Diaphragm Ultrasound in Cardiac Surgery: State of the Art
Abstract
:1. Introduction
2. The Diaphragm
3. Traditional Techniques Used to Assess the Diaphragm Function
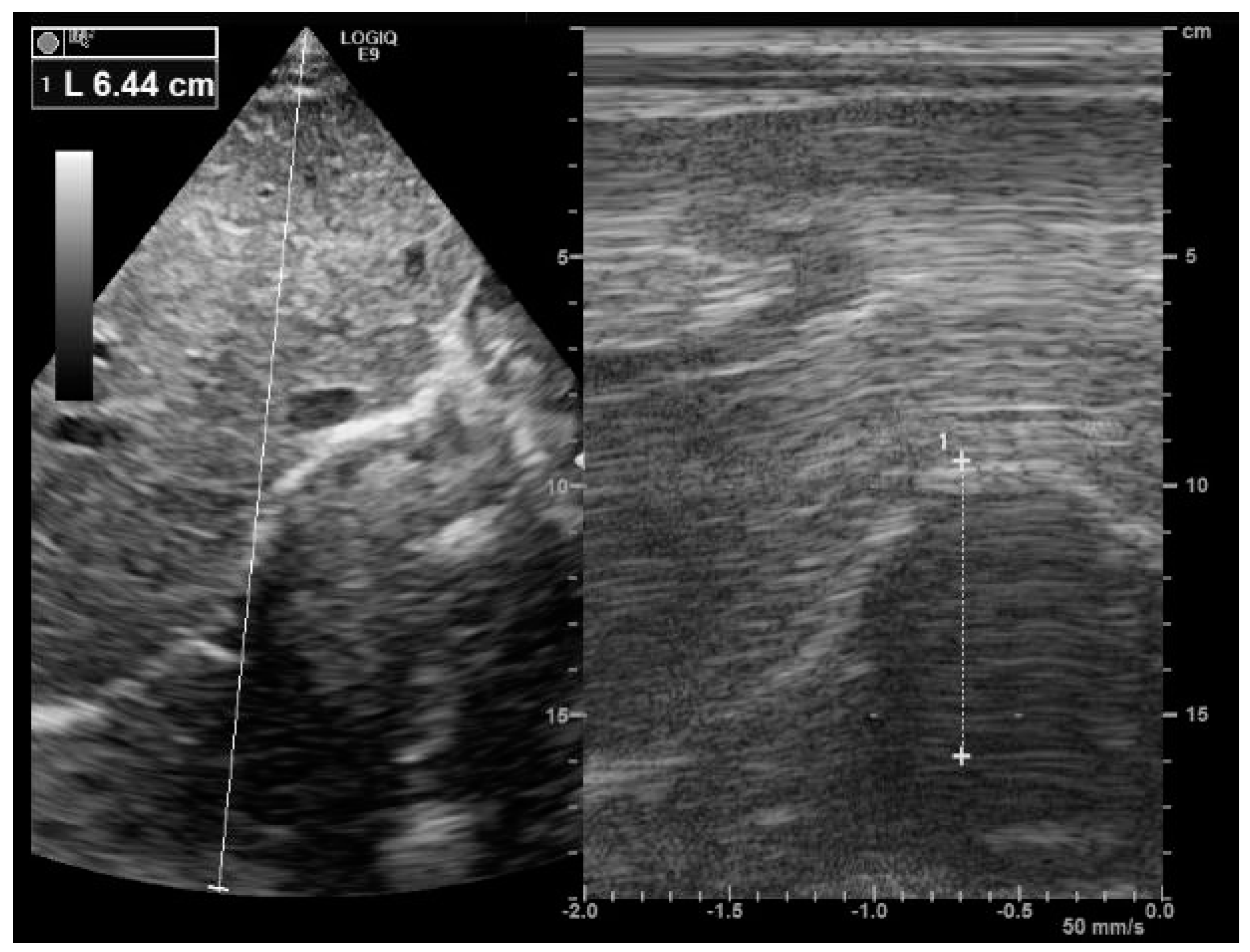
4. Diaphragm Ultrasound Technique
4.1. The Subcostal Approach
4.2. The Apposition Zone Approach
5. Diaphragm Dysfunction in ICU
6. Diaphragm Dysfunction in Cardiac Surgery
- The management of patients in cardiac ICU after surgery, in the context of a weaning trial
- The management of patients with moderate symptoms who may reveal diaphragm features in cardiac rehabilitation unit.
