Impact of Drug Pressure versus Limited Access to Drug in Malaria Control: The Dilemma
Abstract
:1. Introduction
2. Overview of the Current Antimalarial Portfolio
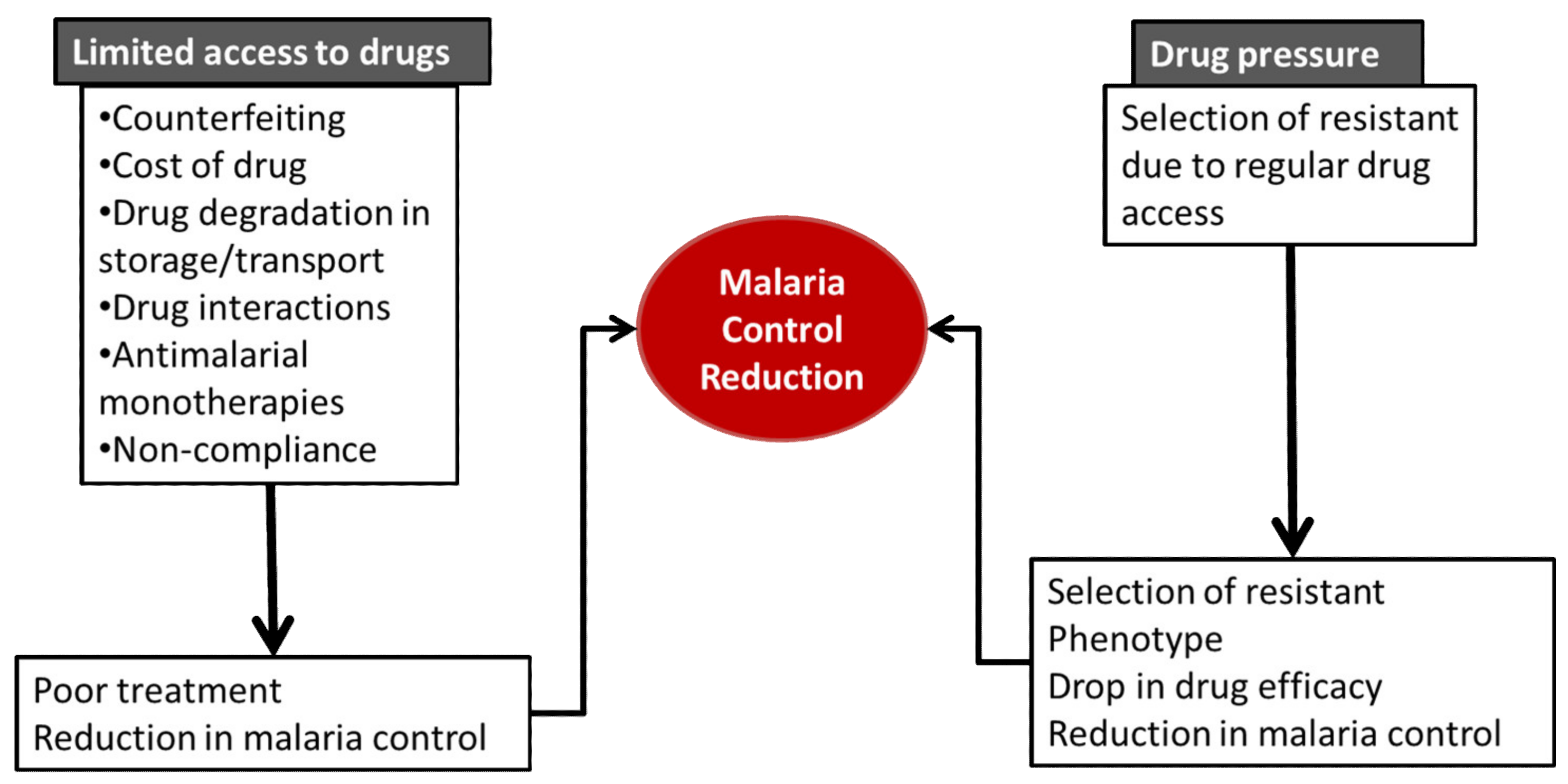
3. Limited Access to Drugs
3.1. Counterfeiting/Drug Quality
3.2. Cost of Drug
3.3. Drug Storage/Transportation
3.4. Drug Interactions
3.5. Use of Antimalarials as Monotherapies
3.6. Hoarding of Drugs by Corrupt Officials
4. Drug Pressure
4.1. Defining the Phenomenon of Drug Pressure
4.2. Genes Affected by Drug Pressure from Antimalarials
4.2.1. Antifolates
4.2.2. Quinolines
4.2.3. Hydroxynaphtoquinones
4.2.4. Endoperoxides
4.3. Cross Resistance Due to Drug Pressure
4.4. Overcoming Drug Pressure
4.4.1. Proper Diagnosis before Treatment
4.4.2. Treatment with Only Optimal Doses
4.4.3. Restrictive Drug Use
4.4.4. Recycling of Antimalarials
4.4.5. Drug Resistance Reversal
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2019; WHO: Geneva, Switzerland, 2019; ISBN 9789241565721. [Google Scholar]
- WHO. World Malaria Report 2020: 20 Years of Global Progress and Challenges; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Payne, D. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol. Today 1987, 3, 241–246. [Google Scholar] [CrossRef]
- Noedl, H.; Se, Y.; Schaecher, K.; Smith, B.L.; Socheat, D.; Fukuda, M.M. Evidence of artemisinin-resistant malaria in Western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620. [Google Scholar] [CrossRef] [PubMed]
- Bloland, P.B. Drug Resistance in Malaria (WHO/CDS/CSR/DRS/2001.4). Available online: https://www.who.int/csr/resources/publications/drugresist/malaria.pdf (accessed on 20 January 2021).
