Cytotoxic Tumour-Selective 1,5-Diaryl-3-Oxo-1,4-Pentadienes Mounted on a Piperidine Ring
Abstract
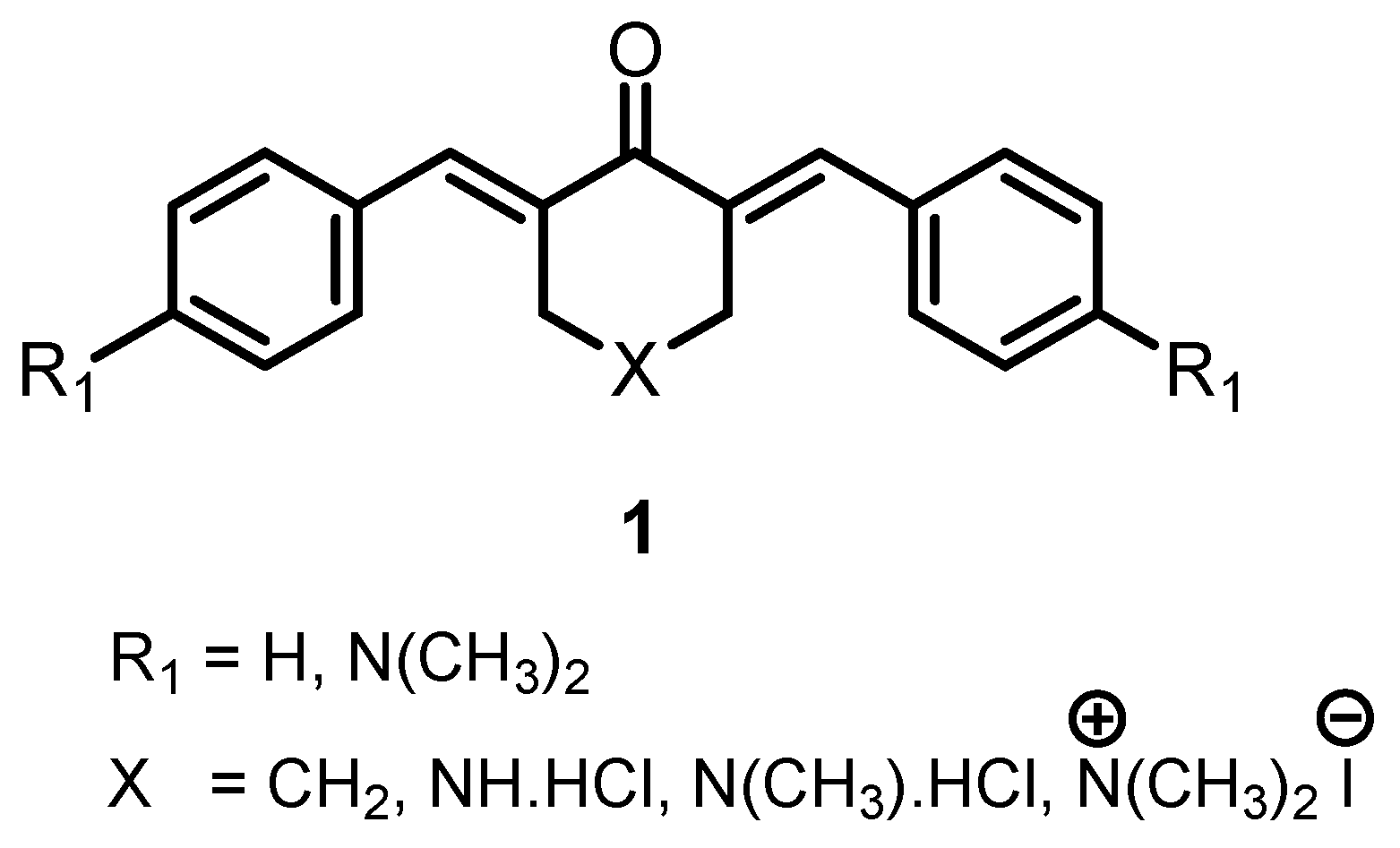
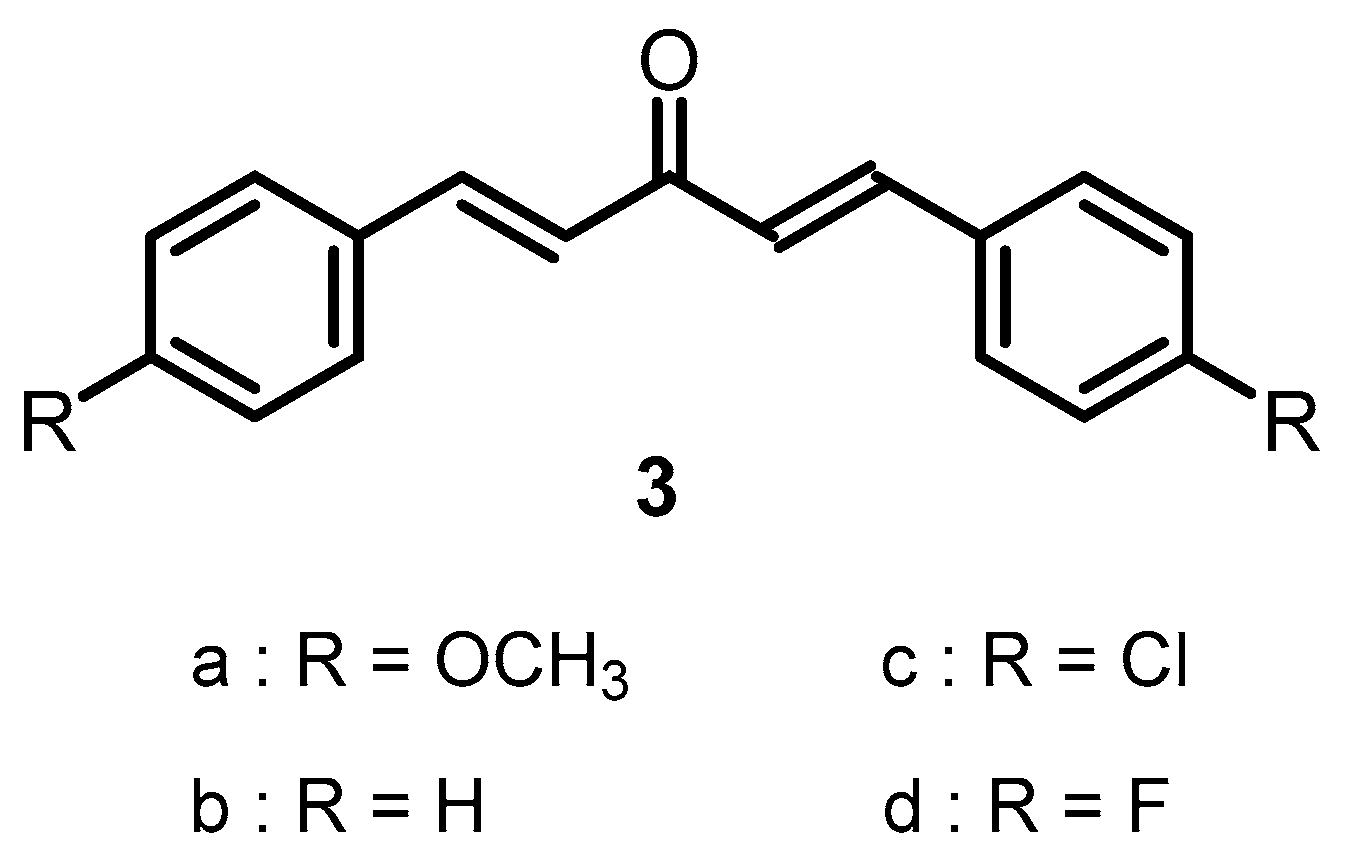
:1. Introduction
2. Materials and Methods
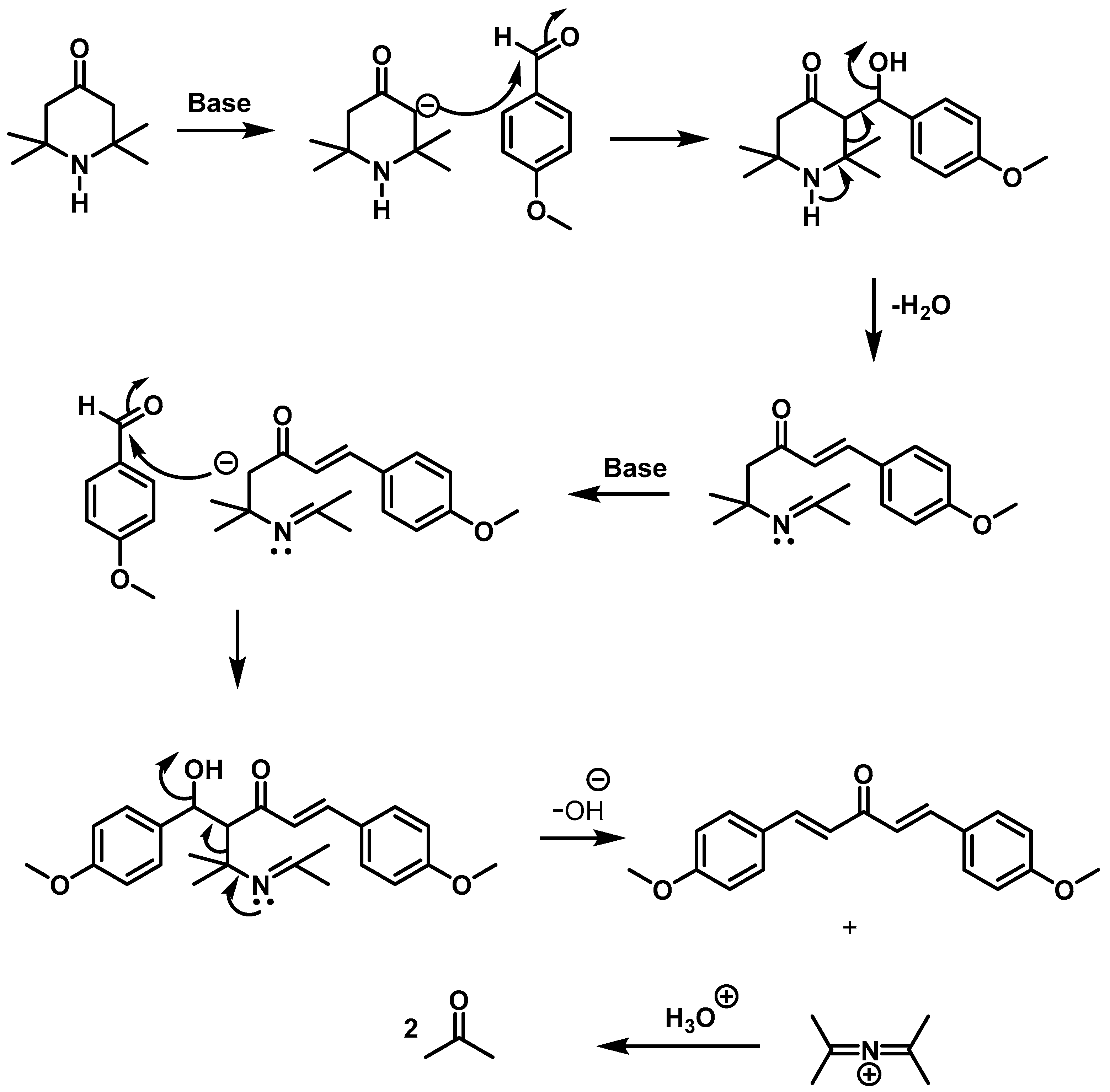
2.1. Synthesis of Compounds
2.2. QSAR Studies
2.3. Cytotoxicity Assays
3. Results
4. Discussion
| Human Oral Squamous Cell Carcinoma Cell Lines | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca9-22 | HSC-2 | HSC-4 | Average | ||||||
| Compound | R | CC50 (µM) a | SI b | CC50 (µM) a | SI b | CC50 (µM) a | SI b | CC50 (µM) a | SI b |
| 2a c | H | 0.27 ± 0.03 | 58.1 | 0.31 ± 0.05 | 50.6 | 0.41 ± 0.13 | 38.3 | 0.33 ± 0.07 | 49.0 |
| 2b | 2-OCH3 | 0.13 ± 0.02 | 40.3 | 0.33 ± 0.09 | 15.9 | 0.50 ± 0.04 | 10.5 | 0.32 ± 0.18 | 22.2 |
| 2c d | 3-OCH3 | 0.10 ± 0.03 | 18.2 | 0.02 ± 0.04 | 91.0 | 0.12 ± 0.02 | 15.2 | 0.08 ± 0.01 | 41.5 |
| 2d e | 4-OCH3 | 3.13 ± 0.40 | 17.8 | 7.97 ± 0.68 | 6.98 | 6.86 ± 0.77 | 8.10 | 5.99 ± 2.53 | 11.0 |
| 2e e | 3,4-(OCH3)2 | 0.02 ± 0.01 | 136 | 0.24 ± 0.04 | 11.3 | 0.07 ± 0.00 | 38.9 | 0.11 ± 0.11 | 62.1 |
| 2f d | 2,5-(OCH3)2 | 0.06 ± 0.01 | 23.3 | 0.17 ± 0.07 | 8.23 | 0.18 ± 0.08 | 7.78 | 0.14± 0.07 | 13.1 |
| 2g e | 2,4,6-(OCH3)3 | 6.90 ± 0.30 | 5.00 | 7.95 ± 0.70 | 4.34 | 8.93 ± 0.75 | 3.86 | 7.93 ± 1.08 | 4.40 |
| 2h e | 3,4,5-(OCH3)3 | 0.04 ± 0.01 | 24.3 | 0.08 ± 0.01 | 12.1 | 0.11 ± 0.03 | 8.82 | 0.08 ± 0.04 | 15.1 |
| 2i d | 3,4-OCH2O | 0.16 ± 0.07 | 102 | 0.53 ± 0.12 | 30.8 | 0.71 ± 0.22 | 23.0 | 0.47 ± 0.28 | 51.9 |
| 2j e | 2-F | 0.16 ± 0.04 | 21.4 | 0.33± 0.23 | 10.4 | 0.23 ± 0.01 | 14.9 | 0.24 ± 0.09 | 15.6 |
| 2k | 3-F | 0.07 ± 0.01 | 26.3 | 0.13 ± 0.03 | 14.2 | 0.12 ± 0.05 | 15.3 | 0.11 ± 0.03 | 18.6 |
| 2l c | 4-F | 0.14 ± 0.06 | 32.9 | 0.94 ± 1.28 | 4.90 | 0.16 ± 0.02 | 28.8 | 0.41± 0.45 | 22.2 |
| 2m | 3,4-F2 | 0.06 ± 0.03 | 41.2 | 0.14 ± 0.05 | 17.6 | 0.07 ± 0.01 | 35.3 | 0.09 ± 0.04 | 31.4 |