7. Management of Diaphragmatic Dysfunction after Cardiac Surgery
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bruni, A.; Garofalo, E.; Pasin, L.; Serraino, G.F.; Cammarota, G.; Longhini, F.; Landoni, G.; Lembo, R.; Mastroroberto, P.; Navalesi, P.; et al. Diaphragmatic Dysfunction after Elective Cardiac Surgery: A Prospective Observational Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3336–3344. [Google Scholar] [CrossRef]
- Cavayas, Y.A.; Eljaiek, R.; Rodrigue, É.; Lamarche, Y.; Girard, M.; Wang, H.T.; Levesque, S.; Denault, A.Y. Preoperative Diaphragm Function is Associated with Postoperative Pulmonary Complications after Cardiac Surgery. Crit. Care Med. 2019, 47, e966–e974. [Google Scholar] [CrossRef]
- Diehl, J.L.; Lofaso, F.; Deleuze, P.; Similowski, T.; Lemaire, F.; Brochard, L. Clinically relevant diaphragmatic dysfunction after cardiac operations. J. Thorac. Cardiovasc. Surg. 1994, 107, 487–498. [Google Scholar] [CrossRef]
- Laghlam, D.; Lê, M.P.; Srour, A.; Monsonego, R.; Estagnasié, P.; Brusset, A.; Squara, P. Diaphragm Dysfunction After Cardiac Surgery: Reappraisal. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, E.R.; Steffens, É.; Windmöller, P.; Fontela, P.C.; da Cruz, D.T.; Battisti, I.D.E. Preoperative expiratory and inspiratory muscle weakness to predict postoperative outcomes in patients undergoing elective cardiac surgery. J. Card. Surg. 2020, 35, 128–134. [Google Scholar] [CrossRef]
- He, S.; Chen, B.; Li, W.; Yan, J.; Chen, L.; Wang, X.; Xiao, Y. Ventilator-associated pneumonia after cardiac surgery: A meta-analysis and systematic review. J. Thorac. Cardiovasc. Surg. 2014, 148, 3148–3155.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moury, P.-H.; Cuisinier, A.; Durand, M.; Bosson, J.-L.; Chavanon, O.; Payen, J.-F.; Jaber, S.; Albaladejo, P. Diaphragm thickening in cardiac surgery: A perioperative prospective ultrasound study. Ann. Intensive Care 2019, 9, 50. [Google Scholar] [CrossRef]
- Aguirre, V.J.; Sinha, P.; Zimmet, A.; Lee, G.A.; Kwa, L.; Rosenfeldt, F. Phrenic nerve injury during cardiac surgery: Mechanisms, management and prevention. Heart Lung Circ. 2013, 22, 895–902. [Google Scholar] [CrossRef]
- Dimopoulou, I.; Daganou, M.; Dafni, U.; Karakatsani, A.; Khoury, M.; Geroulanos, S.; Jordanoglou, J. Phrenic nerve dysfunction after cardiac operations: Electrophysiologic evaluation of risk factors. Chest 1998, 113, 8–14. [Google Scholar] [CrossRef]
- Berrizbeitia, L.D.; Tessler, S.; Jacobowitz, I.J.; Kaplan, P.; Cunningham, J.N. Effect of sternotomy and coronary bypass surgery on postoperative pulmonary mechanics: Comparison of internal mammary and saphenous vein bypass grafts. Chest 1989, 96, 873–876. [Google Scholar] [CrossRef]
- Rochester, D.F. The diaphragm: Contractile properties and fatigue. J. Clin. Investig. 1985, 75, 1397–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benditt, J.O. Esophageal and gastric pressure measurements. Respir. Care 2005, 50, 68–77. [Google Scholar] [PubMed]
- Wilcox, P.G.; Pardy, R.L. Diaphragmatic weakness and paralysis. Lung 1989, 167, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Troyer, A.D.; Wilson, T.A. Action of the diaphragm on the rib cage. J. Appl. Physiol. 2016, 121, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.J. Diaphragmatic paresis: Pathophysiology, clinical features, and investigation. Thorax 1989, 44, 960–970. [Google Scholar] [CrossRef] [Green Version]
