The Antioxidant and Antihyperglycemic Activities of Bottlebrush Plant (Callistemon lanceolatus) Stem Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Extract
2.3. Phytochemical Screening
2.4. Determination of Total Flavonoid Content
2.5. Determination of Total Phenolics
2.6. Free Radical Scavenging Assay
2.7. Reducing Power Assay
2.8. Metal Ion Chelating Activity
2.9. Animal and Maintenance
2.10. Experimental Protocol
- Group 1: Normal control
- Group 2: Diabetic control
- Group 3: Diabetic rats received reference drug glibenclamide (5 mg/kg) orally once a day for 4 weeks.
- Group 4: Diabetic rats received C. lanceolatus methanolic extract (200 mg/kg) orally once a day for 4 weeks.
- Group 5: Diabetic rats received C. lanceolatus methanolic extract (400 mg/kg) orally once a day for 4 weeks.
- Group 6: Extract control rats received C. lanceolatus methanolic extract daily (400 mg/kg) orally once a day for 4 weeks.
2.11. Biochemical Analysis
2.12. Statistical Analysis
3. Results
3.1. Phytochemical Analysis and Quantification of Total Phenolic and Flavonoid Contents
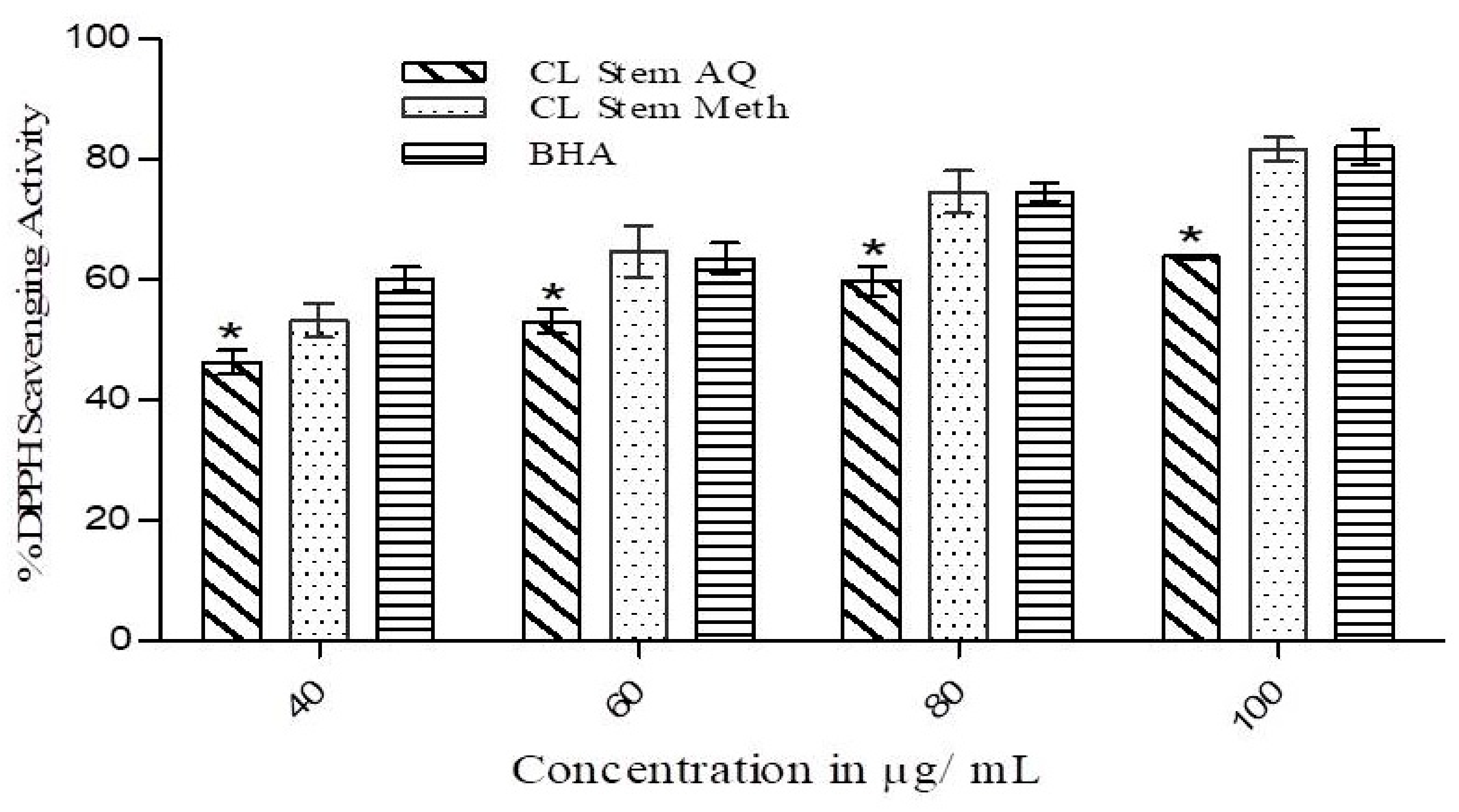
3.2. DPPH Free Radical Scavenging Activity
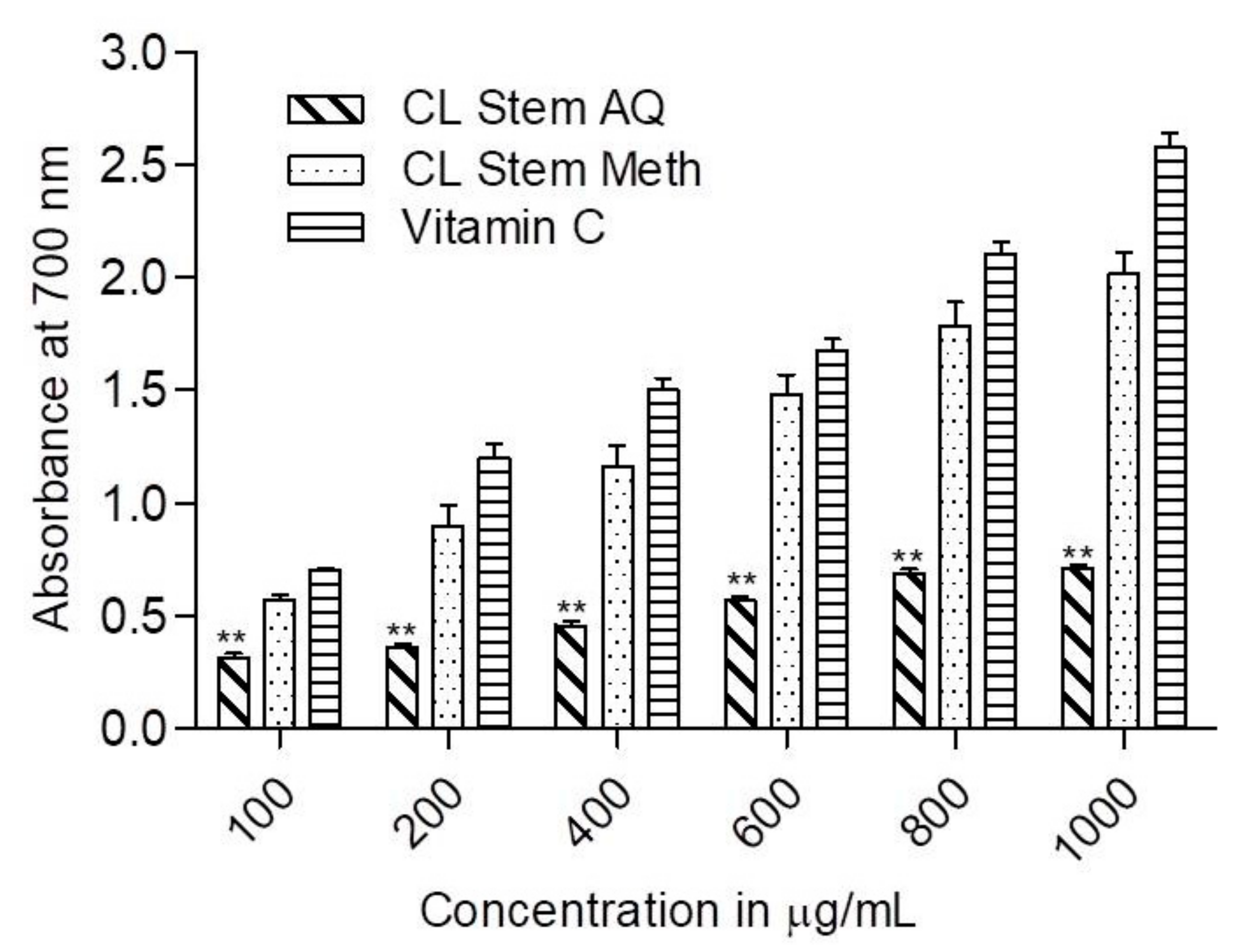
3.3. Reducing Power of the Extract
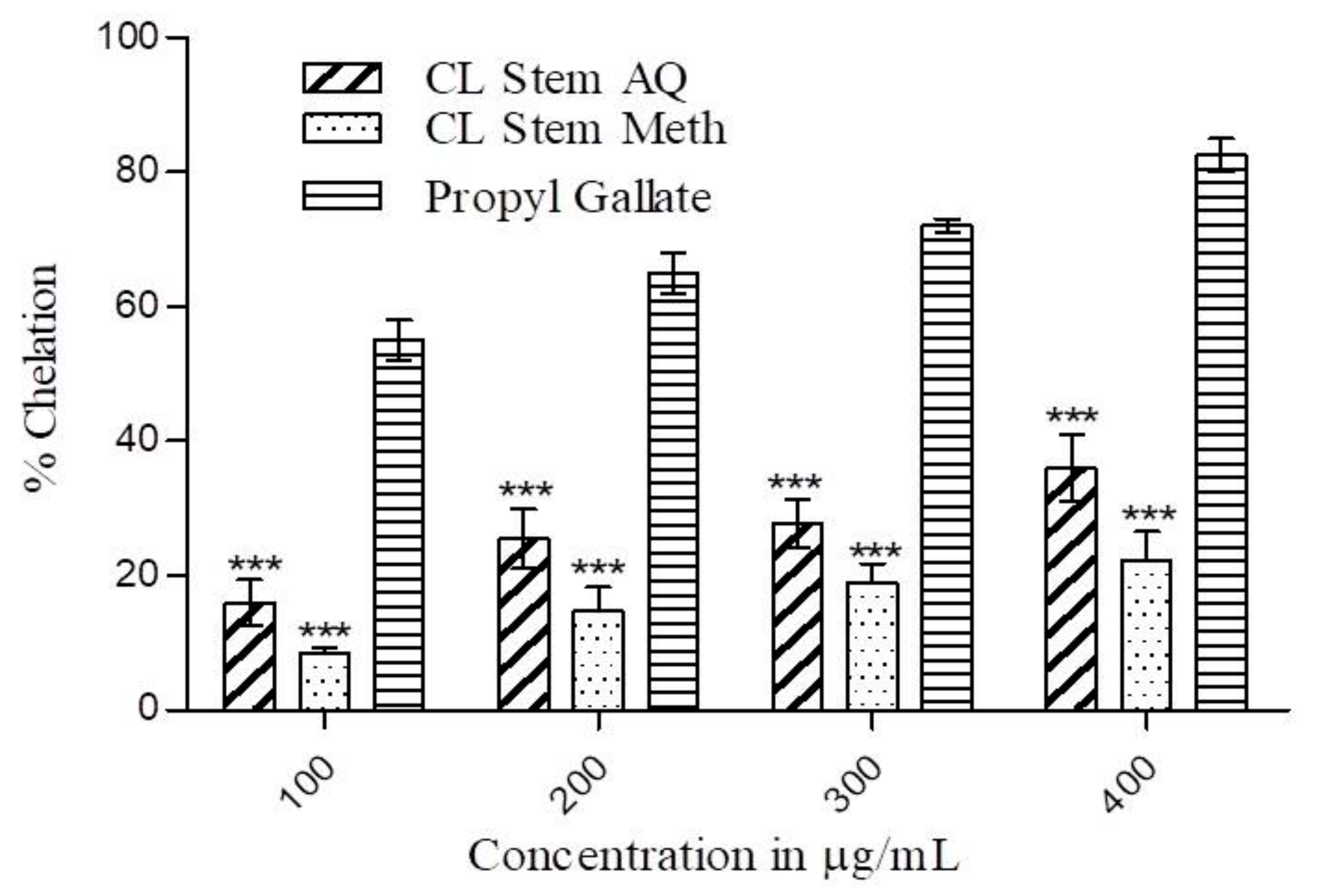
3.4. Metal Ion Chelating Activity
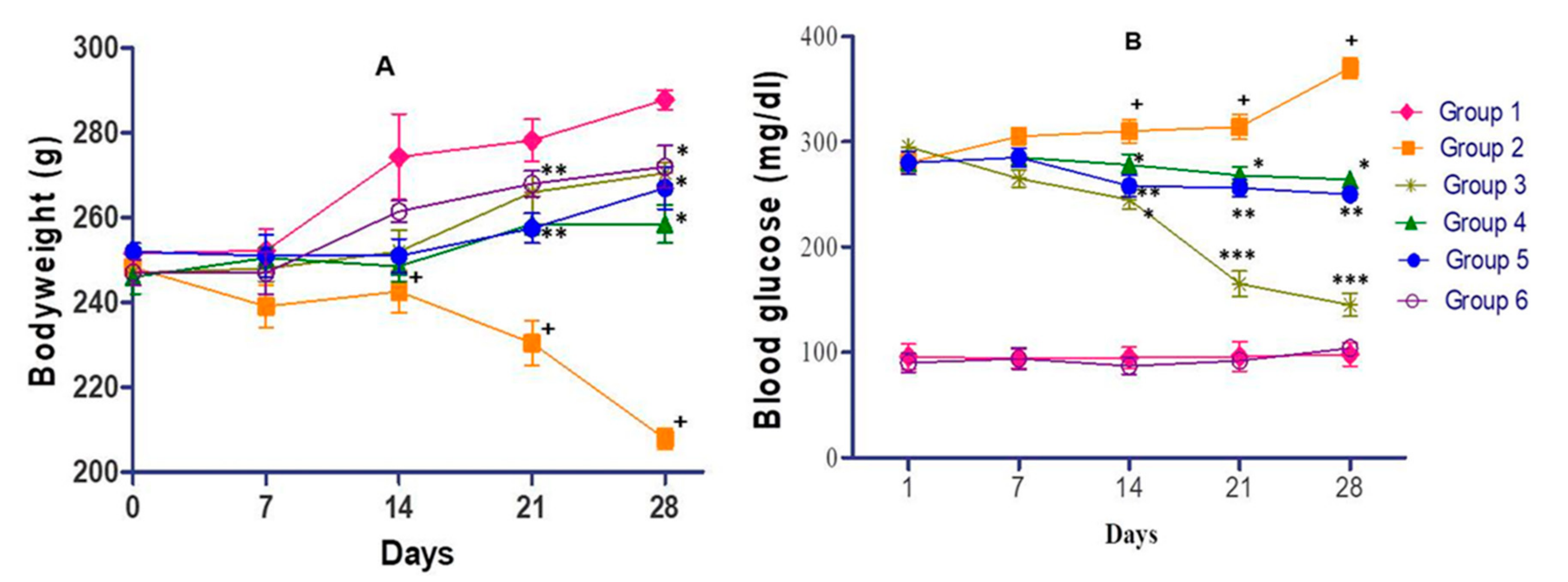
3.5. Effects of C. lanceolatus Extract on Animal Bodyweight and Blood Glucose Level
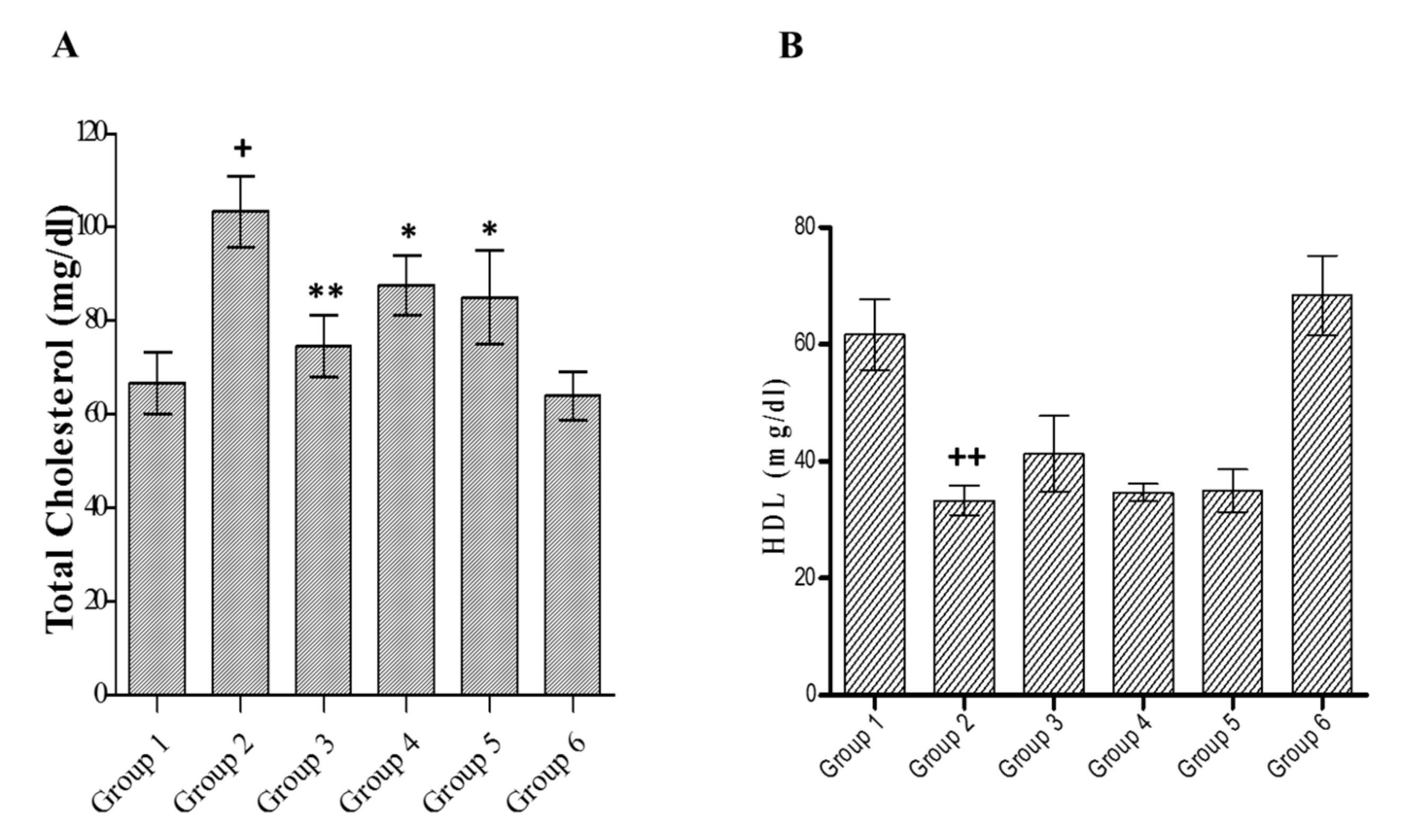
3.6. Effect of C. lanceolatus Extract on Serum Total Cholesterol and Serum HDL
3.7. Effect of C. lanceolatus Extract on Serum Triglyceride and Bilirubin
3.8. Effect of C. lanceolatus Extract on Serum Enzymes
3.9. Effect of C. lanceolatus Extract on Serum Creatinine and Urea
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, U.K.; Kumar, R.; Gupta, A.; Ganguly, R.; Pandey, A.K. Renoprotective effect of cinnamaldehyde in food color induced toxicity. 3Biotech 2018, 8, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, U.K.; Sharma, A.K.; Pandey, A.K. Protective efficacy of Solanumxanthocarpum root extracts against free radical damage: Phytochemical analysis and antioxidant effect. Cell Mol. Biol. 2012, 58, 174–181. [Google Scholar] [PubMed]
- Ademiluyia, A.O.; Oboha, G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef] [PubMed]
- ICMR Guidelines for Management of Type 2 Diabetes 2018. Available online: https://www.icmr.nic.in/content/guidelines-management-type-2-diabetes (accessed on 28 February 2020).