| 2n | 2,6-F2 | 0.17 ± 0.02 | 24.5 | 0.40 ± 0.10 | 10.4 | 0.31 ± 0.21 | 13.5 | 0.29 ± 0.11 | 16.1 |
| 2o | 2-CH3 | 0.45 ± 0.04 | 26.4 | 0.79 ± 0.19 | 15.1 | 1.05 ± 0.34 | 11.3 | 0.76 ± 0.30 | 17.6 |
| 2p | 2-NO2 | 0.13 ± 0.01 | 20.8 | 0.31 ± 0.06 | 8.71 | 0.30 ± 0.15 | 9.00 | 0.25 ± 0.10 | 12.8 |
| 2q | 3-OCH3, 4-OH | 0.61 ± 0.23 | 13.4 | 0.61± 0.08 | 13.4 | 0.63 ± 0.16 | 13.0 | 0.62 ± 0.01 | 13.3 |
| 2r | 3-OH, 4-OCH3 | 0.23 ± 0.01 | 214 | 0.29 ± 0.06 | 170 | 0.31 ± 0.12 | 159 | 0.28 ± 0.04 | 181 |
| 2s e | 4-OH | 1.67 ± 0.13 | 54.1 | 2.52 ± 0.19 | 35.9 | 3.20 ± 0.58 | 28.3 | 2.46 ± 0.77 | 39.4 |
| 2t d | 3-OH | 0.18 ± 0.01 | 28.2 | 0.21 ± 0.01 | 24.1 | 0.20 ± 0.04 | 25.4 | 0.20 ± 0.01 | 25.9 |
| 2u | 2-Cl | 0.10 ± 0.04 | 49.6 | 0.16 ± 0.02 | 31.0 | 0.25 ± 0.12 | 19.8 | 0.17 ± 0.08 | 33.5 |
| Doxorubicin | 0.24 ± 0.04 | 31.5 | 0.07 ± 0.00 | 108 | 0.08 ± 0.01 | 94.5 | 0.13 ± 0.10 | 78.0 | |
| Melphalan | 27.4 ± 6.40 | 6.30 | 13.9 ± 3.80 | 12.4 | 14.4 ± 1.70 | 12.0 | 18.6 ± 7.63 | 10.2 | |
| Human Normal Oral Cell Lines | ||||||
|---|---|---|---|---|---|---|
| HGF | HPLF | HPC | Average | PSE b | ||
| Compound | R | CC50 (µM) a | CC50 (µM) a | CC50 (µM) a | CC50 (µM) a | |
| 2a c | H | 7.78 ± 0.85 | 17.0 ± 2.29 | 22.3 ± 2.61 | 15.7 ± 7.33 | 14,849 |
| 2b | 2-OCH3 | 2.11 ± 0.13 | 4.84 ± 3.52 | 8.77 ± 1.58 | 5.24 ± 3.35 | 6938 |
| 2c d | 3-OCH3 | 2.07 ± 0.11 | 2.47 ± 0.07 | 0.91 ± 0.08 | 1.82 ± 0.81 | 51,875 |
| 2d e | 4-OCH3 | 38.1 ± 12.4 | >100 | 28.8 ± 0.52 | >55.6 ± 38.7 | >184 |
| 2e e | 3,4-(OCH3)2 | 2.06 ± 0.16 | 2.48 ± 0.28 | 3.61 ± 2.50 | 2.72 ± 0.80 | 56,455 |
| 2f d | 2,5-(OCH3)2 | 1.29 ± 0.60 | 2.01 ± 0.27 | 0.91 ± 0.02 | 1.40 ± 0.56 | 9357 |
| 2g e | 2,4,6-(OCH3)3 | 33.9 ± 8.27 | 48.0 ± 10.4 | 21.7 ± 1.72 | 34.5 ± 13.2 | 56 |
| 2h e | 3,4,5-(OCH3)3 | 1.13 ± 0.79 | 1.28 ± 0.71 | 0.51 ± 0.20 | 0.97 ± 0.40 | 18,875 |