- Steier, J.; Kaul, S.; Seymour, J.; Jolley, C.; Rafferty, G.; Man, W.; Luo, Y.M.; Roughton, M.; Polkey, M.I.; Moxham, J. The value of multiple tests of respiratory muscle strength. Thorax 2007, 62, 975–980. [Google Scholar] [CrossRef] [Green Version]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dubé, B.-P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.B.; Desmarais, G.; Falaize, L.; Ogna, A.; Cognet, S.; Louis, B.; Orlikowski, D.; Prigent, H.; Lofaso, F. Twitch mouth pressure for detecting respiratory muscle weakness in suspicion of neuromuscular disorder. Neuromuscul. Disord. 2017, 27, 518–525. [Google Scholar] [CrossRef]
- Boussuges, A.; Gole, Y.; Blanc, P. Diaphragmatic motion studied by m-mode ultrasonography: Methods, reproducibility, and normal values. Chest 2009, 135, 391–400. [Google Scholar] [CrossRef]
- Boon, A.J.; Ba, C.J.H.; Ghahfarokhi, L.S.; Strommen, J.A.; Watson, J.C.; Sorenson, E.J. Two-dimensional ultrasound imaging of the diaphragm: Quantitative values in normal subjects. Muscle Nerve 2013, 47, 884–889. [Google Scholar] [CrossRef]
- Gottesman, E.; McCool, F.D. Ultrasound evaluation of the paralyzed diaphragm. Am. J. Respir. Crit. Care Med. 1997, 155, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Suh, H.J.; Hong, S.-B.; Koh, Y.; Lim, C.-M. Diaphragm dysfunction assessed by ultrasonography: Influence on weaning from mechanical ventilation. Crit. Care Med. 2011, 39, 2627–2630. [Google Scholar] [CrossRef] [PubMed]
- Fayssoil, A.; Nguyen, L.S.; Ogna, A.; Stojkovic, T.; Meng, P.; Mompoint, D.; Carlier, R.; Prigent, H.; Clair, B.; Behin, A.; et al. Diaphragm sniff ultrasound: Normal values, relationship with sniff nasal pressure and accuracy for predicting respiratory involvement in patients with neuromuscular disorders. PLoS ONE 2019, 14, e0214288. [Google Scholar] [CrossRef] [PubMed]
- DiNino, E.; Gartman, E.J.; Sethi, J.M.; McCool, F.D. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 2014, 69, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueki, J.; De Bruin, P.F.; Pride, N.B. In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax 1995, 50, 1157–1161. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Esper, R.; Pérez-Calatayud, Á.A.; Arch-Tirado, E.; Díaz-Carrillo, M.A.; Garrido-Aguirre, E.; Tapia-Velazco, R.; Peña-Pérez, C.A.; Espinoza-de Los Monteros, I.; Meza-Márquez, J.M.; Flores-Rivera, O.I.; et al. Standardization of sonographic diaphragmthickness evaluations in healthy volunteers. Respir. Care 2016, 61, 920–924. [Google Scholar] [CrossRef] [Green Version]
- Goligher, E.C.; Laghi, F.; Detsky, M.E.; Farias, P.; Murray, A.; Brace, D.; Brochard, L.J.; Bolz, S.S.; Rubenfeld, G.D.; Kavanagh, B.P.; et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: Feasibility, reproducibility and validity. Intensive Care Med. 2015, 41, 642–649. [Google Scholar] [CrossRef]
- Wait, J.L.; Nahormek, P.A.; Yost, W.T.; Rochester, D.P. Diaphragmatic thickness-lung volume relationship in vivo. J. Appl. Physiol. 1989, 67, 1560–1568. [Google Scholar] [CrossRef]
- Cohen, E.; Mier, A.; Heywood, P.; Murphy, K.; Boultbee, J.; Guz, A. Excursion-volume relation of the right hemidiaphragm measured by ultrasonography and respiratory airflow measurements. Thorax 1994, 49, 885–889. [Google Scholar] [CrossRef] [Green Version]
- Kantarci, F.; Mihmanli, I.; Demirel, M.K.; Harmanci, K.; Akman, C.; Aydogan, F.; Mihmanli, A.; Uysal, O. Normal diaphragmatic motion and the effects of body composition: Determination with M-mode sonography. J. Ultrasound Med. 2004, 23, 255–260. [Google Scholar] [CrossRef]
