Potential Prognostic Relevance of Left-Ventricular Global Longitudinal Strain and of the Summation of the Mitral and Tricuspid Regurgitation Volume in Patients with Non-Ischemic Dilated Cardiomyopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- during the first phase, patients were enrolled, examined for the first time, and diagnosed with NIDCM (patients without chronic or WHF in their medical history and optimal medical therapy for HF).
- during the second phase, the early primary outcomes of a total of 102 patients with diagnosed NICDM were evaluated after a 1-year follow-up from diagnosis.
2.2. 2D Echocardiographic Data
2.3. Intraobserver and Interobserver Variability
2.4. Statistical Analysis
3. Results
4. Discussion
Clinical Perspectives
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merlo, M.; Caiffa, T.; Gobbo, M.; Adamo, L.; Sinagra, G. Reverse remodeling in Dilated Cardiomyopathy: Insights and future perspectives. Int. J. Cardiol. Heart Vasc. 2018, 18, 52–57. [Google Scholar] [CrossRef] [PubMed]
- McNally, E.M.; Mestroni, L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Díez-López, C.; Salazar-Mendiguchía, J.; García-Romero, E.; Fuentes, L.; Lupón, J.; Bayés-Genis, A.; Manito, N.; de Antonio, M.; Moliner, P.; Zamora, E.; et al. Clinical Determinants and Prognosis of Left Ventricular Reverse Remodelling in Non-Ischemic Dilated Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2022, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, H.Y.; Jian, W.; Yang, Z.J.; Gui, C. Development and validation of a nomogram to predict the risk of death within 1 year in patients with non-ischemic dilated cardiomyopathy: A retrospective cohort study. Sci. Rep. 2022, 12, 8513. [Google Scholar] [CrossRef]
- Morris, D.A.; Otani, K.; Bekfani, T.; Takigiku, K.; Izumi, C.; Yuda, S.; Sakata, K.; Ohte, N.; Tanabe, K.; Friedrich, K.; et al. Multidirectional global left ventricular systolic function in normal subjects and patients with hypertension: Multicenter evaluation. J. Am. Soc. Echocardiogr. 2014, 27, 493–500. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, P.; Bahl, A. Left ventricular size as a predictor of outcome in patients of non-ischemic dilated cardiomyopathy with severe left ventricular systolic dysfunction. Int. J. Cardiol. 2016, 221, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Riffel, J.H.; Keller, M.G.; Rost, F.; Arenja, N.; Andre, F.; Aus, d.S.F.; Fritz, T.; Ehlermann, P.; Taeger, T.; Frankenstein, L.; et al. Left ventricular long axis strain: A new prognosticator in non-ischemic dilated cardiomyopathy? J. Cardiovasc. Magn. Reson. 2016, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Bartko, P.E.; Arfsten, H.; Heitzinger, G.; Pavo, N.; Spinka, G.; Kastl, S.; Prausmüller, S.; Strunk, G.; Mascherbauer, J.; Hengstenberg, C.; et al. Global regurgitant volume: Approaching the critical mass in valvular-driven heart failure. Eur. Heart J. Cardiovasc. Imaging 2020, 2, 168–174. [Google Scholar] [CrossRef]