- Willcox, M.L.; Bodeker, G. Traditional herbal medicines for malaria. Br. Med. J. 2004, 329, 1156–1159. [Google Scholar] [CrossRef] [Green Version]
- Nzila, A. The past, present and future of antifolates in the treatment of Plasmodium falciparum infection. J. Antimicrob. Chemother. 2006, 57, 1043–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, A.; Benoit-Vical, F.; Claparols, C.; Meunier, B. The antimalarial drug artemisinin alkylates heme in infected mice. Proc. Natl. Acad. Sci. USA 2005, 102, 13676–13680. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, D.J.; Matile, H.; Ridley, R.G.; Goldberg, D.E. A common mechanism for blockade of heme polymerization by antimalarial quinolines. J. Biol. Chem. 1998, 273, 31103–31107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golden, E.B.; Cho, H.Y.; Hofman, F.M.; Louie, S.G.; Schönthal, A.H.; Chen, T.C. Quinoline-based antimalarial drugs: A novel class of autophagy inhibitors. Neurosurg. Focus 2015, 38, E12. [Google Scholar] [CrossRef]
- Egwu, C.O.; Augereau, J.-M.; Reybier, K.; Benoit-Vical, F. Reactive Oxygen Species as the Brainbox in Malaria Treatment. Antioxidants 2021, 10, 1872. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, I.K.; Rottenberg, H.; Vaidya, A.B. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J. Biol. Chem. 1997, 272, 3961–3966. [Google Scholar] [CrossRef] [Green Version]
- Dipanjan, B.; Shivaprakash, G.; Balaji, O. Triple Combination Therapy and Drug Cycling—Tangential Strategies for Countering Artemisinin Resistance. Curr. Infect. Dis. Rep. 2017, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; Scott, L.J.; Borrmann, S.; De Vries, P.J.; Hutchinson, D.B.A.; Looareesuwan, S.; Nosten, F.; Price, R.; Shanks, G.D. Atovaquone/proguanil: A review of its use for the prophylaxis of Plasmodium falciparum malaria. Drugs 2003, 63, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Deloron, P.; Bertin, G.; Briand, V.; Massougbodji, A.; Cot, M. Sulfadoxine/Pyrimethamine Intermittent Preventive Treatment for Malaria during Pregnancy. Emerg. Infect. Dis. 2010, 16, 1666. [Google Scholar] [CrossRef]
- Sidhu, A.B.S.; Valderramos, S.G.; Fidock, D.A. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 2005, 57, 913–926. [Google Scholar] [CrossRef]
- Price, R.N.; Uhlemann, A.C.; Brockman, A.; McGready, R.; Ashley, E.; Phaipun, L.; Patel, R.; Laing, K.; Looareesuwan, S.; White, N.J.; et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 2004, 364, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Holmgren, G.; Hamrin, J.; Svärd, J.; Mårtensson, A.; Gil, J.P.; Björkman, A. Selection of pfmdr1 mutations after amodiaquine monotherapy and amodiaquine plus artemisinin combination therapy in East Africa. Infect. Genet. Evol. 2007, 7, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Pickard, A.L.; Wongsrichanalai, C.; Purfield, A.; Kamwendo, D.; Emery, K.; Zalewski, C.; Kawamoto, F.; Miller, R.S.; Meshnick, S.R. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob. Agents Chemother. 2003, 47, 2418–2423. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.E.; Marchetti, R.V.; Cowan, A.I.; Howitt, S.M.; Bröer, S.; Kirk, K. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science 2009, 325, 1680–1682. [Google Scholar] [CrossRef] [Green Version]