| 2i d | 3,4-OCH2O | 6.35 ± 0.12 | 7.91 ± 0.19 | 34.7 ± 12.2 | 16.3 ± 15.9 | 11,043 |
| 2j e | 2-F | 4.40 ± 1.32 | 3.63 ± 0.57 | 2.24 ± 1.17 | 3.42 ± 1.09 | 6500 |
| 2k | 3-F | 2.05 ± 0.16 | 2.70 ± 0.12 | 0.78 ± 0.16 | 1.84 ± 0.98 | 16,909 |
| 2l c | 4-F | 4.22 ± 1.31 | 7.07 ± 0.47 | 2.54 ± 0.14 | 4.61 ± 2.29 | 5415 |
| 2m | 3,4-F2 | 1.68 ± 0.44 | 3.69 ± 0.64 | 2.04 ± 0.97 | 2.47 ± 1.07 | 34,889 |
| 2n | 2,6-F2 | 2.07 ± 0.30 | 7.46 ± 0.38 | 2.97 ± 0.05 | 4.17 ± 2.89 | 5552 |
| 2o | 2-CH3 | 5.58 ± 0.18 | 7.27 ± 0.21 | 22.8 ± 1.85 | 11.9 ± 9.51 | 2316 |
| 2p | 2-NO2 | 1.71 ± 0.23 | 2.57 ± 0.07 | 3.82 ± 1.14 | 2.70 ± 1.06 | 5120 |
| 2q | 3-OCH3, 4-OH | 5.76 ± 1.20 | 10.8 ± 1.10 | 8.08 ± 8.51 | 8.21 ± 2.50 | 2145 |
| 2r | 3-OH, 4-OCH3 | 2.72 ± 0.18 | 54.6 ± 39.1 | 90.7 ± 16.2 | 49.3 ± 44.2 | 64,643 |
| 2s e | 4-OH | 74.0 ± 6.56 | >100 | 97.3 ± 4.62 | >90.4 ± 14.3 | >1602 |
| 2t d | 3-OH | 2.23 ± 0.06 | 3.45 ± 0.30 | 9.53 ± 5.17 | 5.07 ± 3.91 | 12,950 |
| 2u | 2-Cl | 2.25 ± 0.15 | 5.07 ± 1.99 | 7.55 ± 1.62 | 4.96 ± 2.65 | 19,706 |
| Doxorubicin | 2.69 ± 0.35 | >10.0 | >10 ± 0.00 | >7.56 ± 4.22 | >60,000 | |
| Melphalan | 148.0 ± 0.68 | >200 | 169 ± 18.5 | >172 ± 25.8 | >55 | |
| Table | Bioevaluations | Promising Compounds |
|---|---|---|
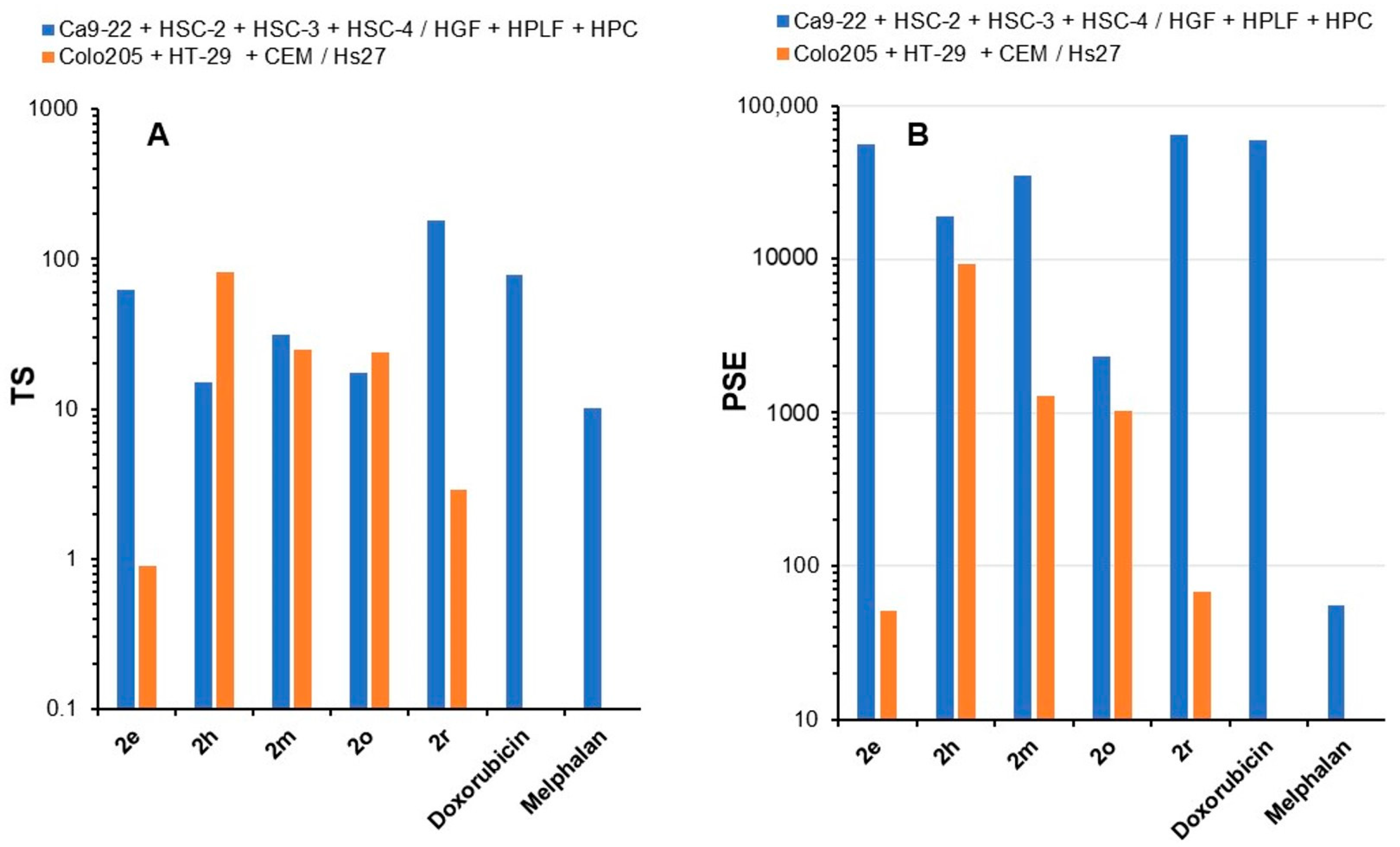
| 1 | Average CC50 values | 2c, h, m |
| 1 | Average SI figures | 2e, i, r |
| 2 | Low toxicity to normal cells | 2d, g, r, s |
| 2 | PSE | 2c, e, m, r |
| Human Tumour Cell Lines | Normal Cell Line | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Colo205 | HT-29 | CEM | Average | Hs27 | |||||||
| Compound | R | CC50 (µM) a | SI b | CC50 (µM) a | SI b | CC50 (µM) a | SI b | CC50 a | SI b | CC50 (µM) a | PSE c |
| 2a d | H | 10.7 ± 0.05 | 1.54 | 1.57 ± 0.21 | 10.5 | 9.29 ± 0.11 | 1.78 | 7.19 | 4.61 | 16.5 ± 0.35 | 64 |
| 2b | 2-OCH3 | 1.32 ± 0.01 | 3.10 | 3.58 ± 0.21 | 1.14 | 0.82 ± 0.09 | 4.99 | 1.91 | 3.08 | 4.09 ± 0.56 | 161 |
