A Predictive Risk Score to Diagnose Adrenal Insufficiency in Outpatients: A 7 Year Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. ACTH Stimulation Test Protocol
2.2. Definitions
2.3. Predictive Variables
2.4. Outcome Variable
2.5. Statistical Analysis
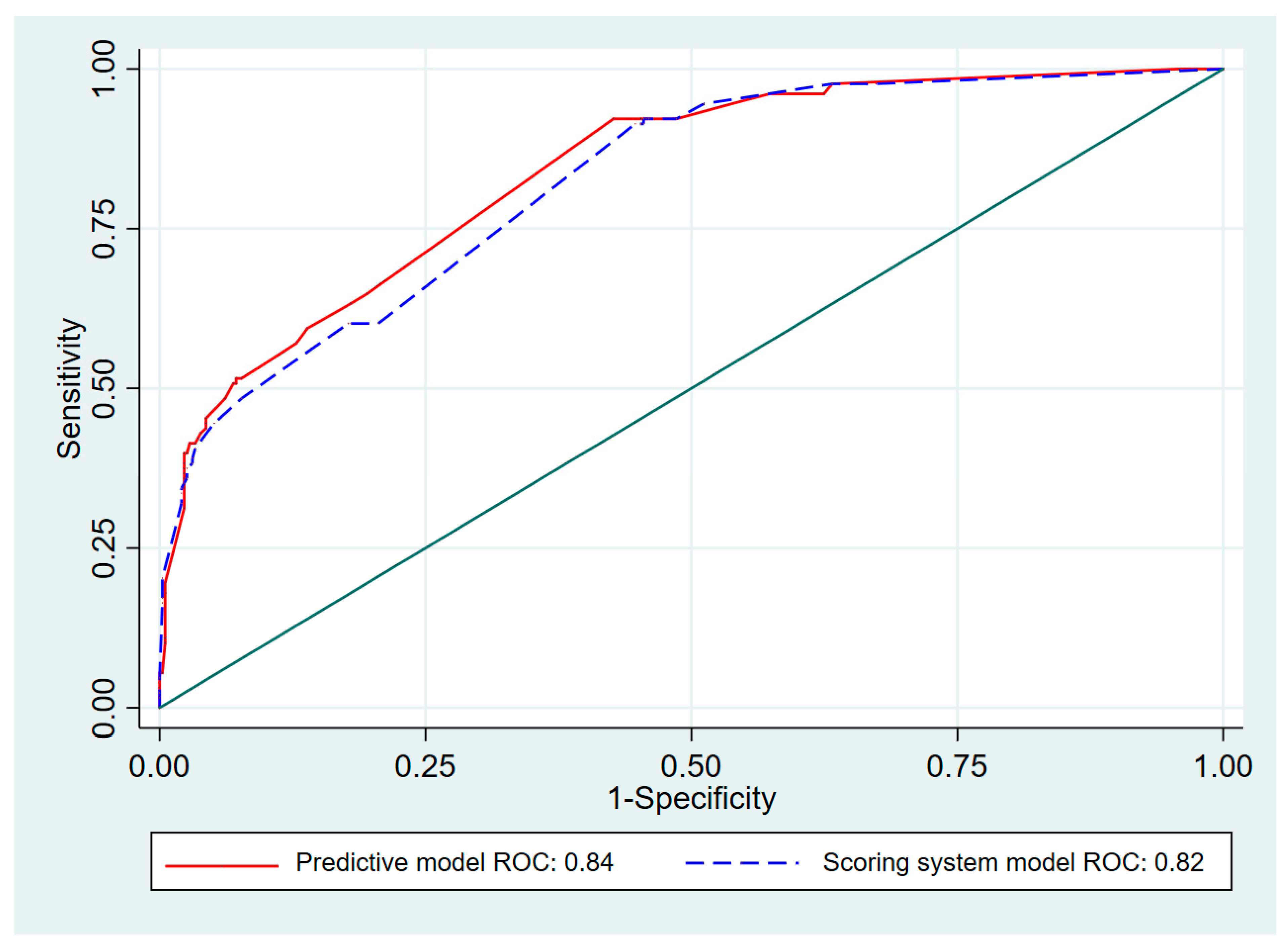
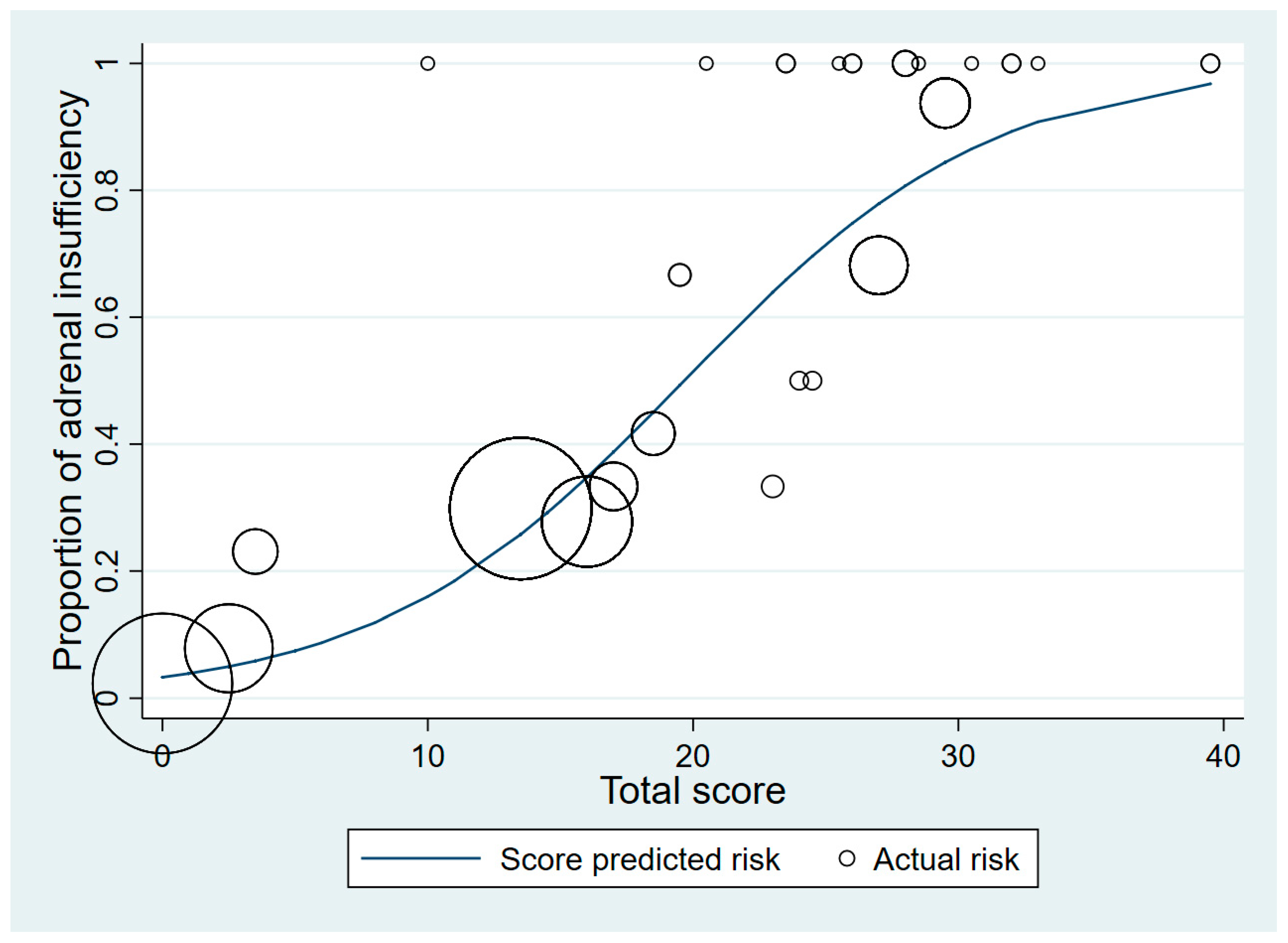
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Betterle, C.; Scarpa, R.; Garelli, S.; Morlin, L.; Lazzarotto, F.; Presotto, F.; Coco, G.; Masiero, S.; Parolo, A.; Albergoni, M.P.; et al. Addison’s disease: A survey on 633 patients in Padova. Eur. J. Endocrinol. 2013, 169, 773–784. [Google Scholar] [CrossRef]
- Erichsen, M.M.; Løvås, K.; Skinningsrud, B.; Wolff, A.B.; Undlien, D.E.; Svartberg, J.; Fougner, K.J.; Berg, T.J.; Bollerslev, J.; Mella, B.; et al. Clinical, Immunological, and Genetic Features of Autoimmune Primary Adrenal Insufficiency: Observations from a Norwegian Registry. J. Clin. Endocrinol. Metab. 2009, 94, 4882–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perton, F.T.; Mijnhout, G.S.; Kollen, B.J.; Rondeel, J.M.; Franken, A.A.; Groeneveld, P.H. Validation of the 1 mug short synacthen test: An assessment of morning cortisol cut-off values and other predictors. Neth. J. Med. 2017, 75, 14–20. [Google Scholar]
- Park, S.H.; Joo, M.S.; Kim, B.H.; Na Yoo, H.; Kim, S.E.; Kim, J.B.; Jang, M.K.; Kim, D.J.; Lee, M.S. Clinical characteristics and prevalence of adrenal insufficiency in hemodynamically stable patients with cirrhosis. Medicine 2018, 97, e11046. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, L.; Noto, D.; Privitera, G.; Tomaselli, T.; Fede, G.; Scicali, R.; Piro, S.; Fayer, F.; Altieri, I.; Averna, M.; et al. Apolipoprotein AI and HDL are reduced in stable cirrhotic patients with adrenal insufficiency: A possible role in glucocorticoid deficiency. Scand. J. Gastroenterol. 2014, 50, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, A.; Mirocha, J.; Gwin, S.M.; Khine, A.K.; Liu, N.-A.; Sheinin, R.C.; Melmed, S. Clinical Factors Associated with Biochemical Adrenal-cortisol Insufficiency in Hospitalized Patients. Am. J. Med. 2014, 127, 754–762. [Google Scholar] [CrossRef] [Green Version]