- Ramachandran, S.; Rajasekaran, A.; Adhirajan, N. In vivo and in vitro anti-diabetic activity of Terminalia paniculata bark: An evaluation of possible phytoconstituents and mechanisms for blood glucose control in diabetes. ISRN Pharmacol. 2013, 2013, 484675. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, R.; Pandey, A.K. Antioxidant and antidiabetic activities of Terminalia bellirica fruit in alloxan induced diabetic rats. S. Afr. J. Bot. 2020, 130, 308–315. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Tewari, S.; Mendes, R.H. The role of oxidative stress in the development of diabetes mellitus and its complications. J. Diabetes Res. 2019, 2019, 4189813. [Google Scholar] [CrossRef]
- Melo da Cunha, J.D.S.; Alfredo, T.M.; Dos Santos, J.M.; Alves, V.V., Jr.; Rabelo, L.A.; Lima, E.S.; Boleti, A.P.A.; Carollo, C.A.; Dos Santos, E.L.; de Picoli Souza, K. Antioxidant, antihyperglycemic, and anti-diabetic activity of Apis mellifera bee tea. PLoS ONE 2018, 13, e0197071. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, S. Perspective on plant product as anti-microbial agents; A review. Pharmacologia 2013, 4, 469–480. [Google Scholar] [CrossRef]
- Marzouk, M.S.A. An acylated flavonol glycoside and hydrolysable tannins from Callistemon lanceolatus flowers and leaves. Phytochem. Anal. 2008, 19, 541–549. [Google Scholar] [CrossRef]
- Arora, D.S.; Nim, L.; Kaur, H. Antimicrobial potential of Callistemon lanceolatus seed extract and its statistical optimization. Appl. Biochem. Biotech. 2016, 180, 289–305. [Google Scholar] [CrossRef]
- Ahmad, K.; Hafeez, Z.B.; Bhat, A.R.; Rizvi, M.A.; Thakur, S.C.; Azam, A.; Athar, F. Antioxidant and apoptotic effects of Callistemon lanceolatus leaves and their compounds against human cancer cells. Biomed. Pharmacother. 2018, 106, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Nazreen, S.; Mahboob, A.M.; Hamid, H.; Ali, M.; SarwarAlam, M. Chemical constituents with antimicrobial and antioxidant activity from the aerial parts of Callistemon lanceolatus (Sm.) Sweet. Nat. Prod. Res. 2019, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; Jeong, W.; Bazer, F.W.; Lee, D.; Song, G. Sideroxylin (Callistemon lanceolatus) suppressed cell proliferation and increased apoptosis in ovarian cancer cells accompanied by mitochondrial dysfunction, the generation of reactive oxygen species, and an increase of lipid peroxidation. J. Cell Physiol. 2018, 233, 8597–8604. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.K.; Karmakar, S.; Naglot, A.; Aich, J.C.; Begam, M. Anticandidial activity of some essential oils of a mega biodiversity hotspot in India. Mycose 2007, 50, 121–124. [Google Scholar] [CrossRef]
- El-Ansary, A.; Sammour, E.M.; Soliman, M.S.; Gawish, F.A. In vivo, attenuation of schistosome cercarial development and disturbance of egg laying capacity in Biomphalaria alexandrina using sublethal concentrations of plant molluscicides. J. Egypt Soc. Parasitol. 2001, 31, 657–669. [Google Scholar]
- Chistokhodova, N.; Nguyen, C.; Calvino, T.; Kachirskaia, I.; Cunningham, G.; Howard Miles, D. Antithrombin activity of medicinal plants from central Florida. J. Ethnopharmacol. 2002, 81, 277–280. [Google Scholar] [CrossRef]
- Park, S.Y.; Lim, J.Y.; Jeong, W.; Hong, S.S.; Yang, Y.T.; Hwang, B.Y.; Lee, D. C-methylflavonoids isolated from Callistemon lanceolatus protect PC12 cells against Abeta-induced toxicity. Planta Med. 2010, 76, 863–868. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Prakash, O. Anti-diabetic hypolipidemic, and antioxidant activities of Callistemon lanceolatus leaves extract. J. Herbs Spices Med. Plants. 2011, 17, 144–153. [Google Scholar] [CrossRef]