| 2c e | 3-OCH3 | 0.32 ± 0.11 | 13.0 | 5.58 ± 0.52 | 0.75 | 4.51 ± 0.11 | 0.92 | 3.47 | 4.89 | 4.16 ± 0.32 | 141 |
| 2d f | 4-OCH3 | 14.0 ± 0.56 | 6.84 | 12.6 ± 0.00 | 7.60 | 4.45 ± 0.03 | 21.5 | 10.4 | 12.0 | 95.7 ± 0.11 | 115 |
| 2e f | 3,4-(OCH3)2 | 1.36 ± 0.17 | 0.65 | 3.46 ± 0.46 | 0.25 | 0.49 ± 0.02 | 1.80 | 1.77 | 0.90 | 0.88 ± 0.05 | 51 |
| 2f e | 2,5-(OCH3)2 | 0.36 ± 0.01 | 1.81 | 0.84 ± 0.01 | 0.77 | 0.42 ± 0.02 | 1.55 | 0.54 | 1.37 | 0.65± 0.02 | 254 |
| 2g f | 2,4,6-(OCH3)3 | 16.9± 0.23 | 1.63 | 9.63 ± 0.32 | 2.87 | 11.0 ± 1.06 | 2.51 | 12.5 | 2.34 | 27.6 ± 0.79 | 19 |
| 2h f | 3,4,5-(OCH3)3 | 0.27 ± 0.03 | 121 | 2.03 ± 0.00 | 16.1 | 0.31 ± 0.09 | 106 | 0.87 | 81.0 | 32.7 ± 0.56 | 9310 |
| 2i e | 3,4-OCH2O | 8.84 ± 0.08 | 2.17 | 7.42± 1.33 | 2.59 | 1.88 ± 0.08 | 10.2 | 6.05 | 4.99 | 19.2 ± 2.38 | 83 |
| 2j f | 2-F | 2.94 ± 0.33 | 0.68 | 3.46 ± 0.33 | 0.58 | 0.78 ± 0.04 | 2.55 | 2.39 | 1.27 | 1.99 ± 0.17 | 53 |
| 2k | 3-F | 4.19 ± 0.15 | 0.44 | 1.73 ± 0.15 | 1.06 | 0.21 ± 0.01 | 8.76 | 2.04 | 3.42 | 1.84 ± 0.32 | 168 |
| 2l d | 4-F | 1.56 ± 0.28 | 1.21 | 5.51 ± 0.56 | 0.34 | 1.68 ± 0.25 | 1.13 | 2.92 | 0.89 | 1.89 ± 0.05 | 31 |
| 2m | 3,4-F2 | 1.81 ± 0.31 | 16.1 | 3.50 ± 0.19 | 8.34 | 0.58 ± 0.05 | 50.3 | 1.96 | 24.9 | 29.2 ± 0.08 | 1270 |
| 2n | 2,6-F2 | 2.22 ± 0.00 | 3.50 | 7.36 ± 0.88 | 1.06 | 7.88 ± 0.68 | 0.99 | 5.82 | 1.85 | 7.77 ± 0.89 | 32 |
| 2o | 2-CH3 | 1.62 ± 0.11 | 30.4 | 3.54 ± 0.42 | 13.9 | 1.82 ± 0.10 | 27.1 | 2.33 | 23.8 | 49.3 ± 0.23 | 1022 |
| 2p | 2-NO2 | 2.09 ± 0.33 | 11.6 | 2.76 ± 0.04 | 8.80 | 6.14 ± 0.56 | 3.96 | 3.66 | 8.12 | 24.3 ± 0.11 | 222 |
| 2q | 3-OCH3, 4-OH | 1.73 ± 0.15 | 1.20 | 17.8 ± 1.48 | 0.12 | 1.92 ± 0.53 | 1.08 | 7.15 | 0.80 | 2.07 ± 0.07 | 11 |
| 2r | 3-OH, 4-OCH3 | 4.58 ± 0.17 | 2.73 | 4.26 ± 0.01 | 2.93 | 4.04 ± 0.17 | 3.09 | 4.29 | 2.92 | 12.5 ± 1.61 | 68 |
| 2s f | 4-OH | 4.17 ± 0.32 | 2.08 | 19.3 ± 0.48 | 0.45 | 1.99 ± 0.10 | 4.36 | 8.49 | 2.30 | 8.68 ± 0.16 | 27 |