- Manosroi, W.; Phimphilai, M.; Khorana, J.; Atthakomol, P.; Pipanmekaporn, T. Predictive Factors of Adrenal Insufficiency in Outpatients with Indeterminate Serum Cortisol Levels: A Retrospective Study. Medicine 2020, 56, 23. [Google Scholar] [CrossRef] [Green Version]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-Wauchope, A.; Hammer, G.D.; Husebye, E.S.; Merke, D.P.; Murad, M.H.; Stratakis, C.A.; et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hägg, E.; Asplund, K.; Lithner, F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin. Endocrinol. 1987, 26, 221–226. [Google Scholar] [CrossRef]
- Deutschbein, T.; Unger, N.; Petersenn, S.; Mann, K. Diagnosis of Secondary Adrenal Insufficiency: Unstimulated Early Morning Cortisol in Saliva and Serum in Comparison with the Insulin Tolerance Test. Horm. Metab. Res. 2009, 41, 834–839. [Google Scholar] [CrossRef]
- Manosroi, W.; Phimphilai, M.; Khorana, J.; Atthakomol, P. Diagnostic performance of basal cortisol level at 0900-1300h in adrenal insufficiency. PLoS ONE 2019, 14, e0225255. [Google Scholar] [CrossRef]
- Gaddey, H.L.; Holder, K. Unintentional weight loss in older adults. Am. Fam. Physician 2014, 89, 718–722. [Google Scholar]
- Finken, M.J.J.; Mul, D. Cushing’s syndrome and adrenal insufficiency after intradermal triamcinolone acetonide for keloid scars. Eur. J. Nucl. Med. Mol. Imaging 2010, 169, 1147–1149. [Google Scholar] [CrossRef] [Green Version]
- Böckle, B.C.; Jara, D.; Nindl, W.; Aberer, W.; Sepp, N.T. Adrenal Insufficiency as a Result of Long-Term Misuse of Topical Corticosteroids. Dermatology 2014, 228, 289–293. [Google Scholar] [CrossRef]