- Beshir, K.; Sutherland, C.J.; Merinopoulos, I.; Durrani, N.; Leslie, T.; Rowland, M.; Hallett, R.L. Amodiaquine resistance in Plasmodium falciparum malaria in Afghanistan is associated with the pfcrt SVMNT allele at codons 72 to 76. Antimicrob. Agents Chemother. 2010, 54, 3714–3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witkowski, B.; Duru, V.; Khim, N.; Ross, L.S.; Saintpierre, B.; Beghain, J.; Chy, S.; Kim, S.; Ke, S.; Kloeung, N.; et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: A phenotype–genotype association study. Lancet Infect. Dis. 2017, 17, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, S.K.; Small-Saunders, J.L.; Ménard, D.; Fidock, D.A. Plasmodium falciparum resistance to piperaquine driven by PfCRT. Lancet Infect. Dis. 2019, 19, 1168–1169. [Google Scholar] [CrossRef]
- Croft, S.L.; Duparc, S.; Arbe-Barnes, S.J.; Craft, J.C.; Shin, C.S.; Fleckenstein, L.; Borghini-Fuhrer, I.; Rim, H.J. Review of pyronaridine anti-malarial properties and product characteristics. Malar. J. 2012, 11, 270. [Google Scholar] [CrossRef] [Green Version]
- Hyde, J.E. Drug-resistant malaria—An insight. FEBS J. 2007, 274, 4688–4698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staines, H.M.; Burrow, R.; Teo, B.H.Y.; Ster, I.C.; Kremsner, P.G.; Krishna, S. Clinical implications of Plasmodium resistance to atovaquone/proguanil: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2018, 73, 581–595. [Google Scholar] [CrossRef]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Musset, L.; Bouchaud, O.; Matheron, S.; Massias, L.; Le Bras, J. Clinical atovaquone-proguanil resistance of Plasmodium falciparum associated with cytochrome b codon 268 mutations. Microbes Infect. 2006, 8, 2599–2604. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.D.; Rosenthal, P.J. Antimalarial drug resistance in Africa: The calm before the storm? Lancet Infect. Dis. 2019, 19, e338–e351. [Google Scholar] [CrossRef]
- Gaillard, T.; Madamet, M.; Pradines, B. Tetracyclines in malaria. Malar. J. 2015, 14, 445. [Google Scholar] [CrossRef] [Green Version]
- Gansané, A.; Moriarty, L.F.; Ménard, D.; Yerbanga, I.; Ouedraogo, E.; Sondo, P.; Kinda, R.; Tarama, C.; Soulama, E.; Tapsoba, M.; et al. Anti-malarial efficacy and resistance monitoring of artemether-lumefantrine and dihydroartemisinin-piperaquine shows inadequate efficacy in children in Burkina Faso, 2017–2018. Malar. J. 2021, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- WHO Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy (Status Report—August 2018). Available online: https://apps.who.int/iris/bitstream/handle/10665/274362/WHO-CDS-GMP-2018.18-eng.pdf?ua=1 (accessed on 26 December 2020).
- WHO Report on Antimalarial Drug Efficacy, Resistance and Response: 10 Years of Surveillance (2010–2019). Available online: https://www.who.int/publications/i/item/9789240012813 (accessed on 27 December 2020).
- Packard, R.M. The Origins of Antimalarial-Drug Resistance. N. Engl. J. Med. 2014, 371, 397–399. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Q8(R2) Pharmaceutical Development|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q8r2-pharmaceutical-development (accessed on 8 June 2021).
- WHO. Quality Assurance of Pharmaceuticals: A Compendium of Guidelines and Related Materials. Available online: https://www.who.int/medicines/areas/quality_safety/quality_assurance/QualityAssurancePharmVol2.pdf (accessed on 24 June 2021).
- WWARN. Access to Quality Antimalarials—Determining the Scale of the Problem. Worldwide Antimalarial Resistance Network. Available online: https://www.wwarn.org/news/news-articles/access-quality-antimalarials-determining-scale-problem (accessed on 8 June 2021).
- WHO. 1 in 10 Medical Products in Developing Countries Is Substandard or Falsified. Available online: https://www.who.int/news-room/detail/28-11-2017-1-in-10-medical-products-in-developing-countries-is-substandard-or-falsified (accessed on 3 July 2021).