| 2t e | 3-OH | 2.12 ± 0.12 | 0.64 | 4.18 ± 0.36 | 0.32 | 0.43 ± 0.04 | 3.14 | 2.24 | 1.37 | 1.35 ± 0.08 | 61 |
| 2u | 2-Cl | 8.19 ± 0.17 | 0.08 | 0.89 ± 0.07 | 0.69 | 4.42 ± 0.35 | 0.14 | 4.50 | 0.30 | 0.61 ± 0.01 | 7 |
| Bioevaluations | Promising Compounds |
|---|---|
| Average CC50 values | 2f, h |
| Average SI values | 2d, h, m, o |
| Low toxicity to normal cells | 2d, g, h, m, o |
| PSE | 2h, m, o |
| Compound | Distribution (%) a | |||
|---|---|---|---|---|
| SubG1 | G1 | S | G2/M | |
| 2e (0.3 µM) | 2.2 | 33.1 | 20.5 | 43.7 |
| 2e (1 µM) | 9.3 * | 26.1 | 14.3 | 50.2 |
| 2r (1 µM) | 11.5 * | 18.5 | 12.0 | 57.9 |
| 2r (3 µM) | 24.5 * | 35.0 | 11.4 | 29.1 |
| AD (1µM) | 15.6 * | 50.9 | 11.5 | 22.1 |
| Control | 1.7 | 33.3 | 17.0 | 47.7 |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Xin, W.Y.; Yao, B.R.; Wang, C.H.; Cong, W.; Zhao, F.; Li, H.J.; Hou, Y.; Meng, Q.G.; Hou, G.G. Novel dissymmetric 3,5-bis(arylidene)-4-piperidones as potential antitumor agents with biological evaluation in vitro and in vivo. Eur. J. Med. Chem. 2018, 147, 21–33. [Google Scholar] [CrossRef]
- Yousif, M.N.M.; Nassar, I.F.; Yousif, N.M.; Awad, H.M.; El-Sayed, W.A. Synthesis and anticancer activity of new substituted piperidinones linked to pyrimidine, thiazole, and triazole glycoside derivatives. Russ. J. Gen. Chem. 2019, 89, 1673–1682. [Google Scholar] [CrossRef]
- Soliman, H.A.; Yousif, M.N.M.; Said, M.M.; Hassan, N.A.; Ali, M.M.; Award, H.M.; Abdel-Megeid, F.M.E. Synthesis of novel 1,6-naphthyridines, pyrano[3,2-c]pyridines and pyrido[4,3-d]pyrimidines derived from 2,2,6,6-tetramethylpiperidin-4-one for in vitro anticancer and antioxidant evaluation. Der Pharma Chem. 2014, 6, 394–410. [Google Scholar]