- Bancos, I.; Hahner, S.; Tomlinson, J.; Arlt, W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015, 3, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Cuesta, M.; Garrahy, A.; Slattery, D.; Gupta, S.; Hannon, A.M.; Forde, H.; McGurren, K.; Sherlock, M.; Tormey, W.; Thompson, C.J. The contribution of undiagnosed adrenal insufficiency to euvolaemic hyponatraemia: Results of a large prospective single-centre study. Clin. Endocrinol. 2016, 85, 836–844. [Google Scholar] [CrossRef]
- Clodi, M.; Riedl, M.; Schmaldienst, S.; Vychytil, A.; Kotzmann, H.; Kaider, A.; Bieglmayer, C.; Mayer, G.; Waldhäusl, W.; Luger, A. Adrenal function in patients with chronic renal failure. Am. J. Kidney Dis. 1998, 32, 52–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raff, H.; Trivedi, H. Circadian rhythm of salivary cortisol, plasma cortisol, and plasma ACTH in end-stage renal disease. Endocr. Connect. 2013, 2, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rotman-Pikielny, P.; Rouach, V.; Chen, O.; Gur, H.G.; Limor, R.; Stern, N.; Roash, V. Serum Cortisol Levels in Patients Admitted to the Department of Medicine: Prognostic Correlations and Effects of Age, Infection, and Comorbidity. Am. J. Med Sci. 2006, 332, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz-Delgadillo, J.; Monreal-Robles, R.; Villarreal-Pérez, J.Z.; Lavalle-González, F.J.; Maldonado-Garza, H.J.; Bosques-Padilla, F.J. Algorithm for Screening of Adrenal Function in Stable Patients with Cirrhosis. Ann. Hepatol. 2017, 16, 788–796. [Google Scholar] [CrossRef]
- Oboni, J.-B.; Marques-Vidal, P.; Pralong, F.; Waeber, G. Predictive factors of adrenal insufficiency in patients admitted to acute medical wards: A case control study. BMC Endocr. Disord. 2013, 13, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, A.S.; Kemp, E.H.; White, A.; Walker, L.; Meredith, S.; Sachdev, P.; Krone, N.P.; Ross, R.J.; Wright, N.P.; Elder, C.J. International survey on high- and low-dose synacthen test and assessment of accuracy in preparing low-dose synacthen. Clin. Endocrinol. 