- Amin, A.A.; Kokwaro, G.O. Antimalarial drug quality in Africa. J. Clin. Pharm. Ther. 2007, 32, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renschler, J.P.; Walters, K.M.; Newton, P.N.; Laxminarayan, R. Estimated under-five deaths associated with poor-quality antimalarials in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2015, 92, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaan, E.; Mathijssen, J.; Tromp, N.; McBain, F.; ten Have, A.; Baltussen, R. The impact of health insurance in Africa and Asia: A systematic review. Bull. World Health Organ. 2012, 90, 685–692. [Google Scholar] [CrossRef]
- WHO. World Health Statistics 2010. Available online: https://apps.who.int/iris/handle/10665/44292 (accessed on 9 June 2021).
- McIntyre, D.; Thiede, M.; Dahlgren, G.; Whitehead, M. What are the economic consequences for households of illness and of paying for health care in low- and middle-income country contexts? Soc. Sci. Med. 2006, 62, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C.J.; Hage, D.S. Factors affecting the stability of drugs and drug metabolites in biological matrices. Bioanalysis 2009, 1, 205–220. [Google Scholar] [CrossRef]
- Tarning, J.; Hoglund, R.M. Clinically Relevant Drug Interactions for Malaria. In Encyclopedia of Malaria; Kremsner, P.G., Krishna, S., Eds.; Springer: New York, NY, USA, 2019. [Google Scholar]
- Kokwaro, G.; Mwai, L.; Nzila, A. Artemether/lumefantrine in the treatment of uncomplicated falciparum malaria. Expert Opin. Pharmacother. 2007, 8, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, W.A.; Balogun, T.; Fehintola, F.A.; Morse, G.D. Drug-Drug Interactions of Antimalarial Drugs. In Drug Interactions in Infectious Diseases: Antimicrobial Drug Interactions; Pai, M.P., Kiser, J.J., Gubbins, P.O., Rodvold, K.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 503–514. [Google Scholar]
- Tsamesidis, I.; Reybier, K.; Marchetti, G.; Pau, M.C.; Virdis, P.; Fozza, C.; Nepveu, F.; Low, P.S.; Turrini, F.M.; Pantaleo, A. Syk Kinase Inhibitors Synergize with Artemisinins by Enhancing Oxidative Stress in Plasmodium falciparum-Parasitized Erythrocytes. Antioxidants 2020, 9, 753. [Google Scholar] [CrossRef]