- Kudo, C.; Yamakoshi, H.; Sato, A.; Nanjo, H.; Ohori, H.; Ishioka, C.; Iwabuchi, Y.; Shibata, H. Synthesis of 86 species of 1,5-diaryl-3-oxo1,4-pentadienes analogs of curcumin can yield a good lead in vivo. BMC Pharmacol. 2011, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Pati, H.N.; Das, U.; Quail, J.W.; Kawase, M.; Sakagami, H.; Dimmock, J.R. Cytotoxic 3,5-bis(benzylidene)piperidin-4-ones and N-acyl analogs displaying selective toxicity for malignant cells. Eur. J. Med. Chem. 2008, 43, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.S.; Michel, D.; Das, U.; Dimmock, J.R.; Alcorn, J. Cytotoxic 1,5-diaryl-3-oxo-1,5-pentadienes: An assessment and comparison of membrane permeability using Caco-2 and MDCK monolayers. Bioorganic Med. Chem. Lett. 2014, 24, 5199–5202. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.; Dandavati, A.; Lee, M.; Tzou, S.; Savagian, M.; Brien, K.A.; Satam, V.; Patil, P.; Lee, M. Synthesis, cytotoxicity, and structure-activity insight of N-H and N-methyl-3,5-bis-(arylidenyl)-4-piperidones. Med. Chem. Res. 2013, 22, 5588–5597. [Google Scholar] [CrossRef]
- Fawzy, I.M.; Youssef, K.M.; Ismail, N.S.; Gullbo, J.; Abouzid, K.A. Newly designed and synthesized curcumin analogs with in vitro cytotoxicity and tubulin polymerization activity. Chem. Biol. Drug Des. 2015, 86, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Eryanti, Y.; Hendra, R.; Herlina, T.; Zamri, A.; Supratman, U. Synthesis of curcumin analogue, N-H and N-benzil-4-piperidone and their cytotoxic activity. Procedia Chem. 2015, 17, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.; Das, S.; Das, U.; Doroudi, A.; Zhu, J.; Dimmock, J.R. Novel hybrid molecules of 3,5-bis(benzylidene)-4-piperidones and dichloroacetic acid which demonstrate potent tumour-selective cytotoxicity. Bioorganic Med. Chem. Lett. 2020, 30, 126878. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Watanabe, T.; Hoshino, T.; Suda, N.; Mori, K.; Yasui, T.; Yamauchi, N.; Kashiwagi, H.; Gomi, T.; Oizumi, T.; et al. Recent progress of basic studies of natural products and their dental application. Medicines 2019, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakagami, H.; Tomomura, M. Dental application of natural products. Medicines 2018, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Maydt, D.; De Spirt, S.; Muschelknautz, C.; Stahl, W.; Muller, T.J.J. Chemical and biological activity of chalcones and other α,β-unsaturated carbonyl compounds. Xenobiotica 2013, 43, 711–718. [Google Scholar] [CrossRef]
- Mutus, B.; Wagner, J.D.; Talpas, C.J.; Dimmock, J.R.; Phillips, O.A.; Reid, R.S. 1-p-Chlorophenyl-4,4-dimethyl-5-diethylamino-1-penten-3-one hydrobromide, a sulfyhydryl-specific compound which reacts irreversibly with protein thiols but reversibly with small molecular weight thiols. Anal. Biochem. 1989, 177, 237–243. [Google Scholar] [CrossRef]
- Xu, Y.; Kalyanaraman, B. Synthesis and ESR studies of novel cyclic nitrone spin trap attached to a phosphonium group—A suitable trap for mitochondria-generated ROS? Free Radic. Res. 2007, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Padmanilayam, M.P.; Puthucode, R.N.; Nazarali, A.J.; Motaganahalli, N.L.; Zello, G.A.; Quail, J.W.; Oloo, E.O.; Kraatz, H.B.; Prisciak, J.S. A conformational and structure-activity relationship study of cytotoxic 3,5-bis(arylidene)-4-piperidones and related N-acryloyl analogues. J. Med. Chem. 2001, 44, 586–593. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.J. Substituent Constants for Correlation Analysis in Chemistry and Biology; John Wiley and Sons: New York, NY, USA, 1979; p. 49. [Google Scholar]
- Taft, R.W., Jr. Separation of polar, steric and resonance effects in reactivity. In Steric Effects in Organic Chemistry; Newman, M.S., Ed.; John Wiley and Sons: New York, NY, USA, 1956; p. 591. [Google Scholar]
- Structural Package for Social Sciences, SPSS for Windows; Release 26.0; SPSS: Chicago, IL, USA, 2008.