2018, 88, 744–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Adrenal Insufficiency (n = 125) | Normal Adrenal Response (n = 382) | p-Value |
|---|---|---|---|
| Baseline Characteristics | |||
| Age | |||
| - <50 years, n (%) | 40 (32.0) | 170 (45.5) | |
| - ≥50 years, n (%) | 85 (68.0) | 212 (55.5) | 0.016 * |
| Male, n (%) | 67 (53.6) | 194 (50.8) | 0.607 |
| ACTH stimulation dose, n (%) | |||
| - 1 µg | 41 (32.8) | 148 (38.7) | |
| - 250 µg | 87 (67.2) | 234 (61.3) | 0.243 |
| Underlying disease, n (%) | |||
| - Diabetes mellitus | 19 (15.2) | 56 (14.7) | 0.885 |
| - Hypertension | 40 (32.0) | 83 (21.8) | 0.023 * |
| - Chronic kidney disease | 11 (8.8) | 5 (1.3) | <0.001 * |
| - Autoimmune disease | 24 (19.2) | 52 (13.6) | 0.149 |
| - Cancer | 3 (2.4) | 12 (3.1) | 0.671 |
| Symptom, n (%) | |||
| - Fatigue | 36 (28.8) | 70 (18.3) | 0.016 * |
| - Weight loss | 4 (3.2) | 22 (5.8) | 0.352 |
| - Orthostatic hypotension | 14 (11.2) | 36 (9.4) | 0.604 |
| - Nausea/vomiting | 4 (3.2) | 5 (1.3) | 0.234 |
| Indication for ACTH testing, n (%) | |||
| - Exogenous steroid use | 57 (45.6) | 73 (19.1) | <0.001 * |
| - Post-surgery of pituitary | 18 (14.4) | 85 (22.3) | 0.072 |
| - Pituitary tumor | 20 (16.0) | 94 (24.6) | 0.049 * |
| - Pituitary hormonal deficiencies | 32 (25.6) | 115 (30.1) | 0.365 |
| - Symptoms of adrenal insufficiency | 40 (32.0) | 130 (34.0) | 0.744 |
| - Hyponatremia | 10 (8.0) | 8 (2.1) | 0.004 * |
| - Hypoglycemia | 2 (1.6) | 12 (3.1) | 0.534 |
| Cushingoid appearance in exogenous steroid use | 49 (39.2) | 15 (3.9) | <0.001 * |
| History of pituitary surgery, n (%) | |||
| - Microadenoma | 6 (17.6) | 31 (18.4) | |
| - Macroadenoma | 28 (82.4) | 137 (81.6) | 0.912 |
| Other hormonal deficiencies, n (%) | |||