- White, N. Antimalarial drug resistance and combination chemotherapy. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 739–749. [Google Scholar] [CrossRef] [Green Version]
- Von Seidlein, L.; Greenwood, B.M. Mass administrations of antimalarial drugs. Trends Parasitol. 2003, 19, 452–460. [Google Scholar] [CrossRef]
- Zuber, J.A.; Takala-Harrison, S. Multidrug-resistant malaria and the impact of mass drug administration. Infect. Drug Resist. 2018, 11, 299. [Google Scholar] [CrossRef] [Green Version]
- Nzila, A.M.; Nduati, E.; Mberu, E.K.; Sibley, C.H.; Monks, S.A.; Winstanley, P.A.; Watkins, W.M. Molecular evidence of greater selective pressure for drug resistance exerted by the long-acting antifolate pyrimethamine/sulfadoxine compared with the shorter-acting chlorproguanil/dapsone on Kenyan Plasmodium falciparum. J. Infect. Dis. 2000, 181, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Lelièvre, J.; Berry, A.; Benoit-Vical, F. Artemisinin and chloroquine: Do mode of action and mechanism of resistance involve the same protagonists? Curr. Opin. Investig. Drugs 2007, 8, 117–124. [Google Scholar]
- Noedl, H.; Socheat, D.; Satimai, W. Artemisinin-Resistant Malaria in Asia. N. Engl. J. Med. 2009, 361, 540–541. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uwimana, A.; Legrand, E.; Stokes, B.H.; Ndikumana, J.L.M.; Warsame, M.; Umulisa, N.; Ngamije, D.; Munyaneza, T.; Mazarati, J.B.; Munguti, K.; et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, B.; Khim, N.; Chim, P.; Kim, S.; Ke, S.; Kloeung, N.; Chy, S.; Duong, S.; Leang, R.; Ringwald, P.; et al. Reduced artemisinin susceptibility of plasmodium falciparum ring stages in western cambodia. Antimicrob. Agents Chemother. 2013, 57, 914–923. [Google Scholar] [CrossRef] [Green Version]
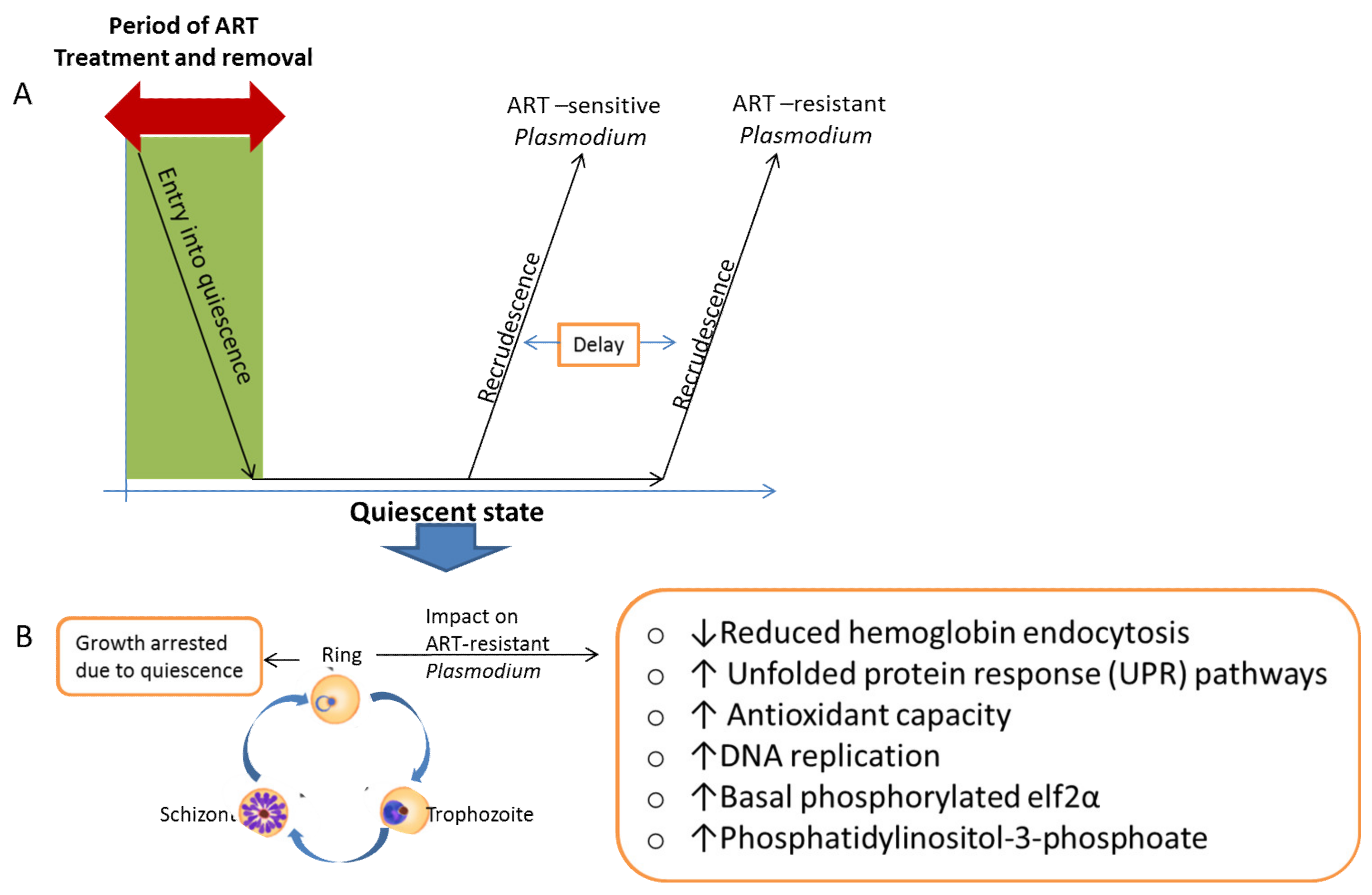
- Witkowski, B.; Lelièvre, J.; Barragán, M.J.L.; Laurent, V.; Su, X.Z.; Berry, A.; Benoit-Vical, F. Increased tolerance to artemisinin in plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob. Agents Chemother. 2010, 54, 1872–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witkowski, B.; Menard, D.; Amaratunga, C.; Fairhurst, R. Ring Stage Survival Assays (RSA) to Evaluate the In Vitro and Ex Vivo Susceptibility of Plasmodium falciparum to Artemisinins; National Institutes of Health Procedure: Phnom Penh, Cambodia, 2013.