- Merlo, M.; Gigli, M.; Poli, S.; Stolfo, D.; Brun, F.; Lardieri, G.; Pinamonti, B.; Zecchin, M.; Pivetta, A.; Sinagra, G.; et al. Dilated cardiomyopathy: A dynamic disease-clinical course, reverse remodeling and prognostic stratification. G. Ital. Cardiol. 2016, 17, 15–23. [Google Scholar]
- Xu, X.R.; Han, M.M.; Yang, Y.Z.; Wang, X.; Hou, D.Y.; Meng, X.C.; Wang, H.; Zhao, W.S.; Zhang, L.; Xu, L. Fifteen-year mortality and prognostic factors in patients with dilated cardiomyopathy: Persistent standardized application of drug therapy and strengthened management may bring about encouraging change in an aging society. J. Geriatr. Cardiol. 2022, 19, 335–342. [Google Scholar]
- Raafs, A.G.; Boscutti, A.; Henkens, M.T.H.M.; van den Broek, W.W.A.; Verdonschot, J.A.J.; Weerts, J.; Stolfo, D.; Nuzzi, V.; Manca, P.; Hazebroek, M.R.; et al. Global Longitudinal Strain is Incremental to Left Ventricular Ejection Fraction for the Prediction of Outcome in Optimally Treated Dilated Cardiomyopathy Patients. J. Am. Heart Assoc. 2022, 11, e024505. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, 367 hypokinetic non-dilated cardiomyopathy, and its implications for the clinical practice: A position 368 statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Sciomer, S.; Moscucci, F.; Salvioni, E.; Marchese, G.; Bussotti, M.; Corrà, U.; Piepoli, M.F. Role of gender, age, and BMI in prognosis of heart failure. Eur. J. Prev. Cardiol. 2020, 27, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Deswal, A. Obesity as a prognostic factor in chronic symptomatic heart failure. Am. Heart J. 2005, 150, 1233–1239. [Google Scholar] [CrossRef]
- Wang, J.; Yang, F.; Wan, K.; Mui, D.; Han, Y.; Chen, Y. Left ventricular midwall fibrosis as a predictor of sudden cardiac death in non-ischaemic dilated cardiomyopathy: A meta-analysis. ESC Heart Fail. 2020, 7, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Dziewiecka, E.; Gliniak, M.; Winiarczyk, M.; Karapetyan, A.; Wiśniowska-Śmiałek, S.; Karabinowska, A.; Dziewięcki, M.; Podolec, P.; Rubiś, P. Mortality risk in dilated cardiomyopathy: The accuracy of heart failure prognostic models and dilated cardiomyopathy-tailored prognostic model. ESC Heart Fail. 2020, 7, 2455–2467. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Bergamaschi, L.; Pizzi, C.; Tuttolomondo, D. Resting global longitudinal strain and stress echocardiography to detect coronary artery disease burden. Eur. Heart J. Cardiovasc. Imaging 2023, 24, e86–e88. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. Authors/Task Force Members: 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar]