| - Gonadotropin | 10 (12.8) | 45 (20.9) | 0.148 |
| - Thyroid | 26 (32.1) | 77 (32.9) | 0.894 |
| - Growth hormone | 4 (5.4) | 13 (6.7) | 0.787 |
| - Diabetes insipidus | 5 (6.9) | 28 (13.9) | 0.143 |
| Baseline biochemical investigations | |||
| Serum morning cortisol (µg/dL) | |||
| - <9 µg/dL, n (%) | 81 (64.8) | 184 (48.2) | |
| - ≥9 µg/dL, n (%) | 44 (35.2) | 198 (51.8) | 0.001 * |
| Serum basal cortisol (µg/dL) | |||
| - <9 µg/dL, n (%) | 101 (80.8) | 158 (41.4) | |
| - ≥9 µg/dL, n (%) | 24 (19.2) | 224 (58.6) | <0.001* |
| Serum albumin (g/dL) | |||
| - <3 g/dL | 20 (16.0) | 16 (4.2) | |
| - ≥3 g/dL | 105 (84.0) | 366 (95.8) | <0.001 * |
| Total cholesterol (mg/dL) | |||
| - <150 mg/dL | 38 (30.4) | 79 (20.7) | |
| - ≥150 mg/dL | 87 (69.6) | 303 (79.3) | 0.028 * |
| Serum sodium (mEq/L) | |||
| - <135 mEq/L | 19 (15.2) | 54 (14.1) | |
| - ≥135 mEq/L | 106 (84.8) | 328 (85.9) | 0.770 |
| Predictive Factors | RR | 95% CI for RR | Coefficient | Transformed Coefficients | Assigned Score | p-Value |
|---|---|---|---|---|---|---|
| Chronic kidney disease | ||||||
| - No | - | |||||
| - Yes | 2.5 | 2.02–3.10 | 0.92 | 9.17 | 9 | <0.001 |
| Cushingoid appearance in exogenous steroid use | ||||||
| - No | ||||||
| - Yes | - | |||||
| 3.38 | 2.10–5.44 | 1.22 | 12.17 | 12 | <0.001 | |
| Nausea and/or vomiting | ||||||
| - No | - | |||||
| - Yes | 1.82 | 1.20–2.76 | 0.6 | 6 | 6 | 0.005 |
| Fatigue | ||||||
| - No | - | |||||
| - Yes | 1.24 | 1.14–1.35 | 0.22 | 2.15 | 2 | <0.001 |
| Basal cortisol | ||||||
| - ≥9 µg/dL | - | |||||
| - <9 µg/dL | 3.4 | 3.28–3.53 | 1.22 | 12.25 | 12.5 | <0.001 |
| Cholesterol | ||||||
| - ≥150 mg/dL | - | |||||
| - <150 mg/dL | 1.28 | 1.26–1.30 | 0.25 | 2.5 | 2.5 | <0.001 |
| Sodium | ||||||
| - ≥135 mEq/L | - | |||||
| - <135 mEq/L | 1.11 | 1.04–1.19 | 0.1 | 1 | 1 | 0.003 |
| Criteria | Score |
|---|---|
| Basal cortisol < 9 µg/dL | |
| Yes | 12.5 |