- Chen, N.; LaCrue, A.N.; Teuscher, F.; Waters, N.C.; Gatton, M.L.; Kyle, D.E.; Cheng, Q. Fatty acid synthesis and pyruvate metabolism pathways remain active in dihydroartemisinin-induced dormant ring stages of plasmodium falciparum. Antimicrob. Agents Chemother. 2014, 58, 4773–4781. [Google Scholar] [CrossRef] [Green Version]
- Peatey, C.L.; Chavchich, M.; Chen, N.; Gresty, K.J.; Gray, K.A.; Gatton, M.L.; Waters, N.C.; Cheng, Q. Mitochondrial Membrane Potential in a Small Subset of Artemisinin-Induced Dormant Plasmodium falciparum Parasites in Vitro. J. Infect. Dis. 2015, 212, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, J.; Scharf, S.; Schmidt, S.; Jonscher, E.; Maria Hoeijmakers, W.A.; Flemming, S.; Toenhake, C.G.; Schmitt, M.; Sabitzki, R.; Bergmann, B.; et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science 2020, 367, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mbengue, A.; Bhattacharjee, S.; Pandharkar, T.; Liu, H.; Estiu, G.; Stahelin, R.V.; Rizk, S.S.; Njimoh, D.L.; Ryan, Y.; Chotivanich, K.; et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 2015, 520, 683–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocamora, F.; Zhu, L.; Liong, K.Y.; Dondorp, A.; Miotto, O.; Mok, S.; Bozdech, Z. Oxidative stress and protein damage responses mediate artemisinin resistance in malaria parasites. PLoS Pathog. 2018, 14, e1006930. [Google Scholar] [CrossRef]
- Cazelles, J.; Robert, A.; Meunier, B. Alkylation of heme by artemisinin, an antimalarial drug. Comptes Rendus l’Academie des Sci. Ser. IIC Chem. 2001, 4, 85–89. [Google Scholar] [CrossRef]
- Witkowski, B.; Lelièvre, J.; Nicolau-Travers, M.L.; Iriart, X.; Soh, P.N.; Bousejra-ElGarah, F.; Meunier, B.; Berry, A.; Benoit-Vical, F. Evidence for the contribution of the hemozoin synthesis pathway of the murine plasmodium yoelii to the resistance to artemisinin-related drugs. PLoS ONE 2012, 7, e32620. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Wang, Z.; Miao, J.; Miao, M.; Chandra, R.; Jiang, H.; Su, X.Z.; Cui, L. Mechanisms of in vitro resistance to dihydroartemisinin in Plasmodium falciparum. Mol. Microbiol. 2012, 86, 111–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsel, S.; Gustafson, S.; Friedlander, E.; Shnyra, A.; Adegbulu, A.; Liu, Y.; Parrish, N.; Jamal, S.; Lofthus, E.; Ayuk, L.; et al. Malaria over-diagnosis in Cameroon: Diagnostic accuracy of Fluorescence and Staining Technologies (FAST) Malaria Stain and LED microscopy versus Giemsa and bright field microscopy validated by polymerase chain reaction. Infect. Dis. Poverty 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
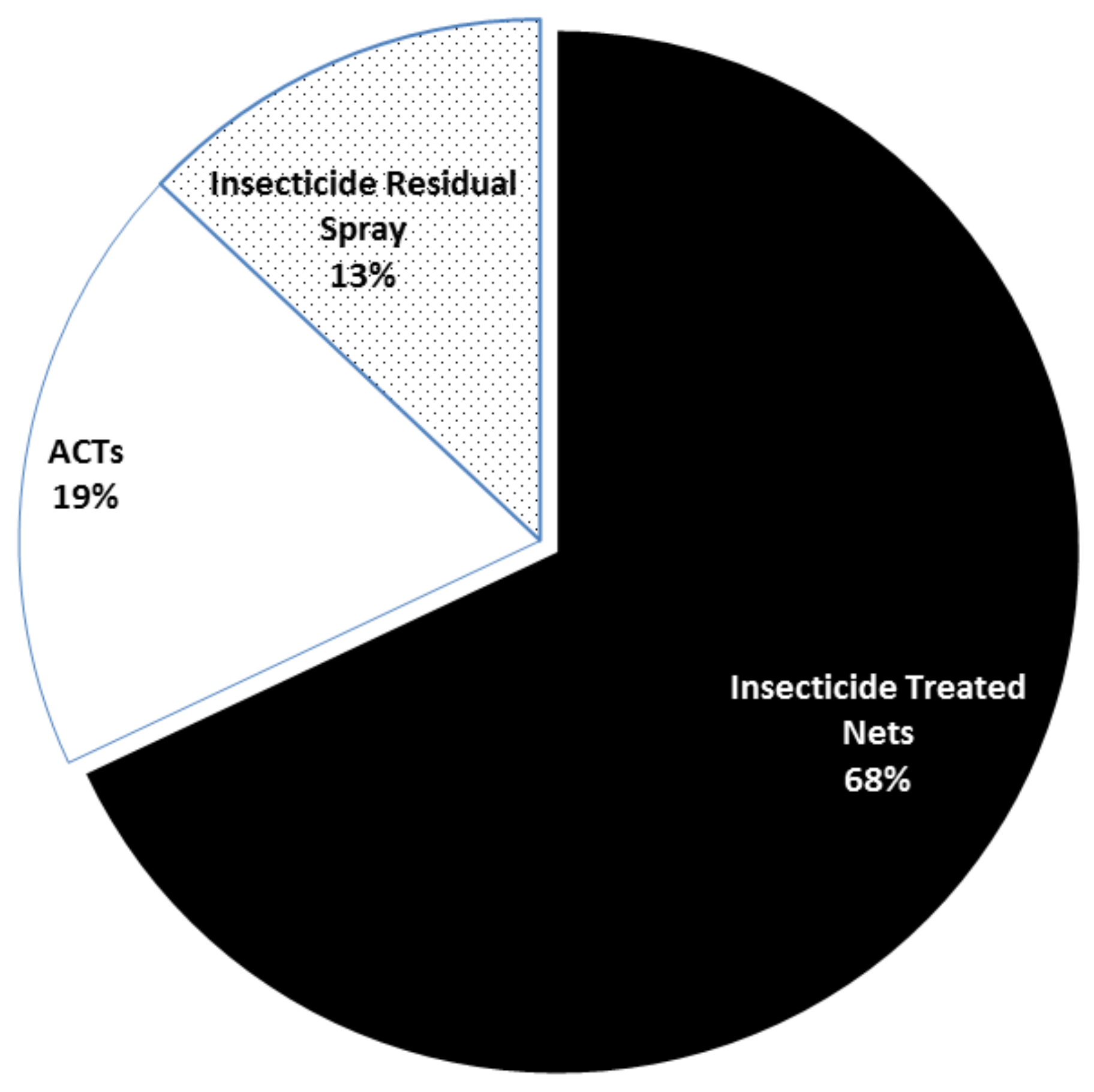
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Kublin, J.G.; Cortese, J.F.; Njunju, E.M.; Mukadam, R.A.G.; Wirima, J.J.; Kazembe, P.N.; Djimdé, A.A.; Kouriba, B.; Taylor, T.E.; Plowe, C.V. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J. Infect. Dis. 2003, 187, 1870–1875. [Google Scholar] [CrossRef] [Green Version]