- Catapano, A.L.; Reiner, Z.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.; Durrington, P.; et al. European Society of Cardiology (ESC); European Atherosclerosis Society (EAS). ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 2011, 217, 3–46. [Google Scholar]
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Greene, S.J.; Bauersachs, J.; Brugts, J.; Ezekowitz, J.A.; Lam, C.S.P.; Lund, L.H.; Ponikowski, P.; Voors, A.A.; Zannad, F.; Zieroth, S.; et al. Worsening Heart Failure: Nomenclature, Epidemiology, and Future Directions. J. Am. Coll. Cardiol. 2023, 81, 413–424. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Bo, K.; Gao, Y.; Zhou, Z.; Gao, X.; Liu, T.; Zhang, H.; Li, Q.; Wang, H.; Xu, L. Incremental prognostic value of left atrial strain in patients with heart failure. ESC Heart Fail. 2022, 6, 3942–3953. [Google Scholar] [CrossRef]
- Sjöland, H.; Silverdal, J.; Bollano, E.; Pivodic, A.; Dahlström, U.; Fu, M. Temporal trends in outcome and patient characteristics in dilated cardiomyopathy, data from the Swedish Heart Failure Registry 2003–2015. BMC Cardiovasc. Disord. 2021, 21, 307. [Google Scholar] [CrossRef] [PubMed]
- Agrinier, N.; Thilly, N.; Briancon, S.; Juillière, Y.; Mertes, P.M.; Villemot, J.P.; Alla, F.; Zannad, F.; EPICAL group. Prognostic factors associated with 15-year mortality in patients with hospitalized systolic HF: Results of the observational community-based EPICAL cohort study. Int. J. Cardiol. 2017, 228, 940–947. [Google Scholar] [CrossRef]
- Lupón, J.; Díez-López, C.; de Antonio, M.; Domingo, M.; Zamora, E.; Moliner, P.; González, B.; Santesmases, J.; Troya, M.I.; Bayés-Genís, A. Recovered heart failure with reduced ejection fraction and outcomes: A prospective study. Eur. J. Heart Fail. 2017, 19, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Karatolios, K.; Holzendorf, V.; Richter, A.; Schieffer, B.; Pankuweit, S.; Competence Network Heart Failure Germany. Long-term outcome and predictors of outcome in patients with non-ischemic dilated cardiomyopathy. Int. J. Cardiol. 2016, 220, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Orphanou, N.; Papatheodorou, E.; Anastasakis, A. Dilated cardiomyopathy in the era of precision medicine: Latest concepts and developments. Heart Fail. Rev. 2022, 27, 1173–1191. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gao, Y.; Wang, H.; Zhou, Z.; Wang, R.; Chang, S.S.; Liu, Y.; Sun, Y.; Rui, H.; Yang, G.; et al. Association between right ventricular strain and outcomes in patients with dilated cardiomyopathy. Heart 2020, 107, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.J.; Breuninger, K.; Lehrke, S.; Voss, A.; Galuschky, C.; Lossnitzer, D.; Andre, F.; Ehlermann, P.; Franke, J.; Taeger, T.; et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Park, J.B.; Park, J.H.; Cho, G.Y. Global Longitudinal Strain to Predict Mortality in Patients With Acute Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 1947–1957. [Google Scholar] [CrossRef]