- Testa, A.; Soldati, G.; Giannuzzi, R.; Berardi, S.; Portale, G.; Silveri, N.G. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med. Biol. 2011, 37, 44–52. [Google Scholar] [CrossRef]
- Orde, S.R.; Boon, A.J.; Firth, D.G.; Villarraga, H.R.; Sekiguchi, H. Diaphragm assessment by two dimensional speckle tracking imaging in normal subjects. BMC Anesthesiol. 2016, 16, 43. [Google Scholar] [CrossRef] [Green Version]
- Scarlata, S.; Mancini, D.; Laudisio, A.; Benigni, A.; Incalzi, R.A. Reproducibility and Clinical Correlates of Supine Diaphragmatic Motion Measured by M-Mode Ultrasonography in Healthy Volunteers. Respiration 2018, 96, 259–266. [Google Scholar] [CrossRef]
- Spiesshoefer, J.; Herkenrath, S.; Henke, C.; Langenbruch, L.; Schneppe, M.; Randerath, W.; Young, P.; Brix, T.; Boentert, M. Evaluation of Respiratory Muscle Strength and Diaphragm Ultrasound: Normative Values, Theoretical Considerations, and Practical Recommendations. Respiration 2020, 99, 369–381. [Google Scholar] [CrossRef]
- Demoule, A.; Jung, B.; Prodanovic, H.; Molinari, N.; Chanques, G.; Coirault, C.; Matecki, S.; Duguet, A.; Similowski, T.; Jaber, S. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am. J. Respir. Crit. Care Med. 2013, 188, 213–219. [Google Scholar] [CrossRef]
- Jung, B.; Moury, P.H.; Mahul, M.; De Jong, A.; Galia, F.; Prades, A.; Albaladejo, P.; Chanques, G.; Molinari, N.; Jaber, S. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016, 42, 853–861. [Google Scholar] [CrossRef]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef]
- Dres, M.; Dubé, B.-P.; Mayaux, J.; Delemazure, J.; Reuter, D.; Brochard, L.; Similowski, T.; Demoule, A. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am. J. Respir. Crit. Care Med. 2017, 195, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Pirompanich, P.; Romsaiyut, S. Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. J. Intensive Care 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, G.; De Filippi, G.; Elia, F.; Panero, F.; Volpicelli, G.; Aprà, F. Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit. Ultrasound J. 2014, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dres, M.; Goligher, E.C.; Dubé, B.-P.; Morawiec, E.; Dangers, L.; Reuter, D.; Mayaux, J.; Similowski, T.; Demoule, A. Diaphragm function and weaning from mechanical ventilation: An ultrasound and phrenic nerve stimulation clinical study. Ann. Intensive Care 2018, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Dres, M.; Demoule, A. Diaphragm dysfunction during weaning from mechanical ventilation: An underestimated phenomenon with clinical implications. Crit. Care 2018, 22, 73. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.R.; Tsai, T.H.; Jerng, J.S.; Yu, C.J.; Wu, H.D.; Yang, P.C. Ultrasonographic evaluation of liver/spleen movements and extubation outcome. Chest 2004, 126, 179–185. [Google Scholar] [CrossRef]
- Yoo, J.W.; Lee, S.J.; Lee, J.D.; Kim, H.C. Comparison of clinical utility between diaphragm excursion and thickening change using ultrasonography to predict extubation success. Korean J. Intern. Med. 2018, 33, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Palkar, A.; Narasimhan, M.; Greenberg, H.; Singh, K.; Koenig, S.; Mayo, P.; Gottesman, E. Diaphragm Excursion-Time Index: A New Parameter Using Ultrasonography to Predict Extubation Outcome. Chest 2018, 153, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Grasso, S.; Mauri, T.; Corte, F.D.; Alvisi, V.; Ragazzi, R.; Cricca, V.; Biondi, G.; Di Mussi, R.; Marangoni, E.; et al. Can diaphragmatic ultrasonography performed during the T-tube trial predict weaning failure? The role of diaphragmatic rapid shallow breathing index. Crit. Care 2016, 20, 305. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, K.; Kato, H.; Tsujimoto, S.; Kitamura, R. Diabetes mellitus, internal thoracic artery grafting, and risk of an elevated hemidiaphragm after coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 1994, 8, 437–440. [Google Scholar] [CrossRef]