- Nazreen, S.; Kaur, G.; Alam, M.M.; Shafi, S.; Hamid, H.; Ali, M.; Alam, M.S. New flavones with antidiabetic activity from Callistemon lanceolatus DC. Fitoterapia 2012, 83, 1623–1627. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.; Wen, H. Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Sharma, U.K.; Sharma, A.K.; Pandey, A.K. Medicinal attributes of major phenylpropanoids present in cinnamon. BMC Complement. Altern Med. 2016, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Chidambara, M.K.N.; Jayapakash, G.K. Studies on the antioxidant activity of Pomegranate (Punicagranatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. JAP J. Nut. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, S.; Bhargava, A.; Sharma, B.; Pandey, A.K. Studies on in vitro antioxidant and antistaphylococcal activites of some important medicinal plants. Cell Mol. Biol. 2011, 57, 16–25. [Google Scholar] [PubMed]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivates (acetaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Kondhare, D.; Lade, H. Phytochemical profile, aldose reductase inhibitory, and antioxidant activities of Indian traditional medicinal Cocciniagrandis (L.) fruit extract. 3Biotech 2017, 7, 378. [Google Scholar]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant and anticancer activities. BioMed Res. Int. 2013, 2013, 915436. [Google Scholar] [CrossRef]
- Mahmoud, I.I.; Moharram, F.A.; Marzouk, M.S.; Linscheid, M.W.; Saleh, M.I. Polyphenolic constituents of Callistemon lanceolatus leaves. Pharmazie 2002, 57, 494–496. [Google Scholar]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K.; Bhargav, V.K. Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J. Food Sci. Technol. 2014, 51, 2568–2575. [Google Scholar] [CrossRef]
- Kumar, S.; Mishra, A.; Pandey, A.K. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement. Altern Med. 2013, 13, 120. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Medicinal attributes of Solanum xanthocarpum fruit consumed by several tribal communities as food: An in vitro antioxidant, anticancer and anti HIV perspective. BMC Complement Altern Med. 2014, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Jamzad, M.; Kazembakloo, A.; Tehrani, A.D.; Rostami, F. Essential oil composition and antioxidant activity of hydromethanolic extract from the flowers, leaves and stems of Callistemon citrinus (Curtis) Skeels. Ind. J. Nat. Prod. Res. 2014, 5, 308–312. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Chidinma, A.O.; Vincent, C.E.; Chukwuka, C.O.; Ihechiluru, E.I.; Daniel, D.N.; Augustine, N.O. Hexane extract of dacryodesedulis fruits possesses anti-diabetic and hypolipidaemic potentials in alloxan diabetes of rats. Afr. J. Trad Complement. Alt. Med. 2016, 13, 132–144. [Google Scholar]
- Wang, J.J.; Jin, H.; Zheng, S.L.; Xia, P.; Cai, Y.; Ni, X.J. Phytoecdysteroids from Ajugaiva act as potential antidiabetic agent against alloxan-induced diabetic male albino rats. Biomed. Pharmacother. 2017, 96, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Sadat, A.; Sayem, M.; Arya, A.; Karimian, H.; Krishnasamy, N.; Hasamnis, A.A.; Hossain, C.F. Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules 2018, 23, 258. [Google Scholar]