- Takao, K.; Hoshi, K.; Sakagami, H.; Shi, H.; Bandow, K.; Nagai, J.; Uesawa, Y.; Tomomura, A.; Tomomura, M.; Sugita, Y. Further quantitative structure-cytotoxicity relationship analysis of 3-styrylchromones. Anticancer Res. 2020, 40, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Sakagami, H.; Okudaira, N.; Masuda, Y.; Amano, O.; Yokose, S.; Kanda, Y.; Suguro, M.; Natori, T.; Oizumi, H.; Oizumi, T. Induction of apoptosis in human oral keratinocyte by doxorubicin. Anticancer Res. 2017, 37, 1023–1029. [Google Scholar]
- Sugita, Y.; Takao, K.; Uesawa, Y.; Nagai, J.; Iijima, Y.; Sano, M.; Sakagami, H. Development of newly synthesized chromone derivatives with high tumor specificity against human oral squamous cell carcinoma. Medicines 2020, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Lema, C.; Varela-Ramirez, A.; Aguilera, R.J. Differential nuclear staining assay for high-throughput screening to identify cytotoxic compounds. Curr. Cell Biochem. 2011, 1, 1–14. [Google Scholar]
- Santiago-Vázquez, Y.; Das, U.; Varela-Ramirez, A.; Baca, S.T.; Ayala-Marin, Y.; Lema, C.; Das, S.; Baryyan, A.; Dimmock, J.R.; Aguilera, R.J. Tumor-selective cytotoxicity of a novel pentadiene analogue on human leukemia/lymphoma cells. Clin. Cancer Drugs 2016, 3, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Varela-Ramirez, A.; Costanzo, M.; Carrasco, Y.P.; Pannell, K.H.; Aguilera, R.J. Cytotoxic effects of two organotin compounds and their mode of inflicting cell death on four mammalian cancer cells. Cell. Biol. Toxicol. 2011, 27, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakagami, H.; Furukawa, T.; Satoh, K.; Amano, S.; Iijima, Y.; Koshikawa, T.; Asai, D.; Fukuchi, K.; Takemura, H.; Kanamoto, T.; et al. Re-evaluation of chemotherapeutic potential of pyoktanin blue. Medicines 2021, 8, 33. [Google Scholar] [CrossRef]
- Yamali, C.; Sakagami, H.; Uesawa, Y.; Kurosaki, K.; Satoh, K.; Masuda, Y.; Yokose, S.; Ece, A.; Bua, S.; Angeli, A.; et al. Comprehensive study on potent and selective carbonic anhydrase inhibitors: Synthesis, bioactivities and molecular modelling studies of 4-(3-(2-arylidenehydrazine-1-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-1-yl) benzenesulfonamides. Eur. J. Med. Chem. 2021, 217, 113351. [Google Scholar] [CrossRef]
- Contreras, L.; Calderon, R.J.; Varela-Ramirez, A.; Zhang, H.-Y.; Quan, Y.; Das, U.; Dimmock, J.R.; Skouta, R.; Aguilera, R.J. Induction of apoptosis via proteasome inhibition in leukemia/lymphoma cells by two potent piperidones. Cell. Oncol. 2018, 41, 628–636. [Google Scholar] [CrossRef]
- Das, S.; Das, U.; Michel, D.; Gorecki, D.K.J.; Dimmock, J.R. Novel bis(arylidene)-4-piperidone dimers: Potent cytotoxins against colon cancer cells. Eur. J. Med. Chem. 2013, 64, 321–328. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Cai, Y.; Wang, J.; Weng, B.; Tang, Q.; Chen, X.; Pan, Z.; Liang, G.; Yang, S. Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogs of curcumin as anti-inflammatory agents. Bioorganic Med. Chem. 2013, 21, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Sheshkin, D. Handbook of Parametric and Nonparametric Statistical Procedures; Chapman and Hall: London, UK, 2004; pp. 1093–1107. [Google Scholar]
- Boudreau, M.W.; Peh, J.; Hergenrother, P.J. Procaspase-3 overexpression in cancer: A paradoxical observation with therapeutic potential. ACS Chem. Biol. 2019, 14, 2335–2348. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Arora, V.K.; Wonko, S.L.; Hamon, N.W.; Quail, J.W.; Jia, Z.; Warrington, R.C.; Fang, W.D.; Lee, J.S. 3,5-bis-benzylidene-4-piperidones and related compounds with high activity towards P388 leukemia cells. Drug Des. Deliv. 1990, 6, 183–194. [Google Scholar] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roayapalley, P.K.; Sakagami, H.; Satoh, K.; Amano, S.; Bandow, K.; Aguilera, R.J.; Hernandez, K.G.C.; Schiaffino Bustamante, A.Y.; Dimmock, S.G.; Sharma, R.K.; et al. Cytotoxic Tumour-Selective 1,5-Diaryl-3-Oxo-1,4-Pentadienes Mounted on a Piperidine Ring. Medicines 2021, 8, 78. https://doi.org/10.3390/medicines8120078
Roayapalley PK, Sakagami H, Satoh K, Amano S, Bandow K, Aguilera RJ, Hernandez KGC, Schiaffino Bustamante AY, Dimmock SG, Sharma RK, et al. Cytotoxic Tumour-Selective 1,5-Diaryl-3-Oxo-1,4-Pentadienes Mounted on a Piperidine Ring. Medicines. 2021; 8(12):78. https://doi.org/10.3390/medicines8120078
Chicago/Turabian StyleRoayapalley, Praveen K., Hiroshi Sakagami, Keitaro Satoh, Shigeru Amano, Kenjiro Bandow, Renato J. Aguilera, Karla G. Cano Hernandez, Austre Y. Schiaffino Bustamante, Stephen G. Dimmock, Rajendra K. Sharma, and et al. 2021. "Cytotoxic Tumour-Selective 1,5-Diaryl-3-Oxo-1,4-Pentadienes Mounted on a Piperidine Ring" Medicines 8, no. 12: 78. https://doi.org/10.3390/medicines8120078
APA StyleRoayapalley, P. K., Sakagami, H., Satoh, K., Amano, S., Bandow, K., Aguilera, R. J., Hernandez, K. G. C., Schiaffino Bustamante, A. Y., Dimmock, S. G., Sharma, R. K., Das, U., & Dimmock, J. R. (2021). Cytotoxic Tumour-Selective 1,5-Diaryl-3-Oxo-1,4-Pentadienes Mounted on a Piperidine Ring. Medicines, 8(12), 78. https://doi.org/10.3390/medicines8120078