| No | 0 |
| Cushingoid appearance in exogenous steroid use | |
| Yes | 12 |
| No | 0 |
| Chronic kidney disease | |
| Yes | 9 |
| No | 0 |
| Nausea and/or vomiting | |
| Yes | 6 |
| No | 0 |
| Cholesterol < 150 mg/dL | |
| Yes | 2.5 |
| No | 0 |
| Fatigue | |
| Yes | 2 |
| No | 0 |
| Sodium < 135 mEq/L | |
| Yes | 1 |
| No | 0 |
| High risk of adrenal insufficiency if the total score is >20.5 | |
| Risk Level | Score | Adrenal Insufficiency (n = 125), n (%) | Normal Adrenal Response (n = 382), n (%) | LR+ | 95% CI of LR+ | p-Value | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|---|---|---|---|
| Low | 0–20.0 | 77 (15.2) | 370 (72.9) | 0.08 | 0.04–0.15 | <0.001 | 61.6 | 3.1 |
| High | 20.5–50.0 | 48 (9.5) | 12 (2.4) | 12.22 | 6.71–22.26 | <0.001 | 96.9 | 38.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manosroi, W.; Pipanmekaporn, T.; Khorana, J.; Atthakomol, P.; Phimphilai, M. A Predictive Risk Score to Diagnose Adrenal Insufficiency in Outpatients: A 7 Year Retrospective Cohort Study. Medicines 2021, 8, 13. https://doi.org/10.3390/medicines8030013
Manosroi W, Pipanmekaporn T, Khorana J, Atthakomol P, Phimphilai M. A Predictive Risk Score to Diagnose Adrenal Insufficiency in Outpatients: A 7 Year Retrospective Cohort Study. Medicines. 2021; 8(3):13. https://doi.org/10.3390/medicines8030013
Chicago/Turabian StyleManosroi, Worapaka, Tanyong Pipanmekaporn, Jiraporn Khorana, Pichitchai Atthakomol, and Mattabhorn Phimphilai. 2021. "A Predictive Risk Score to Diagnose Adrenal Insufficiency in Outpatients: A 7 Year Retrospective Cohort Study" Medicines 8, no. 3: 13. https://doi.org/10.3390/medicines8030013
APA StyleManosroi, W., Pipanmekaporn, T., Khorana, J., Atthakomol, P., & Phimphilai, M. (2021). A Predictive Risk Score to Diagnose Adrenal Insufficiency in Outpatients: A 7 Year Retrospective Cohort Study. Medicines, 8(3), 13. https://doi.org/10.3390/medicines8030013