- Chan, C.K.; Loke, J.; Virgulto, J.A.; Mohsenin, V.; Ferranti, R.; Lammertse, T. Bilateral diaphragmatic paralysis: Clinical spectrum, prognosis, and diagnostic approach. Arch. Phys. Med. Rehabil. 1988, 69, 976–979. [Google Scholar] [PubMed]
- Markand, O.N.; Moorthy, S.; Mahomed, Y.; King, R.D.; Brown, J.W. Postoperative phrenic nerve palsy in patients with open-heart surgery. Ann. Thorac. Surg. 1985, 39, 68–73. [Google Scholar] [CrossRef]
- Canbaz, S.; Turgut, N.; Halici, U.; Balci, K.; Ege, T.; Duran, E. Electrophysiological evaluation of phrenic nerve injury during cardiac surgery--a prospective, controlled, clinical study. BMC Surg. 2004, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVita, M.A.; Robinson, L.R.; Rehder, J.; Hattler, B.; Cohen, C. Incidence and natural history of phrenic neuropathy occurring during open heart surgery. Chest 1993, 103, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Merino-Ramirez, M.A.; Juan, G.; Ramón, M.; Cortijo, J.; Rubio, E.; Montero, A.; Morcillo, E. Electrophysiologic evaluation of phrenic nerve and diaphragm function after coronary bypass surgery: Prospective study of diabetes and other risk factors. J. Thorac. Cardiovasc. Surg. 2006, 132, 530–536.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tralhão, A.; Cavaleiro, P.; Arrigo, M.; Lopes, J.-P.; Lebrun, M.; Rivas-Lasarte, M.; Le Pimpec-Barthes, F.; Latrémouille, C.; Achouh, P.; Pirracchio, R.; et al. Early changes in diaphragmatic function evaluated using ultrasound in cardiac surgery patients: A cohort study. J. Clin. Monit. 2019, 34, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Doorduin, J.; van Hees, H.W.; van der Hoeven, J.G.; Heunks, L.M. Monitoring of the respiratory muscles in the critically ill. Am. J. Respir. Crit. Care Med. 2013, 187, 20–27. [Google Scholar] [CrossRef]
- McCool, F.D.; Oyieng’o, D.O.; Koo, P. The Utility of Diaphragm Ultrasound in Reducing Time to Extubation. Lung 2020, 198, 499–505. [Google Scholar] [CrossRef]



| Author (Ref) | Year | N | Diaphragm Motion | Diaphragm Thickness | Diaphragm TF (%) | Diaphragm TDI or 2D Strain |
|---|---|---|---|---|---|---|
| Wait [28] | 1989 | 10 | 2.2 ± 0.4 mm (FRC) | |||
| Cohen [29] | 1994 | 10 | Deep inspiratory motion: 60 ± 7 mm | |||
| Ueki [25] | 1995 | 13 | 1.7 ± 0.2 mm (FRC) 4.5 ± 0.9 mm (TLC) | |||
| Kantarci [30] | 2004 | 164 | Right DM: 49.2 ± 10.9 mm Left DM: 50.17 ± 11.7 mm | |||
| Boussuges [19] | 2009 | 210 | QB: 9 mm (F) and 10 mm (M) DB: 37 mm (F) and 47 mm (M) Sniff echo: 16 mm (F) and 18 mm (M) | |||
| Testa [31] | 2011 | 40 | QB: 18.4 ± 7.6 mm DB: 78.8 ± 13.3 mm | |||
| Boon [20] | 2013 | 150 | Right: 3.3 ± 1 mm (FRC) Left: 3.4 ± 1.8 mm (FRC) | |||
| Orde [32] | 2016 | 50 | Right: 49 ± 10 mm | Right: 2.4 ± 1 mm | Right TF: 45.1% ± 12% | Right diaphragm Strain: −40.3% ± 9% |
| Carrillo-Esper [26] | 2016 | 109 | Female: 1.4 ± 0.3 mm(FRC) Male: 1.9 ± 04 mm (FRC) | |||
| Scarlata [33] | 2018 | 100 | QB: 17.6 ± 5.4 mm DB: 62 ± 15.5 mm | |||