- Laufer, M.K.; Thesing, P.C.; Eddington, N.D.; Masonga, R.; Dzinjalamala, F.K.; Takala, S.L.; Taylor, T.E.; Plowe, C.V. Return of Chloroquine Antimalarial Efficacy in Malawi. N. Engl. J. Med. 2006, 355, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Bitonti, A.J.; Sjoerdsma, A.; Mccann, P.P.; Kyle, D.E.; Oduola, A.M.J.; Rossan, R.N.; Milhous, W.K.; Davidson, D.E. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science 1988, 242, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.K.; Oduola, A.M.J.; Milhous, W.K. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science 1987, 235, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Tripathi, L.M.; Saxena, J.K.; Puri, S.K. Implication of intracellular glutathione and its related enzymes on resistance of malaria parasites to the antimalarial drug arteether. Parasitol. Int. 2011, 60, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.; Diez, A.; Radfar, A.; Pérez-Benavente, S.; do Rosario, V.E.; Puyet, A.; Bautista, J.M. Early transcriptional response to chloroquine of the Plasmodium falciparum antioxidant defence in sensitive and resistant clones. Acta Trop. 2010, 114, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Kumar, S.; Puri, S. Buthionine sulfoximine increases the efficacy of arteether antimalarial activity in arteether-resistant Plasmodium vinckei by glutathione depletion. Malar. World J. 2015, 6, 4. [Google Scholar]
| Antimalarials | Introduction Date | Resistance Date | Genetic Marker | Mechanism of Action | Mechanism of Resistance | References |
|---|---|---|---|---|---|---|
| Aryl-amino alcohol | ||||||
| Quinine | 1820 | 1910 | Pfmdr1 | Inhibition of heme detoxification | Gene amplification and mutation leading to drug efflux and/or non-binding to target site | [16] |
| Mefloquine | 1977 | 1982 | Pfmdr1 | [17] | ||
| Lumefantrine | 1976 | Pfmdr1 | [18] | |||
| 4-aminoquinolines | ||||||
| Chloroquine | 1945 | 1957 | Pfcrt, Pfmdr | Inhibition of heme detoxification and redox cycling of heme to and fro the cytosol | Gene mutation: efflux of molecules from food vacuole | [19,20] |
| Amodiaquine | 1948 | 1990s | Pfcrt, Pfmdr | [18,21] | ||
| Bis-quinoline | ||||||
| Piperaquine | 1960s | 2010 | Pfcrt, Pfplasmepsin 2-3 copy number | Inhibition of heme detoxifica-tion and redox cycling of heme to and fro the cytosol | Gene amplification | [22,23] |
| Naphthyridine | ||||||
| Pyronaridine | 1980 | - | Inhibition of heme detoxification | [24] | ||
| Antifolates | ||||||
| Sulfadoxine | 1937 | 1960s | Pfdhps | Inhibition of folate metabolism and DNA replication | Gene mutation leading to drug binding site modification | [25] |
| Proguanil/cycloguanil | 1948 | 1949 | Pfdhfr | [26] | ||
| Pyrimethamine | 1952 | 1960s | ||||
| Endoperoxide | ||||||
| Artemisinin and its derivatives (DHA, ATS, ATM) | 1972 | 2008 | PfKelch13 | Inhibition of heme detoxification and C-C radical formation | Entry into quiescent state | [27] |
| Hydroxynaphtoquinones | ||||||
| Atovaquone | 1996 | 1996 | Pfcytb | Competitive inhibition of Complex iii of the ETC | Modification of binding site on Complex iii/cytochrome b | [26,28] |