- Romano, S.; Judd, R.M.; Kim, R.J.; Kim, H.W.; Klem, I.; Heitner, J.F.; Shah, D.J.; Jue, J.; White, B.E.; Indorkar, R.; et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients With Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2018, 11, 1419–1429. [Google Scholar] [CrossRef]
- Bartko, P.E.; Arfsten, H.; Heitzinger, G.; Pavo, N.; Toma, A.; Strunk, G.; Hengstenberg, C.; Hülsmann, M.; Goliasch, G. A Unifying Concept for the Quantitative Assessment of Secondary Mitral Regurgitation. J. Am. Coll. Cardiol. 2019, 73, 2506–2517. [Google Scholar] [CrossRef]
- Bartko, P.E.; Arfsten, H.; Frey, M.K.; Heitzinger, G.; Pavo, N.; Cho, A.; Neuhold, S.; Tan, T.C.; Strunk, G.; Hengstenberg, C.; et al. Natural History of Functional Tricuspid Regurgitation: Implications of Quantitative Doppler Assessment. JACC Cardiovasc. Imaging 2019, 12, 389–397. [Google Scholar] [CrossRef]
- Rossi, A.; Dini, F.L.; Faggiano, P.; Agricola, E.; Cicoira, M.; Frattini, S.; Simioniuc, A.; Gullace, M.; Ghio, S.; Enriquez-Sarano, M.; et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 1675–1680. [Google Scholar] [CrossRef]
- La Vecchia, L.; Zanolla, L.; Varotto, L.; Bonanno, C.; Spadaro, G.L.; Ometto, R.; Fontanelli, A. Reduced right ventricular ejection fraction as a marker for idiopathic dilated cardiomyopathy compared with ischemic left ventricular dysfunction. Am. Heart J. 2001, 142, 181–189. [Google Scholar] [CrossRef]
- Gulati, A.; Ismail, T.F.; Jabbour, A.; Alpendurada, F.; Guha, K.; Ismail, N.A.; Raza, S.; Khwaja, J.; Brown, T.D.; Morarji, K.; et al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation 2013, 128, 1623–1633. [Google Scholar] [CrossRef]
- Juillière, Y.; Barbier, G.; Feldmann, L.; Grentzinger, A.; Danchin, N.; Cherrier, F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur. Heart J. 1997, 18, 276–280. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, L.; Paccanaro, M.; Bonanno, C.; Varotto, L.; Ometto, R.; Vincenzi, M. Left ventricular versus biventricular dysfunction in idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1999, 83, 120–122. [Google Scholar] [CrossRef] [PubMed]
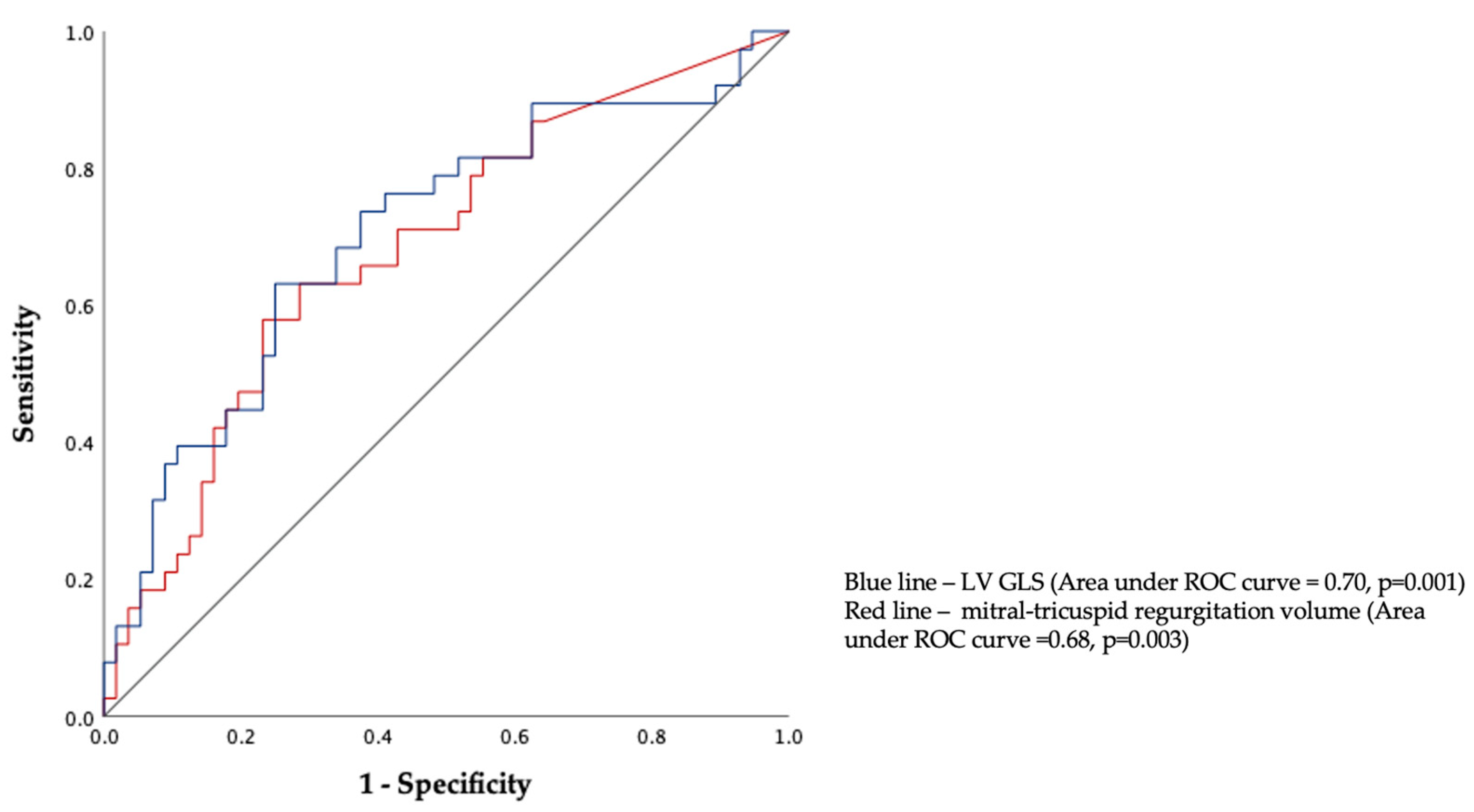
| Variables | Patients with Early Primary Outcomes n = 39 | Patients without Early Primary Outcomes n = 63 | p-Value |
|---|---|---|---|
| Age, y | 48.5 ± 11.7 | 49.8 ± 10.0 | 0.574 |
| Males, n (%) | 26 (36.1) | 46 (63.9) | 0.510 |
| BSA, m2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 0.066 |
| Heart rate, beat/min | 81.3 ± 17.8 | 78.8 ± 15.8 | 0.467 |
| Systolic blood pressure, mmHg | 123.0 ± 14.0 | 127.5 ± 12.6 | 0.104 |
| Dyslipidemia, n (%) | 16 (40.0) | 24 (60.0) | 0.837 |