| Fayssoil [23] | 2019 | 27 | Right DB: 72 mm Left DB: 62 mm | Peak sniff TDI velocity: 13 cm/s (male) 12 cm/s (female) | ||
| Spiesshoefer [34] | 2020 | 70 | QB: 15.6 ± 5.3 mm DB: 80.2 ± 19.1 mm | 1.9 ± 0.6 mm (FRC) 5.3 ± 1.8 mm (TLC) |
| First Author (Ref) | N (Patients) | Diaphragm US Parameter Cut-Off | Sensibility | Specificity | Reference Test |
|---|---|---|---|---|---|
| Pirompanich [39] | 34 | TF ≥ 26% | 96% | 68% | RSBI |
| Dres [41] | 76 | TF > 26% | 79% | 73% | Twitch pressure using phrenic nerve stimulation |
| DiNino [24] | 63 | TF ≥ 30% | 88% | 71% | |
| Ferrari [40] | 46 | TF > 36% | 82% | 88% | RSBI |
| Yoo [44] | 60 | TF ≥ 30% | 68.1% | 61.5% | |
| Yoo [44] | 60 | DM > 10 mm | 80.9% | 69.2% | |
| Jiang [43] | 55 | DM > 11 mm | 84.4% | 82.6% | |
| Kim [22] | 82 | DM < 10 mm * | 83% * | 41% * | RSBI |
| Spadaro [42] | 51 | Diaphragmatic RSBI > 1.3 * | 94% | 64.7% | RSBI |
| Palkar [45] | 73 | Decrease of diaphragm ET index < 3.8% ** | 79.2% | 75% | RSBI |
| First Author (Ref) | Population (N) | Outcome | DD Diag | Prevalence Incidence DD/PNI after Cardiac Surgery | Factors Associated with DD | Prognosis in Patients with DD |
|---|---|---|---|---|---|---|
| Markand [49] | Cardiac surgery (44) | Phrenic nerve palsy after cardiac surgery | EPS | 11% PNI | ||
| Canbaz [50] | Cardiac surgery (78) | Effects on PNI of hypothermia, ice slush, and use of mammary artery harvesting | EPS | 10.2% PNI | Hypothermia Ice slush | |
| Dimopoulou [9] | Cardiac surgery (63) | EPS | 21% PNI | Ice slush | ||
| Yamazaki [47] | CABG (200) | Incidence and factors associated with hemidiaphragm elevation after CABG | radiological study | 14.5% hemi-diaphragm elevation after CABG | Diabetes and use of internal thoracic artery grafting are risk factors | |
| DeVita [51] | Cardiac surgery (92) | Incidence of phrenic neuropathy after cardiac surgery | radiological and EPS studies | Abnormal DM in 54% of patients with abnormal CR 57% PNI among patients with abnormal DM | ||
| Merino-Ramirez [52] | CABG (94) | Incidence of phrenic neuropathy after CABG | EPS | 16% PNI | ||
| Bruni [1] | Cardiac surgery CABG 71% (100) | Rate of post-operative DD | TF < 20% | 38% | Duration of cardiopulmonary bypass | Higher rate of difficult weaning, Longer ICU length of stay |
| Moury [10] | Cardiac surgery CABG 46% (100) | Diaphragm thickening during weaning | TF < 20% | 75% | Length of surgery | |
| Tralhao [53] | (79) | Diaphragm US in patients with cardiac surgery | DM < 10 mm | 36% at D2 after surgery | ||
| Laghlam [4] | 3577 | Incidence, risk factors, and outcomes of patients with postoperative DD | 7.6% | HTA Higher BMI CABG | Post-operative pneumonia Reintubation, ventilation, ICU hospital stay duration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayssoil, A.; Mansencal, N.; Nguyen, L.S.; Orlikowski, D.; Prigent, H.; Bergounioux, J.; Annane, D.; Lofaso, F. Diaphragm Ultrasound in Cardiac Surgery: State of the Art. Medicines 2022, 9, 5. https://doi.org/10.3390/medicines9010005
Fayssoil A, Mansencal N, Nguyen LS, Orlikowski D, Prigent H, Bergounioux J, Annane D, Lofaso F. Diaphragm Ultrasound in Cardiac Surgery: State of the Art. Medicines. 2022; 9(1):5. https://doi.org/10.3390/medicines9010005
Chicago/Turabian StyleFayssoil, Abdallah, Nicolas Mansencal, Lee S. Nguyen, David Orlikowski, Hélène Prigent, Jean Bergounioux, Djillali Annane, and Frédéric Lofaso. 2022. "Diaphragm Ultrasound in Cardiac Surgery: State of the Art" Medicines 9, no. 1: 5. https://doi.org/10.3390/medicines9010005