| Tetracycline antibiotic | ||||||
| Doxycycline | 1967 | SNPs in Pfmdt and PftetQ | Inhibition of protein, nucleotide and deoxynucleotide synthesis | Not yet described | [29,30] | |
| ACTs | Region Used | Region of Reported ACT Failure |
|---|---|---|
| Artemether-lumefantrine (AL) | Africa, Americas and Middle East | Burkina Faso, Cambodia, Lao People’s Democratic Republic, Thailand and Vietnam |
| Dihydroartemisinin-piperaquine (DHA-PPQ) | Southeast Asia, China and Africa | Cambodia, Lao People’s Democratic Republic, Thailand and Vietnam |
| Artesunate-amodiaquine (AS-AQ) | West Africa | Indonesia, Cambodia |
| Artesunate-mefloquine (AS-MQ) | Southeast Asia and Americas | Cambodia, Lao People’s Democratic Republic, Thailand and Vietnam |
| Artesunate-sulfadoxine-pyrimethamine (AS-SP) | Southeast Asia, Middle-East and South America | Northeastern India, Somalia and Sudan |
| Artesunate—pyronaridine (AS-PY) | Southeast Asia | Cambodia, Vietnam |
| Food/Drug | Class | Probable Mechanism of Interaction | Consequences |
|---|---|---|---|
| Indinavir, nelfinavir | Antiretroviral | Inhibits CYP3A4 | May increase concentrations of ART and LUM |
| Imatinib | Anticancer | Inhibits Syk, Lyn, Bcr-Abl | Decrease artemisinin concentration and accelerate ART efficacy |
| Ritonavir | Antiretroviral | Inhibits CYP2D6 and CYP3A4 | May increase concentrations of ART and LUM |
| Ketoconazole | Antifungal | Inhibits CYP3A4 | Shown to cause modest increase in concentration of ART and LUM |
| Fluconazole | Antifungal | Inhibits CYP3A4 | May cause increase in concentration of ART and LUM |
| Rifampicin, isoniazid | Anti-tuberculosis | Induces CYP3A4 | May decrease concentrations of ART and LUM |
| Nevirapine, efavirenz | Antiretrovirals | Induces CYP3A4 | May decrease concentrations of ART and LUM |
| Phentytoin/phenobarbital /carbamazepine | Anticonvulsants | Induces CYP3A4 | May decrease concentrations of ART and LUM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egwu, C.O.; Obasi, N.A.; Aloke, C.; Nwafor, J.; Tsamesidis, I.; Chukwu, J.; Elom, S. Impact of Drug Pressure versus Limited Access to Drug in Malaria Control: The Dilemma. Medicines 2022, 9, 2. https://doi.org/10.3390/medicines9010002
Egwu CO, Obasi NA, Aloke C, Nwafor J, Tsamesidis I, Chukwu J, Elom S. Impact of Drug Pressure versus Limited Access to Drug in Malaria Control: The Dilemma. Medicines. 2022; 9(1):2. https://doi.org/10.3390/medicines9010002
Chicago/Turabian StyleEgwu, Chinedu Ogbonnia, Nwogo Ajuka Obasi, Chinyere Aloke, Joseph Nwafor, Ioannis Tsamesidis, Jennifer Chukwu, and Sunday Elom. 2022. "Impact of Drug Pressure versus Limited Access to Drug in Malaria Control: The Dilemma" Medicines 9, no. 1: 2. https://doi.org/10.3390/medicines9010002
APA StyleEgwu, C. O., Obasi, N. A., Aloke, C., Nwafor, J., Tsamesidis, I., Chukwu, J., & Elom, S. (2022). Impact of Drug Pressure versus Limited Access to Drug in Malaria Control: The Dilemma. Medicines, 9(1), 2. https://doi.org/10.3390/medicines9010002