- Joshi, T.; Singh, A.K.; Haratipour, P.; Sah, A.N.; Pandey, A.K.; Naseri, R.; Juyal, V.; Farzaei, M.H. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J. Cell Physiol. 2019, 27, 1–20. [Google Scholar] [CrossRef]
- Vitek, L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol. 2012, 3, 55. [Google Scholar] [CrossRef]
- Yasuda, M.; Kiyohara, Y.; Wang, J.J.; Arakawa, S.; Yonemoto, K.; Doi, Y.; Ninomiya, T.; Ishibashi, T. High serum bilirubin levels and diabetic retinopathy: The Hisayama Study. Ophthalmology 2011, 118, 1423–1428. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, S.; Chashoo, G.; Saxena, A.K.; Pandey, A.K. Cell cycle inhibitory activity of Piper longum against A549 cell line and its protective effect against metal-induced toxicity in rats. Ind. J. Biochem. Biophys. 2014, 51, 358–364. [Google Scholar]
- Sharma, U.K.; Kumar, R.; Gupta, A.; Ganguly, R.; Singh, A.K.; Ojha, A.K.; Pandey, A.K. Ameliorating efficacy of eugenol against metanil yellow induced toxicity in albino Wistar rats. Food Chem. Toxicol. 2019, 126, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Larcan, A.; Lambert, A.; Laprevote-Heully, M.C.; Delorme, N. Light and electron microscopic study of hepatic lesions in the course of hyperlactatemia in diabetic patients. Diabete Metab. 1979, 5, 103–112. [Google Scholar] [PubMed]
- Lucchesi, A.N.; Cassettari, L.L.; Spadella, C.T. Alloxan-induced diabetes causes morphological and ultrastructural changes in rat liver that resemble the natural history of chronic fatty liver disease in humans. J. Diabetes Res. 2015, 2015, 494578. [Google Scholar] [CrossRef]
- Huq, F.; Misra, L.N. An alkenol and C-methylated flavones from Callistemonlanceolatusleaves. Planta Med. 1997, 63, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Hong, S.S.; Kim, N.; Yang, Y.T.; Shin, Y.S.; Lee, C.; Hwang, B.Y.; Lee, D. Bioactive triterpenoids from Callistemon lanceolatus. Arch Pharm Res. 2009, 32, 845–849. [Google Scholar] [CrossRef] [PubMed]



| Phytochemicals | Extract | Quantitative Analysis | ||||
|---|---|---|---|---|---|---|
| Meth | AQ | |||||
| Tannins | + | + | Total Phenols (µgPGE/mg) | Total Flavonoids (µgQE/mg) | ||
| Flavonoids | + | + | ||||
| Terpenoids | + | + | AQ | Meth | AQ | Meth |
| Cardiac glycosides | + | + | ||||
| Anthraquinones | - | - | 72.27 ± 0.55 | 227.96 ± 0.17 * | 10.13 ± 0.27 | 10.49 ± 0.42 |
| Reducing sugars | + | - | ||||
| Phlobatannins | + | + | ||||
| Saponins | + | + | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Gupta, A.; Singh, A.K.; Bishayee, A.; Pandey, A.K. The Antioxidant and Antihyperglycemic Activities of Bottlebrush Plant (Callistemon lanceolatus) Stem Extracts. Medicines 2020, 7, 11. https://doi.org/10.3390/medicines7030011
Kumar R, Gupta A, Singh AK, Bishayee A, Pandey AK. The Antioxidant and Antihyperglycemic Activities of Bottlebrush Plant (Callistemon lanceolatus) Stem Extracts. Medicines. 2020; 7(3):11. https://doi.org/10.3390/medicines7030011
Chicago/Turabian StyleKumar, Ramesh, Ashutosh Gupta, Amit Kumar Singh, Anupam Bishayee, and Abhay K. Pandey. 2020. "The Antioxidant and Antihyperglycemic Activities of Bottlebrush Plant (Callistemon lanceolatus) Stem Extracts" Medicines 7, no. 3: 11. https://doi.org/10.3390/medicines7030011
APA StyleKumar, R., Gupta, A., Singh, A. K., Bishayee, A., & Pandey, A. K. (2020). The Antioxidant and Antihyperglycemic Activities of Bottlebrush Plant (Callistemon lanceolatus) Stem Extracts. Medicines, 7(3), 11. https://doi.org/10.3390/medicines7030011