan” in Rats with Acute Inflammatory Pain. Medicines. 2020; 7(12):75. https://doi.org/10.3390/medicines7120075
Chicago/Turabian StyleAkanuma, Yuko, Mami Kato, Yasunori Takayama, Hideshi Ikemoto, Naoki Adachi, Yusuke Ohashi, Wakako Yogi, Takayuki Okumo, Mana Tsukada, and Masataka Sunagawa. 2020. "Analgesic Efficacy of a Combination of Fentanyl and a Japanese Herbal Medicine “Yokukansan” in Rats with Acute Inflammatory Pain" Medicines 7, no. 12: 75. https://doi.org/10.3390/medicines7120075
APA StyleAkanuma, Y., Kato, M., Takayama, Y., Ikemoto, H., Adachi, N., Ohashi, Y., Yogi, W., Okumo, T., Tsukada, M., & Sunagawa, M. (2020). Analgesic Efficacy of a Combination of Fentanyl and a Japanese Herbal Medicine “Yokukansan” in Rats with Acute Inflammatory Pain. Medicines, 7(12), 75. https://doi.org/10.3390/medicines7120075