| Arterial hypertension, n (%) | 24 (39.3) | 37 (60.7) | 0.510 |
| Smoking, n (%) | 19 (45.2) | 23 (54.8) | 0.302 |
| Diabetes mellitus, n (%) | 4 (57.1) | 3 (42.9) | 0.425 |
| Chronic kidney disease, n (%) | 5 (55.6) | 4 (44.4) | 0.302 |
| Genetic analysis: positive, n (%) Uncertain significance, n (%) Refused the genetic test, n (%) | 5 (20.0) 5 (41.7) 11 (91.7) | 20 (80.0) 7 (58.3) 1 (8.3) | <0.001 |
| Pharmacotherapy (at baseline), n (%) | |||
| ACE-I/ARB | 11 (35.4) | 20 (64.5) | 0.742 |
| Betablocker | 9 (42.8) | 12 (57.1) | 0.381 |
| CCB | 5 (41.6) | 7 (58.3) | 0.402 |
| Aldosterone antagonist | 2 (40) | 3 (60) | 0.453 |
| Statins | 3 (37.5) | 5 (62.5) | 0.291 |
| VT, n (%) | 23 (65.7) | 12 (34.3) | <0.001 |
| Atrial fibrillation, n (%) | 21 (50.0) | 21 (50.0) | 1.000 |
| LBBB, n (%) | 19 (43.2) | 25 (56.8) | 0.419 |
| Prevalence of CA stenosis Without any CA stenosis, n (%) CA stenosis <50%, n (%) | 24 (31.6) 15 (57.7) | 52 (68.4) 11 (42.3) | p > 0.05 |
| QRS duration, ms | 125.5 ± 31.2 | 118.1 ± 27.6 | 0.226 |
| NYHA class III-IV, n (%) | 45 (62.3) | 30 (37.7) | 0.057 |
| 6MWT (<300 m), n (%) | 10 (66.7) | 5 (33.3) | 0.602 |
| Hs-CRP | 3.0 ± 1.5 | 3.1 ± 1.6 | 0.854 |
| BNP, ng/L | 1812.1 ± 844.3 | 822.7 ± 425.6 | 0.006 |
| Heart failure death, n (%) | 10 (25.6) | - | - |
| Hospitalization for HF worsening at 1 year, n (%) | 36 (92.3) | - | - |
| Variables | Patients with Early Primary Outcomes n = 39 | Patients without Early Primary Outcomes n = 63 | p-Value |
|---|---|---|---|
| LVESD, mm | 57.8 ± 9.0 | 55.1 ± 7.2 | 0.118 |
| LVESDi, mm/m2 | 29.1 ± 5.1 | 26.7 ± 3.9 | 0.013 |
| LVEDD, mm | 66.1 ± 7.0 | 64.2 ± 5.8 | 0.128 |
| LVEDDi, mm/m2 | 34.1 ± 4.4 | 31.1 ± 3.4 | 0.009 |
| LAV, mL | 134.3 ± 89.3 | 104. ± 36.6 | 0.064 |
| LAVi, mL/m2 | 66.3 ± 40.6 | 50.2 ± 16.9 | 0.027 |
| RAV, mL | 81.6 ± 27.4 | 78.9 ± 23.1 | 0.585 |
| RAVi, mL/m2 | 40.5 ± 10.3 | 38.1 ± 10.2 | 0.266 |
| MRV, mL | 33.3 ± 14.1 | 24.3 ± 18.0 | 0.005 |
| TRV, mL | 27.8 ± 14.7 | 16.6 ± 15.7 | 0.002 |
| Mitral–tricuspid regurgitation volume, mL | 61.1 ± 20.4 | 40.9 ± 22.9 | 0.003 |
| LVEDV, mL | 233.4 ± 75.2 | 230.7 ± 70.0 | 0.865 |
| LVEDVi, mL/m2 | 117.4 ± 39.6 | 112.3 ± 35.3 | 0.526 |
| LVESV, mL | 173.8± 68.6 | 156.8 ± 59.5 | 0.219 |
| LVESVi, mL/m2 | 90.0 ± 39.2 | 79.5 ± 36.1 | 0.198 |
| RVEDVi, mL | 76.8 ± 32.4 | 69.5 ± 26.8 | 0.069 |
| RVESVi, mL | 49.7 ± 26.4 | 42.1 ± 17.0 | 0.105 |
| LV GLS, % | −7.4 ± 2.7 | −10.3 ± 2.6 | 0.001 |
| LV GCS, % | −12.2 ± 5.6 | −14.7 ± 6.0 | 0.097 |
| LV GRS, % | 18.1 ± 9.2 | 21.6 ± 9.3 | 0.249 |
| GSI, % | 12.8 ± 4.5 | 12.7 ± 5.4 | 0.929 |
| Longitudinal–circumferential systolic index, % | 9.9 ± 3.7 | 9.7 ± 4.5 | 0.826 |
| LVEF, % | 27.3 ± 9.6 | 29.1 ± 8.5 | 0.356 |
| RVFWLS, % | −17.7 ± 3.1 | −18.5 ± 2.0 | 0.257 |
| RVEF, % | 37.3 ± 10.1 | 42.5 ± 7.9 | 0.099 |
| RVGLS, % | −10.5 ± 3.7 | −12.9 ± 3.2 | 0.053 |
| FAC, % | 30.9 ± 6.6 | 31.7 ± 5.5 | 0.868 |
| LAScd, % | −12.1 ± 5.0 | −13.7 ± 4.3 | 0.551 |
| LASr, % | 21.7 ± 4.1 | 24.8 ± 4.0 | 0.047 |
| LASct, % | −9.3 ± 3.6 | −10.3 ± 3.4 | 0.322 |
| RAScd, % | −14.3 ± 5.8 | −16.5 ± 5.1 | 0.065 |
| RASr, % | 28.9 ± 5.8 | 29.1 ± 6.5 | 0.875 |
| RASct, % | −12.2 ± 5.2 | −12.5 ± 6.1 | 0.771 |
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| LV GLS, % | 0.876 | 0.855–0.998 | 0.034 | 0.778 | 0.650–0.923 | 0.034 |
| LASr, % | 1.005 | 0.955–1.057 | 0.858 | - | - | - |
| RVGLS, % | 0.986 | 0.890–1.092 | 0.780 | - | - | - |
| RVFWLS, % | 0.979 | 0.881–1.089 | 0.701 | - | - | - |
| LVEF, % | 1.061 | 0.998–1.128 | 0.060 | - | - | - |
| LAVi, mL/m2 | 0.976 | 0.955–0.998 | 0.034 | 0.971 | 0.932–1.012 | 0.161 |
| TAPSE, mm | 1.150 | 0.976–1.355 | 0.094 | - | - | - |
| Mitral–tricuspid regurgitation volume, mL | 0.976 | 0.955–0.998 | 0.034 | 1.098 | 1.012–1.295 | 0.008 |
| MRV, mL | 0.966 | 0.938–0.996 | 0.026 | 1.064 | 0.984–1.151 | 0.120 |
| TRV, mL | 0.971 | 0.942–1.001 | 0.061 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mėlinytė-Ankudavičė, K.; Ereminienė, E.; Mizarienė, V.; Šakalytė, G.; Plisienė, J.; Jurkevičius, R. Potential Prognostic Relevance of Left-Ventricular Global Longitudinal Strain and of the Summation of the Mitral and Tricuspid Regurgitation Volume in Patients with Non-Ischemic Dilated Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2023, 10, 410. https://doi.org/10.3390/jcdd10100410
Mėlinytė-Ankudavičė K, Ereminienė E, Mizarienė V, Šakalytė G, Plisienė J, Jurkevičius R. Potential Prognostic Relevance of Left-Ventricular Global Longitudinal Strain and of the Summation of the Mitral and Tricuspid Regurgitation Volume in Patients with Non-Ischemic Dilated Cardiomyopathy. Journal of Cardiovascular Development and Disease. 2023; 10(10):410. https://doi.org/10.3390/jcdd10100410
Chicago/Turabian StyleMėlinytė-Ankudavičė, Karolina, Eglė Ereminienė, Vaida Mizarienė, Gintarė Šakalytė, Jurgita Plisienė, and Renaldas Jurkevičius. 2023. "Potential Prognostic Relevance of Left-Ventricular Global Longitudinal Strain and of the Summation of the Mitral and Tricuspid Regurgitation Volume in Patients with Non-Ischemic Dilated Cardiomyopathy" Journal of Cardiovascular Development and Disease 10, no. 10: 410. https://doi.org/10.3390/jcdd10100410
APA StyleMėlinytė-Ankudavičė, K., Ereminienė, E., Mizarienė, V., Šakalytė, G., Plisienė, J., & Jurkevičius, R. (2023). Potential Prognostic Relevance of Left-Ventricular Global Longitudinal Strain and of the Summation of the Mitral and Tricuspid Regurgitation Volume in Patients with Non-Ischemic Dilated Cardiomyopathy. Journal of Cardiovascular Development and Disease, 10(10), 410. https://doi.org/10.3390/jcdd10100410






